Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.105 n.11-12 Pretoria Nov./Dec. 2009
RESEARCH LETTERS
Anti-inflammatory and antibacterial profiles of selected compounds found in South African propolis
K. Du ToitI; S. ButheleziI; J. BodensteinII, *
IDepartment of Pharmaceutical Chemistry, School of Pharmacy and Pharmacology, University of KwaZulu-Natal, Private Bag X54001, Durban 4000, South Africa
IIDepartment of Pharmacology, School of Pharmacy and Pharmacology, University of KwaZulu-Natal, Private Bag X54001, Durban 4000, South Africa
ABSTRACT
Propolis is a complex resinous substance manufactured by honey bees to scaffold and protect the hive against pathogens. Although it has been widely used for its medicinal properties, it is unknown whether the activity depends on the concentrations of specific constituents or on potentiation between these. This study describes (1) the individual topical anti-inflammatory activities of selected flavonoids commonly found in propolis, and (2) their antibacterial activities, alone or in combination with the non-flavonoid caffeic acid phenethyl ester (CAPE). For the anti-inflammatory activities, the reduction in croton oil-induced oedema in a mouse model, after topical application of quercetin and galangin for 3 h, was more than 50%, while after6hof treatment the reduction was less then 50%. By contrast, the suppressive activity of luteolin was about 30% and 50%, for treatments of 3 h and 6 h, respectively. The maximum inhibition of the growth of Staphylococcus aureus by each of CAPE, eriodictyol and quercetin was about 20%, while luteolin was inactive. When combined with CAPE, potentiation of the antibacterial effect was observed in the case of luteolin, but antagonism was observed when combined with either eriodictyol or quercetin. The propolis flavonoids each appear to have significant anti-inflammatory activity while their antibacterial activities are somewhat weaker and significant only when luteolin was combined with CAPE.
Key words: propolis, flavonoids, antibacterial, anti-inflammatory, potentiation, antagonism
Introduction
Propolis is a complex resinous substance manufactured by honeybees. It consists of exudates collected from the parts of various plant species, substances secreted from bee metabolism, and materials which are introduced during propolis elaboration.1–3 In general, it is composed of 50% resin and vegetable balsam, 30% wax, 10% essential and aromatic oils, 5% pollen and 5% various other organic substances including debris. Exudates collected from leaves and leaf buds, mucilages, gums, resins and latices contribute the most to the constituents of propolis.2 Of these, the largest group of compounds is the flavonoid pigments, such as luteolin, eriodictyol, galangin and quercetin. Flavonoids are ubiquitous in the plant kingdom, occurring naturally in foods and at concentrations of <0.1–0.7% in propolis.2,4–7 Other non-flavonoid constituents include caffeic acids and their esters (2–20%), such as caffeic acid phenethyl ester (CAPE).8
Since the chemical composition of propolis is largely dependent on plant exudates, the geographical region in which particular plants are present and the season during which they are harvested play an important role.1 This chemical diversity becomes more apparent as it has been reported that between ten and two hundred different compounds, at varying concentrations, were detected in propolis from different collection sites.2 In addition, it has been shown that the species of bee influences the composition of propolis.9 Thus the chemical standardisation of propolis has been difficult to achieve.
Bees use propolis to scaffold the hive and embalm killed invader organisms. Propolis has been recognised by man for its medicinal properties since ancient times. Claims to promote propolis as a 'global remedy' are supported by its numerous medicinal uses that include, amongst others, antibacterial10 and anti-inflammatory properties. The list of preparations and uses is nearly endless. For example, propolis is used in toothpaste to prevent gingivitis and in cosmetic products to promote tissue regeneration.4
Considering the striking variability in the chemical composition of propolis and its claimed therapeutic uses, it is unknown whether observed activity is dependent on the concentrations of specific constituents or on potentiation between these. The goal of this study was to investigate the pharmacological properties of some components commonly found in propolis, taking into consideration that in South African propolis, flavonoids are the main constituents.11 Specifically, the ability of each of the flavonoids luteolin, quercetin and galangin to reduce croton oil-induced swelling in a mouse model was investigated. In addition, the susceptibility of the important invasive pathogen Staphylococcus aureus to each of the flavonoids luteolin, eriodictyol and quercetin was tested. Since these flavonoids exhibited little antibacterial activity alone, it was sought to identify potential potentiation of activity in combination with CAPE. Whereas antibacterial activity was less pronounced and variable when flavonoids were combined with CAPE, the topical anti-inflammatory activity of all the flavonoids was significant on their own.
Materials and methods
Chemicals
(S)-3',4',5,7-Tetrahydroxyflavanone (eriodictyol); 3',4',5,7tetrahydroxyflavone (luteolin); 3,5,7-trihydroxyflavone (galangin); p-iodonitrotetrazolium chloride violet (p-INT); 3',4',3,5,7-pentahydroxyflavone dihydrate (quercetin) and Mueller Hinton broth were obtained from Fluka (Buchs, Switzerland). Caffeic acid phenethyl ester (CAPE), croton oil, indomethacin and neomycin solution (10 mg ml–1) were obtained from Sigma-Aldrich (St Louis, MO, U.S.A.). Acetone and dimethyl sulphoxide (DMSO) were obtained from Merck (Darmstadt, Germany).
Assessment of croton oil-induced oedema
Ethical approval (003/09/Animal) from the University of KwaZulu-Natal Animal Ethics Subcommittee was obtained prior to the investigation of croton oil-induced oedema12 in a mouse model. Guidelines by the University of KwaZulu-Natal Animal Ethics Subcommittee and Biomedical Resources Unit for the maintenance and treatment of laboratory animals were followed.
This topical study design was based on previous studies where the inhibition of croton oil-induced auricular oedema by emu oil was investigated.13,14 Briefly, 8-week-old male Balb/c mice of approximately 30 g each were used. Equal volumes of croton oil were mixed with acetone as vehicle and 50 µl was applied for 1 h onto the inner surface of the right auricle of each mouse to induce oedema. Acetone has not been documented to have an independent effect.15 Thereafter, the flavonoids in acetone were applied (0.5 mg in 50 µl;3hor6h) onto the right auricle to assess the reduction in oedema. The non-steroidal anti-inflammatory drug indomethacin (0.5 mg; 6 h) was included as a control.
Mice were euthanised after treatment. From each mouse, left and right auricle biopsy specimens were obtained with a 6-mm biopsy punch and then weighed. Oedema was quantified by calculating the difference in weights of the right and left auricle biopsy specimens and expressed as a percentage of the croton oil control.
Assessment of bacterial susceptibility
Mueller Hinton broth was inoculated with Staphylococcus aureus (ATCC strain 12600, Manassas, VA, U.S.A.) and grown in an incubator (37ºC; optical density of 0.8 at 490 nm). The broth was prepared according to the manufacturer's protocol.
The bacterial susceptibility assay was based on a microplate method16 but with modifications. Briefly, 100 µl of sterile broth was added in each well of a clear, sterile 96-well microtitre plate (Corning Life Sciences, Acton, MA, U.S.A.), after which 100 µl of the appropriate drug or combination of drugs (at 0, 30, 90, 150, 210 or 300 µM in 12% v/v DMSO) was added to each well. This was followed by addition of 100 µl bacterial culture to each well. Thus, with a dilution factor of three, the final concentration of drug or combination of drugs in the wells was 0, 10, 30, 50, 70 or 100 µM in a final concentration of 4% v/v DMSO. The plate was then tapped to mix the contents and incubated at 37ºC for 18 h. After incubation, 40 µl of p-INT (400 µg ml–1 in water) was added to each well, the plate was tapped to mix the contents, and incubated at 37ºC for 15 min.
Bacterial growth was quantified by colourimetry (490 nm) in a microplate reader (BioTek ELx800, Winooski, VT, U.S.A.). Bacterial growth was quantified as a percentage of the control without any drugs. Growth of S. aureus was not significantly inhibited by DMSO (data not shown).
Data analysis
Data are reported as the mean ± s.e.m. of four to five independent experiments performed in duplicate. GraphPad Prism (version 5.02; GraphPad Software, San Diego, CA, U.S.A.) was used to present and analyse the data. Statistical comparisons were made by one-way ANOVA followed by Bonferroni's post-test to determine P values. A value of P < 0.05 was considered significant.
Results and discussion
Anti-inflammatory activity
Reduction of croton oil-induced oedema by flavonoids
The anti-inflammatory effects of plant extracts and flavonoids have predominantly been investigated systemically in animal models where inflammation was locally induced (e.g. cotton pellet-induced granuloma and carrageenan-induced mouse paw oedema) but the flavonoids have been administered by injection.17,18 Little is known about the acute anti-inflammatory effects of the flavonoids luteolin, quercetin and galangin when topically applied. Thus, to assess potential differences in the ability of these flavonoids to reduce croton oil-induced oedema and to sustain a topical anti-inflammatory response, swelling of the mice ears was measured after two treatment times. After 3 h and6hof topical treatment, oedema was significantly reduced with 0.5 mg of each of indomethacin, luteolin (3 h: 31.6 ± 6.9%; 6 h: 49.5 ± 7.8%), quercetin (3 h: 55.9 ± 7.7%; 6 h: 42.6 ± 4.7%) and galangin (3 h: 55.1 ± 4.5%; 6 h: 40.4 ± 4.3%). After 3 h of treatment, the reduction of oedema by quercetin was more pronounced than by luteolin (1.6-fold difference; P < 0.05) (Fig. 1, middle panel). Although the differences in response to the flavonoids after 3 h and 6 h were not significant, quercetin and galangin became less active after 6 h of treatment (Fig. 1, right panel).
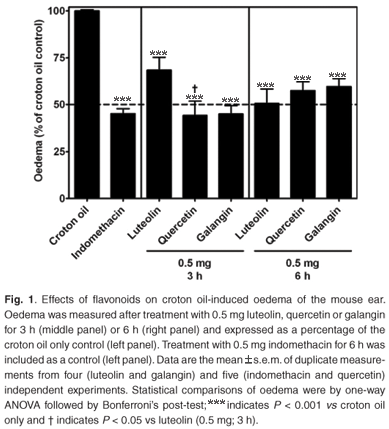
These results suggest that quercetin and galangin at 0.5 mg were topically active and faster acting than luteolin in reducing inflammation after 3 h treatment. The activity of luteolin increased from 3 h to 6 h treatment, while those of quercetin and galangin decreased. The molecular mechanisms of anti-inflammatory activity (possibly through inhibition of IL-1β and TNF-α) are not completely understood and perhaps future research should focus on this.
Antibacterial activity
Antibacterial activity of CAPE
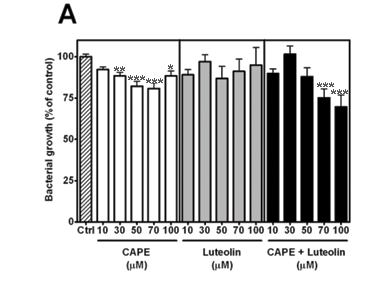
It has been reported that the antibacterial action of caffeic acid can be attributed to its esters, such as 3,3-dimethyl caffeate and isopent-3-enyl caffeate,19 but little is known about the antibacterial activity of the phenethyl ester (CAPE). To assess the susceptibility of Staphylococcus aureus to CAPE, bacterial growth was measured against increasing concentrations of CAPE. Growth was inhibited in a concentration-dependent fashion and a maximum (19.2 ± 3.4%) was reached at 70 µM (Fig. 2A–C, left panel).
Antibacterial activity of CAPE and luteolin
It has been suggested that the combined and specific concentrations of its compounds contribute to the pharmacological activity of propolis.2 Arima and coworkers demonstrated the synergistic effects of rutin (which has no independent antibacterial activity) on the activity of the flavonoids quercetin and eriodictyol against Bacillus cereus and Salmonella enteritidis.20 Although luteolin,21 eriodictyol and quercetin20 have each previously been reported to exhibit antibacterial activity,22 it is not known whether their activity against S. aureus is potentiated in the presence of CAPE. Since luteolin did not significantly inhibit growth at the concentrations tested (Fig. 2A, middle panel), it was combined with CAPE. The combination significantly inhibited growth at both 70 µM (24.8 ± 5.4%) and 100 µM (30.3 ± 7.3%), suggesting potentiation (Fig. 2A, right panel).
Antibacterial activity of CAPE and eriodictyol
Eriodictyol significantly inhibited growth at 50 µM (18.2 ± 6.1%) and 70 µM (19.8 ± 8.2%) (Fig. 2B, middle panel). The combination of CAPE and eriodictyol reduced the activity, however, suggesting antagonism (Fig. 2B, right panel).
Antibacterial activity of CAPE and quercetin
Quercetin significantly inhibited growth at 10 µM (16.9 ± 3.4%), 70 µM (16.8 ± 2.6%) and 100 µM (17.8 ± 2.5%) (Fig. 2C, middle panel). Like eriodictyol, when quercetin was combined with CAPE, the activity was reduced, suggesting antagonism.
Conclusion
Each of the propolis flavonoids tested exerted anti-inflammatory effects, but differed in their durations of the effect. In contrast, their weaker antibacterial activities appeared to be critically dependent on the constituent concerned, as well as on its combination with other constituents, and their concentrations. The highly variable composition of propolis may thus influence its medicinal activity. Hence, some types of propolis may be more active as anti-inflammatory agents than as antibacterial agents.
We thank Rolexsi (Pty) Ltd and the University of KwaZulu-Natal Competitor Fund for financial support. We thank Mr Dennis Ndwandwe of the UKZN Biomedical Resource Centre for assistance with the mice.
1 Bankova V., De Castro S. and Marcucci M. (2000). Propolis: recent advances in chemistry and plant origin. Apidologie 31, 3–15. [ Links ]
2 Burdock G.A. (1998). Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 36, 347–363. [ Links ]
3 Castaldo S. and Capasso F. (2002). Propolis, an old remedy used in modern medicine. Fitoterapia 73(suppl. 1), S1–S6. [ Links ]
4 Chen J., Long Y., Han M., Wang T., Chen Q. and Wang R. (2008). Water-soluble derivative of propolis mitigates scopolamine-induced learning and memory impairment in mice. Pharmacol. Biochem. Behav. 90, 441–446. [ Links ]
5 Uzel A., Sorkun K., Önçag Ö., Çogulu D., Gençay Ö. and Salih B. (2005). Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 160, 189–195. [ Links ]
6 Jiang L., Fang G., Zhang Y., Cao G. and Wang S. (2008). Analysis of flavonoids in propolis and Ginkgo biloba by micellar electrokinetic capillary chromatography. J. Agric. Food Chem. 56, 11571–11577. [ Links ]
7 Wang S-P., Fu M-D. and Wang M-H. (2007). Separation mechanism and determination of flavanones with capillary electrophoresis and highperformance liquid chromatography. J. Chromatogr. A. 1164, 306–312. [ Links ]
8 Gardana C., Scaglianti M., Pietta P. and Simonetti P. (2007). Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 45, 390–399. [ Links ]
9 Silici S. and Kutluca S. (2005). Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 99, 69–73. [ Links ]
10 Lu L-C., Chen Y-W. and Chou C-C. (2005). Antibacterial activity of propolis against Staphylococcus aureus. Int. J. Food Microbiol. 102, 213–220. [ Links ]
11 Shigemi T., Tsutomu W. and Tadataka N. (2000). On the chemical evaluation of propolis. Nat. Med. (Tokyo). 54, 306–313. [ Links ]
12 Tubaro A., Dri P., Delbello G., Zilli C. and Della Loggia R. (1986). The croton oil ear test revisited. Agents Actions 17, 347–349. [ Links ]
13 López A., Sims D., Ablett R., Skinner R., Léger L., Lariviere C., Jamieson L., Martínez-Burnes J. and Zawadzka G. (1999). Effect of emu oil on auricular inflammation induced with croton oil in mice. Am. J. Vet. Res. 60, 1558–1561. [ Links ]
14 Yoganathan S., Nicolosi R., Wilson T., Handelman G., Scollin P., Tao R., Binford P. and Orthoefer F. (2003). Antagonism of croton oil inflammation by topical emu oil in CD-1 mice. Lipids 38, 603–607. [ Links ]
15 Fretland D., Widomski D., Zemaitis J., Walsh R., Levin S., Djuric S., Shone R., Tsai B. and Gaginella T. (1990). Inflammation of guinea pig dermis. Effects of leukotriene B4 receptor antagonist, SC-41930. Inflammation 14, 727–739. [ Links ]
16 Eloff J.N. (1998). A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica 64, 711–713. [ Links ]
17 Guardia T., Rotelli A.E., Juarez A.O. and Pelzer L.E. (2001). Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmaco. 56, 683–687. [ Links ]
18 Pelzer L.E., Guardia T., Juarez A.O. and Guerreiro E. (1998). Acute and chronic anti-inflammatory effects of plant flavonoids. Il Farmaco. 53, 421–424. [ Links ]
19 Kartal M., Yildz S., Kaya S., Kurucu S. and Topçu G. (2003). Antimicrobial activity of propolis samples from two different regions of Anatolia. J. Ethno pharmacol. 86, 69–73. [ Links ]
20 Arima H., Ashida H. and Danno G. (2002). Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 66, 1009–1014. [ Links ]
21 Lv P-C., Li H-Q., Xue J-Y., Shi L. and Zhu H-L. (2009). Synthesis and biological evaluation of novel luteolin derivatives as antibacterial agents. Eur. J. Med. Chem. 44, 908–914. [ Links ]
22 Cushnie T. and Lamb A. (2005). Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26, 343–356. [ Links ]
Received 19 August.
Accepted 3 November 2009.
* Author for correspondence E-mail: bodensteinj@ukzn.ac.za