Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.105 no.11-12 Pretoria nov./dic. 2009
RESEARCH LETTERS
Were Malagasy Uncarina fruits dispersed by the extinct elephant bird?
J.J. MidgleyI, *; N. IllingII
IDepartment of Botany, University of Cape Town, Private Bag, Rondebosch 7701, South Africa
IIDepartment of Molecular and Cell Biology, University of Cape Town, Private Bag, Rondebosch 7701, South Africa
ABSTRACT
We hypothesise that the spiny fruits of the endemic Madagascar genus Uncarina (Pedaliaceae) are trample burrs that evolved to be dispersed on the feet of the extinct elephant bird (Aepyornis). Our evidence is: i) the morphology of the fruit with its large grapple hooks is more likely to attach to a foot than to adhere to fur and ii) the presentation of mature fruits on the ground rather than in the canopy. These differences to adhesive burrs make lemurs unlikely dispersers. We argue, given the absence of other large terrestrial mammals in Madagascar, that the most likely dispersers of Uncarina fruits were the extinct large birds. If correct, our hypothesis has implications for conservation of Uncarina, the biogeography of the elephant birds and dispersal biology. For example, we predict that the demography of Uncarina will be skewed towards adult plants, and that the dispersal mutualism could possibly be rescued by domestic animals.
Key words: Madagascar, Aepyornis, burrs, seed dispersal
Some species of Uncarina (Pedaliaceae), an endemic genus of Malagasy plants, have large prickly fruits (Fig. 1a,b,d). It has been speculated that these fruits are adhesive burrs that are dispersed by attachment to the furry coat of arboreal animals.1 Ihlenfeldt2 did not specify which animals; however, in the context of Madagascar, these would presumably be extinct or extant lemurs. In contrast, we speculate that these fruits are trample burrs which represent an anachronistic dispersal attribute for the extinct elephant bird, Aepyornis.
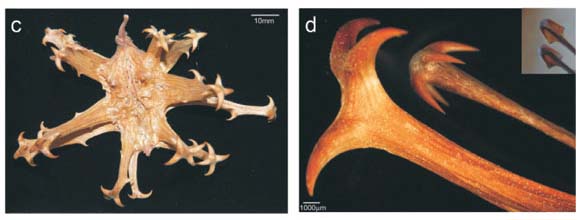
There are two kinds of burrs—adhesive burrs (which may be sticky or covered in small spines) and trample burrs that stick into or around hooves or feet.3 Both are well described structurally and taxonomically, but almost nothing is known about the animal species for which various trample burrs evolved.3 The Pedaliaceae is a family well known for spiny fruit1 and includes the devil's claw (or grapple thorn) fruits of Harpagophytum species (Fig. 1c,d) found in the arid regions of central-southern Africa. These burrs are clearly trample burrs because they occur on prostrate plants and obviously evolved for animal dispersal.3 However there are no data on dispersal of this species by animals. Questions such as which indigenous species disperses these fruits and whether they are hard-or soft-footed, remain unanswered. The only evidence available is a film sequence of a running ostrich apparently unbothered by the Harpagophytum burr surrounding its foot.4 A trample burr dispersed in this way would gradually break up and its seeds would be liberated. Since the burr is tough and indehiscent, few seeds would be liberated in the absence of dispersal.
Several lines of evidence suggest that the fruits of some Uncarina species are also trample burrs. First, at the Arboretum d'Antsokay near Tulear, we observed that fruits of Uncarina stellulifera fall to the ground when mature and accumulate around the base of adult plants, often in large numbers (>10). Also, the attachment hooks are only exposed after the fruit dries out (compare Fig. 1a,d). The fruit is thus not spiny when on the plant. Furthermore, the sharp terminal spines of the mature U. stellulifera fruits are too large and far apart to clasp fur (Fig. 1b,d). The spiny arms of these fruits are hairless (Fig. 1d) whereas adhesive burrs typically have multiple small spines or hairs (<0.5 mm) per arm (Fig. 1e–h), which are also close enough to each other to trap, rather than to stick into, hairs. Finally, most Uncarina species are unsuitable for climbing by lemurs large enough to disperse their fruits. They are swollen-stem succulent shrubs with fragile, narrow terminal fruit-bearing branches.5 We suggest therefore that Uncarina has trample burrs not adhesive burrs.
The only terrestrial animals large enough to trample the fruit would be elephant birds and possibly the extinct giant lemur (Archaeoindris fontoynontii). This lemur was considered to be about 200 kg in mass and to spend time equally between climbing and terrestrial quadrupedialism.6 It is unlikely that a primate with feet flexible enough to be capable of climbing, would also have had feet hard enough to tolerate a trample burr. We have not considered pygmy hippopotami as possible dispersers on account of their association with mesic habitats.7
This leaves the elephant bird as the most likely disperser. The two Malagasy genera of large ratite birds, Aepyornis and Mulleromis, became extinct in the 19th century.8 These birds were possibly 3–4 m tall and weighed up to 400 kg.8 The distribution of Aepyornis7,9 essentially matches that of dry deciduous forest and arid spiny bush,10 as well as that of Uncarina5 (Fig. 2b). Remains of the giant lemur A. fontoynontii have only been found in central Madagascar6,7 and not in the west or south, which further suggests that it was not a likely disperser (Fig. 2b).
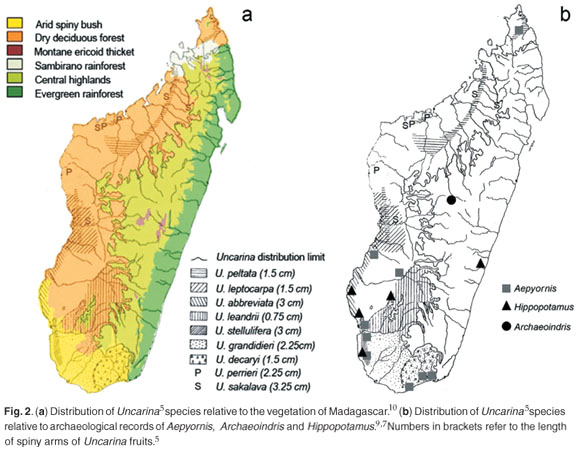
If the speculation that Uncarina evolved to be dispersed by elephant birds is correct, this has three implications. First, it strengthens the arguments that features of the flora of Madagascar need to be seen in the light of coevolution with extinct birds.11,12 Bond and Silander12 studied Malagasy plants with peculiar branching patterns (termed 'spring and wire plants'). Their analysis suggests the branching pattern represents an anachronistic defence against large avian herbivores. Uncarina is especially diverse in the southwest and this coincides with the distribution of spring and wire plants.12 Grubb11 noted that plants of the eastern rainforests, dry evergreen forests and deciduous forests were apparently undefended against elephant birds (i.e. low spinescence, or absence of the above densely-branched, small-leaved species), suggesting this was not part of the range or habitat of elephant birds. Dransfield and Beentje13 were the first to suggest a relationship between dispersal of Malagasy plants and Aepyornis, in this case with some palm species. These palms, such as Satranala decussilvae and Voanioala gerrardii, are from wet, east coast rainforests13 (Fig. 2a), often found on steep slopes and in nutrient-poor soils. On biogeographical grounds, we suggest that these palms were not dispersed by Aepyornis.
Second, it also suggests that the evolution of the Harpago phytum-Uncarina type of trample burr was in part for dispersal by large birds in open environments; these being the ostrich in Africa and the elephant bird in Madagascar. The contribution of recent dispersal versus ancient vicariance and ecological factors to the richness of the Malagasy biota is a contentious issue10,14.We suggest that the similarities in fruits of Harpagophytum and Uncarina demonstrate the importance of ecological convergence rather than recent Africa–Madagascar dispersal of sister taxa. This lack of close relatedness is suggested by the significant differences between these genera in many aspects, for example the growth form (prostrate plants in the former to medium-sized shrubs in the latter), type of vascular cylinder and other features, such as the presence of hydathodes.1 Ihlenfeldt1 considered Uncarina to be basal in the tribe Pedalieae which he placed with Rogeria from central and southwest Africa. In contrast, Harpagophytum was placed in a different group of closely related, more advanced genera. In addition, Harpagophytum is not found on the east coast of central Africa. It is restricted to west and central areas of southern Africa1 and thus is unlikely to have dispersed to Madagascar. This argues against recent dispersal and should be tested using molecular techniques. The absence of similar trample burrs in New Zealand, despite a recent history of many, large ratite birds and the presence of spring and wire plants,15 could be because the Pedaliaceae are absent in New Zealand.1
There is considerable unexplained variation in the fruits in Uncarina (Fig. 2). For example U. leandrii fruits have very short (<7.5 mm) spiny arms,5 whereas they may reach 50 mm in U. stellulifera (pers. obs.). This matches similar variation within Harpagophytum. Harpagophytum zeyheri has small spiny arms and H. prostratum has much larger (>50 mm) spiny arms. It is not clear what the disperser of the smaller fruits would be, certainly not around the feet of large birds, but possibly by the extinct Geochelone tortoises, which occurred in the arid southwest.7
Finally, considering Uncarina persistence in the light of a collapsed dispersal process has conservation implications. We surveyed recruitment underneath adult plants in May 2009 in a semi-natural area of spiny forest in the Arboretum d'Antsokay (23º24'S, 43º48'E). Recruits (plants <0.5 m tall) were found under only 6 of 25 adult (>1.5 tall) plants with a total of 9 recruits. No recruits were observed in 25 matched plots each2m×2m,5m away from these adults. The adult plants largely existed on old domestic animal paths (of Zebu cattle and goats). We suspect that the demography of Uncarina will be skewed towards large plants in many areas. Ironically, low levels of anthropocentric disturbance, such as that by domestic livestock, may increase dispersal and recruitment of Uncarina, in the same way that modern horses have replaced extinct dispersers of some Central American plants.16
We thank Andry Petignat for permission to work in his Arboretum d'Antsokay, Tulear, Madagascar. This work was funded by the National Research Foundation, South Africa.
1 Ihlenfeldt H.D. (ed.) (2004). Pedaliaceae. Springer-Verlag, Berlin. [ Links ]
2 Ihlenfeldt H. (1994). Diversification in an arid world: the Mesembryanthemaceae. Annu. Rev. Ecol. Syst. 25, 521–546. [ Links ]
3 Van der Pijl L. (1982). Principles of Dispersal in Higher Plants. Springer-Verlag, Berlin. [ Links ]
4 Attenborough D. The Private Life of Plants, BBC nature documentary, released 1995, United Kingdom. [ Links ]
5 Humbert H. (1962). Les Pedaliacees de Madagascar. Adansonia 2, 200–215. [ Links ]
6 Godfrey L.R., Jungers W.L., Reed K.E., Simons E.L. and Chatrath P.S. (1997). Subfossil lemurs: inferences about past and present primate communities. In Natural Change and Human Impact in Madagascar, eds S.M. Goodman and B. Patterson, pp. 218–256. Smithsonian Institute, Washington D.C. [ Links ]
7 Burney D.A., Burney L.P., Godfrey L.R., Jungers W.L., Goodman S.M., Wright H.T. and Jull A.J. (2004). A chronology for late prehistoric Madagascar. J. Hum. Evol. 47, 25–63. [ Links ]
8 Hawkins A.F.A. and Goodman S.M. (2003). Introduction to the birds. In The Natural History of Madagascar, eds S.M. Goodman and J.P. Benstead, pp. 1026– 1029. University of Chicago Press, Chicago. [ Links ]
9 Clarke S.J., Miller G.H., Fogel M.L., Chivas A.R. and Murray-Wallace C.V. (2006). The amino acid and stable isotope biogeochemistry of elephant bird (Aepyornis) eggshells from southern Madagascar. Quat. Sci. Rev. 25, 2343–2356. [ Links ]
10 Yoder A.D. and Nowak M.D. (2006). Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell. Annu. Rev. Ecol. Evol. Syst. 37, 405–431. [ Links ]
11 Grubb P.J. (2003). Interpreting some outstanding features of the flora and vegetation of Madagascar. Perspect. Plant Ecol. Evol. Syst. 6, 125–146. [ Links ]
12 Bond W.J. and Silander J.A. (2007). Springs and wire plants: anachronistic defences against Madagascar's extinct elephant birds. Proc. R. Soc. B 274, 1985– 1992. [ Links ]
13 Dransfield J. and Beentje H. (1995). The Palms of Madagascar. Royal Botanic Gardens, Kew and The International Palm Society, Austin, TX. [ Links ]
14 Vences M., Wollenberg K.C., Vieites D.R. and Lees D.C. (2009). Madagascar as a model region of species diversification. Trends Ecol. Evol. 24, 456–465. [ Links ]
15 Bond W.J., Lee W.G. and Craine J.M. (2004). Plant structural defences against browsing birds: a legacy of New Zealand's extinct moas. Oikos 104, 500–508. [ Links ]
16 Janzen D.H. (1981). Enterolobium cyclocarpum seed passage rate and survival in horses, Costa Rican Pleistocene seed dispersal agents. Ecology 62, 593–601. [ Links ]
Received 5 August.
Accepted 4 November 2009.
* Author for correspondence E-mail: jeremy.midgley@uct.ac.za