Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.105 n.11-12 Pretoria Nov./Dec. 2009
REVIEW ARTICLES
Mechanisms by which circadian rhythm disruption may lead to cancer
M. Beckett; L.C. Roden*
Department of Molecular and Cell Biology, University of Cape Town, Private Bag, Rondebosch 7701, South Africa
ABSTRACT
Humans have evolved in a rhythmic environment and display daily (circadian) rhythms in physiology, metabolism and behaviour that are in synchrony with the solar day. Modern lifestyles have compromised the exposure to bright light during the day and dark nights, resulting in the desynchronisation of endogenously generated circadian rhythms from the external environment and loss of coordination between rhythms within the body. This has detrimental effects on physical and mental health, due to the misregulation and uncoupling of important cellular and physiological processes. Long-term shift workers who are exposed to bright light at night experience the greatest disruption of their circadian rhythms. Studies have shown an association between exposure to light at night, circadian rhythm disruption and an increased risk of cancer. Previous reviews have explored the relevance of light and melatonin in cancer, but here we explore the correlation of circadian rhythm disruption and cancer in terms of molecular mechanisms affecting circadian gene expression and melatonin secretion.
Key words: circadian rhythm, carcinogenesis, shift work
Introduction
Life on earth has evolved in the presence of a rhythmically changing environment. There are daily rhythms of light and dark, seasonal rhythms of daylength, temperature and precipitation, as well as longer-term rhythms of climatic conditions such as El Niño. The ability of organisms to anticipate the changes and adjust their physiology to adapt to, survive or exploit prevailing conditions provides a distinct adaptive advantage to an organism.1,2 As a result, most eukaryotes, and some prokaryotes, have developed a molecular time-keeping mechanism that can be synchronised with the external environment, to ensure optimal timing of cellular functions, metabolism and physiology (for review3–7).
The internal time-keeping mechanism is referred to as the circadian oscillator or clock. The term 'circadian' is derived from the Latin circa (about) and dies (day). The oscillator generates rhythms via an autoregulatory transcription-translation feedback loop. Although the individual components may not be conserved across the phyla, the basic mechanism of long-period rhythm generation is conserved. A feature of circadian rhythms is that they persist even in conditions of constant darkness or light, with periods close to 24 h.3–7 However, since the period of circadian rhythms is not exactly 24 h, the circadian clocks have the important function of using environmental cues to cause phase shifts and entrain or synchronise to the external environment. Light is the most important synchronising agent or zeitgeber (from the German for 'time giver '). Temperature changes, social cues, meal times and activity also play roles in setting our internal clock. Integration of the information from the various zeitgebers ensures synchrony of the internal physiology with the external 24-h cycle of day and night.8
Many fundamental metabolic, cellular and physiological processes occur with a circadian rhythm. The cell cycle has a 24-h rhythm and may be gated in Gap 1 (G1) phase by the circadian clock.9,10 The 24-h sleep-wake cycle is an obvious function of our innate rhythmic behaviour. Body temperature displays regular circadian changes in relation to the sleep-wake schedule and is the best characterised biological rhythm.8 Certain hormones are also secreted in an oscillatory manner. For example, melatonin synthesis and secretion by the pineal gland surges during the night and is low during the day.11 Cortisol, growth hormone and testosterone also display daily rhythms of secretion.12–15
Lifestyles in industrialised societies, where artificial light and activity at night are commonplace, result in mismatches between external planetary time and internal organismal time. We are most familiar with this kind of mismatch or desynchronisation in the phenomenon of jet lag, in which one experiences symptoms of poor sleep, fatigue, poor appetite and gastrointestinal disturbances for some time following extensive transmeridian travel. This desynchronisation of external and internal time also causes dysregulation of physiological processes within the body, such that the subjective time of different organs may be out of phase with one another. Recent studies suggest a link between circadian disturbances resulting from night shift work and jet lag, and an increased risk of cancer. An expert working group for the WHO International Agency for Research on Cancer (IARC) recently concluded that shift work that causes circadian rhythm disruption is 'probably carcinogenic to humans (Group 2A)'.16 To put this in context, Group 2A agents as evaluated in the IARC Monographs on the Evaluation of Carcinogenic Risks to Humans include acrylamide, chloramphenicol, nitrogen mustard, lead compounds and diesel engine exhaust fumes.17 It has been shown that the circadian clock-timing mechanism is vital for cell cycles, DNA damage responses and tumour suppression.18 Since a persistent desynchrony could contribute to an increased risk of cancer, this article reviews the current evidence for this connection and discusses the disruptions at the molecular level that may lead to cancer development.
Molecular basis of rhythms
The molecular basis of the circadian clock is the oscillatory transcription and translation of 'clock genes'. Current understanding of molecular rhythm generation has come from studying model organisms such as cyanobacteria (Synechococcus elongatus), bread mould (Neurospora crassa), fruit flies (Drosophila melanogaster), mice (Mus musculus) and thale cress (Arabidopsis thaliana).3–7,19 The mammalian circadian clock model has been derived predominantly from studies carried out in mice.
The molecular clockwork of rhythm generation consists of interlocking positive and negative transcription–translation feedback loops (Fig. 1). Precise regulation of molecular rhythms is achieved through post-transcriptional regulation, post-translational modifications, chromatin remodelling, intracellular localisation and availability of clock protein partners. The positive components are two basic helix-loop-helix PER-ARNT-SIM (PAS) domain-containing transcription factors, CLOCK and BMAL1,19–21 which form heterodimers and bind CACGTG E-box enhancer elements to activate the transcription of three Period genes (designated hPer1, hPer2, and hPer3 in humans), two Cryptochrome genes (hCry1 and hCry2) and the orphan nuclear receptor Rev-erbα gene.20,22–25 CLOCK is a histone acetyltransferase, and its activity is stimulated when it is associated with BMAL1.26 The transcriptional activation of these circadian oscillator genes is achieved through acetylation of histones, but CLOCK is not required for circadian rhythmicity,27 implying that an, as yet unidentified, histone acetyltransferase can substitute for CLOCK. There must also be a histone deacetylase that functions within the circadian clock, but this has not yet been identified. PER proteins contain two tandemly arranged PAS domains, through which they interact with each other and other proteins.28 PER1 and PER2 are central components of the clock while PER3 appears to be clock controlled, but not essential for rhythm generation.29 The PER and CRY proteins negatively regulate the CLOCK:BMAL1-mediated transcription of their own genes.9,24,30 PER proteins also act positively to enhance the transcription of Bmal1, and the greater availability of BMAL1 promotes the heterodimerisation of CLOCK and BMAL1, thus allowing the cycle to restart.31 REV-ERBα represses Bmal1 transcription through a REV-ERBα/ROR response element in the Bmal1 promoter.32 The negative and positive loops are coupled to each other, promoting self-sustained cycling (Fig. 1).

Casein kinase I ε (CKIε) and mitogen-activated protein kinase (MAPK) have been shown to phosphorylate the PER, CRY, CLOCK and BMAL1 proteins, regulating their activity, subcellular organisation and degradation.33–39 The transcriptional activation activity of BMAL1 is enhanced when phosphorylated by CKIε,36 and the phosphorylated forms of CLOCK and BMAL1 are predominantly found in the nucleus.37 PER and CRY proteins must dimerise and be phosphorylated by CKIε prior to nuclear entry.35, 38 The nuclear localisation signal is thought to be unmasked when PER is phosphorylated. Phosphorylated PER is recognised for degradation via the ubiquitin-proteasome pathway.39 Regulated degradation of clock proteins allows the cycle to start again.4 Physiological control, as well as resetting or entraining of circadian rhythms, can thus be achieved through inter-and intracellular signalling.
Where is 'the clock' in mammals?
Circadian clocks are found in most of the tissues and cells of mammals.40,41 There is a hierarchical organisation with a master clock that orchestrates and synchronises the circadian rhythms in other tissues. In mammals, the site of the master pacemaker is in a region of the hypothalamus called the suprachiasmatic nuclei (SCN).42 The role of this anatomical area was recognised when circadian rhythms of activity, drinking and feeding were abolished by electrolytic lesions in the SCN of the rat brain.43 This master clock responds to external light to reset and synchronise rhythms with the ambient 24-h cycle. The SCN are located at an ideal position in the brain to receive visual input via the retinohypothalamic tract for light entrainment.4 As ocular photoreception is the only way for humans to gain information about the light environment, peripheral tissues are synchronised with the light environment indirectly via signals from the SCN.44,45 Rhythms of clock gene expression within peripheral tissues are up to four hours out of phase with the rhythms in the SCN.40,41 These peripheral clocks are synchronised to the external light-dark environment via the central clock, but can also be entrained by meals, glucocorticoids and activity, independently of the SCN40,46–48 (Fig. 2).
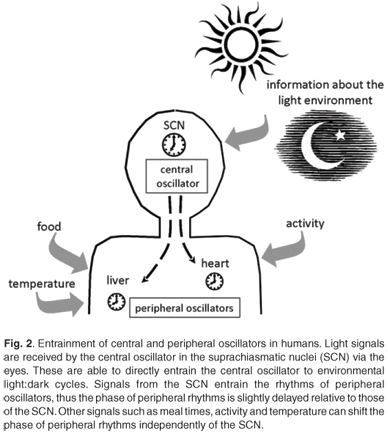
Entrainment of the central and peripheral clocks
Light, especially blue light, is the dominant zeitgeber for setting the phase of circadian clocks.49,50 In humans, only the central clock in the SCN is directly entrained by light received through ocular light exposure. There is no evidence to support the suggestion that extraocular light exposure can affect or reset circadian oscillators in humans.51,52 Rhodopsin and colour opsins that are required for vision are not required for circadian photoreception.53 The retinal photoreceptor system for circadian entrainment requires melanopsin, an opsin-like protein first identified in the skin of frogs, and possibly other opsin family pigments in the inner retina.53,54 In 2000, Provencio and coworkers55 showed that melanopsin was present in a group of retinal ganglion cells. Retinal ganglion cells are the output neurons from the eye which make up the retinohypothalamic tract (RHT) which transmits the photic signal to the SCN.55 In response to light, glutamate is released from the RHT terminal which stimulates receptors in the SCN neurons.56,57 Additionally, the neurotransmitter γ-aminobutyric acid (GABA) and its receptors and transporters are present in almost all cells of the SCN,58 and GABA is implicated in the entrainment of SCN rhythms.
Light input to the SCN can reset the phase of the central circadian pacemaker by inducing Per1 and Per2 gene expression.59–61 Light signals received in the early evening can delay the phase of the circadian clock, while light signals received late in the night serve to advance the phase.61 This information is then relayed via neural and humoral signals to synchronise the clocks of the peripheral tissues. The humoral signals act as chemical zeitgebers to cause phase shifts in the peripheral clocks.46 The pineal hormone melatonin (which peaks during the night) is imperative to the circadian timing system. The SCN regulates melatonin synthesis via GABAergic inhibition and glutaminergic stimulation of the paraventricular nuclei.62 Melatonin receptors are widely distributed, including in areas of the brain, such as the SCN, and in peripheral organs and tissues.63,64 The receptors in the SCN allow melatonin to induce phase shifts in the firing of neurons.65 The melatonin signal provides information to all cells, tissues and organs of the body about the time of day, and increased nocturnal concentrations of melatonin also facilitate sleep.66,67 As ocular light rapidly causes a suppression of melatonin production,68 it is understandable that light at certain times during the night can cause phase shifts in melatonin rhythms and affect circadian rhythm signalling.
Humoral signals from the SCN include transforming growth factor-α and prokineticin 2, which are rhythmically expressed in the SCN and affect locomotor activity.69 Light is not the only zeitgeber: meal times can serve as time cues, and plasma glucose levels that change following meals can affect clock gene expression in peripheral tissues.48 Glucocorticoids and retinoic acid are able to cause phase shifts in circadian gene expression in rat-1 fibroblasts and mouse peripheral tissues, even in the absence of SCN signals.46,70 These internal signals, coupled with the external light signal, synchronise the central and peripheral circadian clocks with external time. Working at night not only requires the presence of light, but also shifts the activity–rest rhythm from a diurnal habit to a nocturnal one, and can lead to altered meal times. These factors compound the loss of coordination between oscillators as they are reset by the different zeitgeber signals.40,46–48
Circadian disruption: desynchronisation of central and peripheral clocks
A phase shift in the melatonin rhythm and other circadian rhythms can be induced by appropriately timed exposure to light or darkness.49,50 Such alterations occur naturally as day length changes with the seasons, and are also induced after transmeridian flights when one rapidly changes time zones, or during night-time shift work. The effects of rapid changes in time zones are collectively referred to as jet lag and symptoms include sleep disruption, poor physical and mental performance and gastrointestinal disturbances.71 Jet lag recovery involves shifting the phases of circadian rhythms and re-entraining the central and peripheral clocks to the new time zone. While the rhythms of the SCN adjust rapidly to the new time zone, rhythms in peripheral tissues remain out of synchrony with the new time zone, and each other, for several cycles.72,73 The significance of this delayed re-entrainment is felt by airline pilots, cabin crew and regular travellers.
Shift work is increasing in the modern, industrialised Western world, and shift workers experience a 'social jet lag' where their circadian rhythms are out of synchrony with their daily schedule. Often, night-time shift workers experience continuous bright light at night, which suppresses natural melatonin production and causes sleep disturbances and phase shifts in metabolic and physiological processes.74–77 Circadian dysregulation in nighttime shift workers, due to light exposure at night, can be further compounded depending on the type of work and their eating schedules. Even if shift workers are on a permanent night shift, they very seldom accomplish complete phase adaptation to a nocturnal activity pattern.72 There are a number of activities that must take place during the day: childcare and schooling responsibilities, some job-related training or physical examinations, domestic administrative duties such as banking, and social events or shopping. This dual pattern of activity can have profound effects on human health in the long term. Effects on health include endocrine disorders, psychiatric illnesses, stressrelated disorders, immune responses and carcinogenesis.75,78 The difference in re-entrainment rates of central and peripheral clocks has been proposed as a compounding factor in tumour development. This is because during the desynchronised period, when phase adaptation is occurring, tissues are out of phase with their normal controlling systems, and abnormal growth and cell proliferation may occur.72,79
Significance of circadian disruption: melatonin and cancer
Melatonin has diverse roles in addition to mediating the phase-shifting of circadian rhythms.65,80 It is proposed to be involved in sleep, retinal physiology, reproductive cycles, cancer development and growth, immune activity, antioxidation and free radical scavenging, mitochondrial respiration, cardiovascular function, bone metabolism, intermediary metabolism and gastrointestinal physiology.66,81–86 Of particular interest, in terms of rhythm disruption and carcinogenesis, are the functions of free radical scavenging and enhancement of immune functions. Studies have shown that melatonin is effective in protecting against oxidative damage.82–84 Melatonin can thus protect macromolecules (particularly DNA in terms of carcinogenesis) from oxidative damage that would otherwise cause detrimental changes.
Studies in animals have shown the influence of melatonin on tumour growth. In 1981, Tamarkin and colleagues demonstrated that removal of the pineal gland in rats enhanced tumour growth, whilst administration of melatonin reversed or inhibited tumourigenesis.87 A proposed mechanism for the suppression of tumourigenesis is melatonin's capacity to suppress the accumulation of DNA adducts.88 DNA adducts are formed by carcinogens, which cause mutations and amplification, which in turn lead to tumour growth. The protective effect could be due to melatonin's role in free radical scavenging and the stimulation of anti-oxidative enzymes. Melatonin also enhances DNA repair, once damage has been done,89 by increasing the expression of the tumour-suppressor gene, p53.90 Numerous studies have shown a wide variety of melatonin's cancer-suppressive actions, including the regulation of oestrogen receptor (ERα) expression and transactivation, calcium/calmodulin activity, protein kinase C activity, cytoskeletal architecture and function, intracellular redox status, melatonin receptor-mediated signal transduction cascades, aromatase and telomerase activities, and fatty acid transport and metabolism.89,91 A recent study found that blind women who were unable to perceive light had a lower incidence of breast cancer than their blind counterparts who were able to perceive light;92 the authors proposed that light perception and melatonin production should be investigated in the blind in terms of the breast cancer-protective effects of the loss of light perception.92
Significance of circadian disruption: circadian genes, proteins and cancer
Light-dependent induction of Per1 and Per2 leads to a phase shift of the central circadian clock.59,60 All of the Per genes are also rhythmically expressed in peripheral tissues, suggesting that they have a role outside of the SCN.18 It has been shown that major biological pathways, such as the cell cycle, DNA damage response and tumour suppression, are under circadian control.9,18 Clock-controlled genes include key cell-cycle regulators, such as c-Myc, CyclinD1, Cyclin A and Wee1. Significantly, it has been shown that there is deregulated expression of these genes in mutant mice lacking functional PER2.18 These mice displayed improper cell division and heightened sensitivity to radiation, suggesting that PER2 functions in regulating the cell cycle and the DNA damage pathway.
Double-stranded breaks in DNA, caused for example by exposure to UV radiation or ionising irradiation, are detrimental to the cell and must be repaired prior to cell division. Cells react to such insults by activating vital replication checkpoints to delay S-phase progression and G2/M transition so that the DNA can be repaired; if the damage is irreparable, apoptosis is induced.93,94 Gery et al.95 investigated the importance of PER1 in cell growth and DNA damage control. They found that PER1 is important in irradiation-induced apoptosis by elevating c-Myc levels and suppressing the cyclin-dependent kinase inhibitor p21Waf1/Cip1(ref. 95).It was also shown that PER1 suppresses the growth and transforming potential of cancer cells by regulating key factors in the cell cycle and DNA damage pathways, which indicates that Per1 could be a tumour-suppression gene. The results showed that the circadian rhythm functions in peripheral tissues, to control mitotic events and the cell cycle at multiple stages.95
Down-regulation of Per2 in vivo and in vitro was associated with an increase in Cyclins D and E and an acceleration of breast cancer cell and tumour growth.96 Decreased expression of Per1 and Per2 was found in sporadic and familial breast cancers.97 It was recently demonstrated that misregulation of Per2 or Bmal1 in cancerous tissues was associated with lymph node metastasis and poor prognosis.98 The same study demonstrated that the promoters of circadian genes Per1, Per2, Cry1 and Bmal1 were hypermethylated in breast cancer tissues, leading to misregulation of gene expression and disruption of circadian rhythms in these tissues. These results demonstrated that tumourigenesis is associated with altered circadian function, whether causal or symptomatic, and suggest that a dysfunctional circadian clock promotes carcinogenesis.
Correlative studies and implications for lifestyle recommendations
Recently, a number of epidemiological studies have been carried out regarding an increased risk of cancer due to lifestyle habits, such as night-time shift work and light pollution at night. The majority of the studies have been carried out with groups of nurses and flight attendants, and consequently most of the study subjects have been women. Investigations into the increased risk of breast cancer are therefore predominant, although there are also studies that show an increased risk of other cancers such as colorectal, endometrial and prostate cancer.90,99 The shift status of workers should be examined as a variable in occupational studies in a broader range of industries in order to determine whether circadian rhythm disruption increases the risk of cancers in general. The first published assessment of shift work and cancer risk showed a 4.3-fold increased risk of breast cancer in women aged 50 and older, who had been exposed to more than three years of shift work before age 30.100 This suggests that the effects of molecular circadian disruption operate early in the process of carcinogenesis in breast tissue. Several studies have indicated an increase in the occurrence of breast cancer in female flight attendants (for review99). It is unclear whether the combination of shift work and transmeridian travel has an additive effect on the carcinogenesis of circadian disruption, thus putting flight crew at a higher risk than other shift workers. It is also difficult to quantify the dose of circadian disruption that is carcinogenic. A means to relate the frequency and duration of shift work to the extent of circadian disruption, and the magnitude and duration of disruption that is harmful, must still be established. It will also be useful to investigate whether there are particular age groups which are more at risk to the cancer-causing effects of circadian disruption.
There are measures that can be taken to reduce the long-term effects of shift work. Adaptation of the circadian rhythm to the night shift pattern would be beneficial for permanent shift workers.72 However, this may not be desirable in rapidly rotating shift workers. If complete adaptation to the night shift is to be achieved, it has been recommended that workers be exposed to bright light during the shift, sleep in a darkened room and keep to a regular sleeping-and-eating cycle.72,101 The proposal for shift workers to wear dark sunglasses or yellow-tinted glasses that exclude blue light when travelling home in daylight is only safe when the shift workers do not drive themselves.102 Generally, these precautions are not feasible or successful, and perhaps it would be more reasonable to implement regular cancer screening in the shift working populations, focusing on early detection and intervention. Although this may add to the healthcare costs to companies, preventative steps or early treatment of disease may prove more cost-effective and beneficial to both the public and business sectors.
Conclusion
Deregulation of clock gene expression and suppression of melatonin at night are consequences of a modern lifestyle, involving both exposure to bright light at night and insufficient exposure to bright light, akin to natural sunlight, during the day. It is highly unlikely that humans, or groups of humans, have adapted biologically to such environmental changes. Recurrent desynchrony between the environment and central and peripheral clocks, as well as a loss of coordination between internal oscillators, have detrimental health effects in the long term. These effects have been recognised as potentially carcinogenic to humans,17 but the observations and suggested mechanisms need to be confirmed and expanded in well-designed human studies. Additional studies are also needed to determine the particular types of shift work and the 'dose' of shift work exposure that are damaging.
We are grateful to David Woods for critical reading of the manuscript and to two anonymous reviewers for their helpful comments and suggestions to improve the manuscript.
1. DeCoursey P.J. and Krulas J.R. (1998). Behavior of SCN-lesioned chipmunks in natural habitat: a pilot study. J. Biol. Rhythms 13, 229–244. [ Links ]
2. Dodd A.N., Salathia N., Hall A., Kévei E., Tóth R., Nagy F., Hibberd J.M., Millar A.J. and Webb A.A. (2005). Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633. [ Links ]
3. Bell-Pedersen D. (1998). Keeping pace with Neurospora circadian rhythms. Microbiology 144, 1699–1711. [ Links ]
4. Reppert S.M. (2000). Cellular and molecular basis of circadian timing in mammals. Semin. Perinatol. 4, 243–246. [ Links ]
5. McWatters H.G., Roden L.C. and Staiger D. (2001). Picking out parallels: plant circadian clocks in context. Phil. Trans. R. Soc. Lond. B 356, 1735–1743. [ Links ]
6. Levine J.D. (2006). Sharing time on the fly. Curr. Opin. Cell Biol. 16, 210–216. [ Links ]
7. Dong G. and Golden S.S. (2008). How a cyanobacterium tells time. Curr. Opin. Microbiol. 11, 1–6. [ Links ]
8. Koukkari W.L. and Sothern R.B. (2006). Introducing Biological Rhythms, chaps 3 and 11, pp. 66–106, pp. 426–525. Springer Science and Business Media, New York. [ Links ]
9. Bjarnason G.A., Jordan R. and Sothern R.B. (1999). Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am. J. Pathol. 154, 613–622. [ Links ]
10. Bjarnason G.A., Jordan R.C.K., Wood P.A., Li Q., Lincoln D.W., Sothern R.B., HrusheskyW.J.M. and Ben-DavidY. (2001). Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am. J. Pathol. 158, 1793–1801. [ Links ]
11. Zeitzer J.M., Duffy J.F., Lockley S.W., Dijk D.J. and Czeisler C.A. (2007). Plasma melatonin rhythms in young and older humans during sleep, sleep deprivation, and wake. Sleep 30, 1437–1443. [ Links ]
12. Abe K., Kroning J., Greer M.A. and Critchlow V. (1979). Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticosterone, body temperature, feeding and plasma thyrotropin. Neuroendocrinology 29, 119–131. [ Links ]
13. Kennaway D.J. and van Dorp C.F. (1991). Free-running rhythms of melatonin, cortisol, electrolytes, and sleep in humans in Antarctica. Am. J. Physiol. Regul. Integr. Comp. Physiol. 260, R1137–R1144. [ Links ]
14. Drobny E.C., Amburn K. and Baumann G. (1983). Circadian variation of basal plasma growth hormone in man. J. Clin. Endocrinol. Metab. 57, 524–528. [ Links ]
15. Diver M.J., Imtiaz K.E., Ahmad A.M., Vora J.P. and FraserW.D. (2003). Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin. Endocrinol. 58, 710–717. [ Links ]
16. Straif K., Baan R., Grosse Y., Secretan B., El Ghissassi F., Bouvard V., Altieri A., Benbrahim-Tallaa L. and Cogliano V. (2007). Carcinogenicity of shift work, painting and fire-fighting. Lancet Oncol. 8, 1065–1066. [ Links ]
17. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. (1972–2009), Vols 1–99. Online at: http://monographs.iarc.fr/ENG/Classification/ crthgr02a.php [ Links ]
18. Lee C.C. (2006). Tumor suppression by the mammalian Period genes. Cancer Causes Control 17, 525–530. [ Links ]
19. Dunlap J.C. (1999). Molecular bases for circadian clocks. Cell 96, 271–290. [ Links ]
20. Gekakis N., Staknis D., Nguyen H.B., Davis F.C., Wilsbacher L.D., King D.P., Takahashi J.S. andWeitz C.J. (1998). Role of the CLOCK protein in themammalian circadian mechanism. Science 280, 1564–1569. [ Links ]
21. Hogenesch J.B., Gu Y.Z., Jain S. and Bradfield C.A. (1998). The basic-helixloop- helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. U.S.A. 95, 5474–5479. [ Links ]
22. Jin X., Shearman L.P.,Weaver D.R., Zylka M.J., de Vries G.J. and Reppert S.M. (1999). A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96, 57–68. [ Links ]
23. Kume K., Zylka M.J., Sriram S., Shearman L.P., Weaver D.R,. Jin X., Maywood E.S., Hastings M.H. and Reppert S.M. (1999). mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205. [ Links ]
24. Sangoram A.M., Saez L., Antoch M.P., Gekakis N., Staknis D., Whiteley A., Fruechte E.M., Vitaterna M.H., Shimomura K., King D.P., Young M.W., Weitz C.J. and Takahashi J.S. (1998). Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1- induced transcription. Neuron 21, 1101–1113. [ Links ]
25. UedaH.R., ChenW.,Adachi A.,WakamatsuH., Hayashi S., Takasugi T.,Nagano M., Nakahama K., Suzuki Y., Sugano S., Iino M., Shigeyoshi Y. and Hashimoto S. (2002). A transcription factor response element for gene expression during circadian night. Nature 418, 534–539. [ Links ]
26. Doi M., Hirayama J. and Sassone-Corsi P. (2006). Circadian regulator CLOCK is a histone acetyltransferase. Cell 125, 497–508. [ Links ]
27. Debruyne J.P., Noton E., Lambert C.M., Maywood E.S., Weaver D.R. and Reppert S.M. (2006).Aclock shock: mouseCLOCKis not required for circadian oscillator function. Neuron 50, 465–477. [ Links ]
28. Hirayama J. and Sassone-Corsi P. (2005). Structural and functional features of transcription factors controlling the circadian clock. Curr. Opin. Genet. Dev. 15, 548–556. [ Links ]
29. Lee C., Weaver D.R. and Reppert S.M. (2004). Direct association between mouse PERIOD and CKIepsilon is critical for a functioning circadian clock. Mol. Cell Biol. 24, 584–594. [ Links ]
30. Harms E., Kivimae S., Young M.L. and Saez L. (2004). Posttranscriptional and posttranslational regulation of clock genes. J. Biol. Rhythms 19, 361–373. [ Links ]
31. Shearman L.P., Sriram S., Weaver D.R., Maywood E.S., Chaves I., Zheng B., Kume K., Lee C.C., van der Horst G.T., Hastings M.H. and Reppert S.M. (2000). Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019. [ Links ]
32. Preitner N., Damiola F., Lopez-Molina L., Zakany J., Duboule D., Albrecht U. and Schibler U. (2002). The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260. [ Links ]
33. Sanada K., Hayashi Y., Harada Y., Okano T. and Fukada Y. (2000). Role of circadian activation of mitogen-activated protein kinase in chick pineal clock oscillation. J. Neurosci. 20, 986–991. [ Links ]
34. Akashi M., TsuchiyaY.,Yoshino T. and Nishida E. (2002). Control of intracellular dynamics of mammalian period proteins by casein kinase I ε (CKIε) and CKIδ in cultured cells. Mol. Cell Biol. 22, 1693–1703. [ Links ]
35. Vielhaber E.L., Duricka D., Ullman K.S. and Virshup D.M. (2001). Nuclear export of mammalian PERIOD proteins. J. Biol. Chem. 276, 45921–45927. [ Links ]
36. Eide E.J., Vielhaber E.L., Hinz W.A. and Virshup D.M. (2002). The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase I epsilon. J. Biol. Chem. 277, 17248–17254. [ Links ]
37. KondratovR.V., ChernovM.V.,Kondratova A.A., GorbachevaV.Y.,Gudkov A.V. and Antoch M.P. (2003). BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 17, 1921–1932. [ Links ]
38. Takano A., Isojima Y. and Nagai K. (2004). Identification of mPer1 phosphorylation sites responsible for the nuclear entry. J. Biol. Chem. 279, 32578–32585. [ Links ]
39. Eide E.J., Woolf M.F., Kang H., Woolf P., Hurst W., Camacho F., Vielhaber E.L., Giovanni A. andVirshup D.M. (2005). Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol. Cell Biol. 25, 2795–2807. [ Links ]
40. Yoo S.H., Yamazaki S., Lowrey P.L., Shimomura K., Ko C.H., Buhr E.D., Siepka S.M., Hong H.K., Oh W.J., Yoo O.J., Menaker M. and Takahashi J.S. (2004). PERIOD 2: Luciferase real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U.S.A 101, 5339–5346. [ Links ]
41. Peirson S.N., Butler J.N., Duffield G.E., Takher S., Sharma P. and Foster R.G. (2006). Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem. Biophys. Res. Comm. 351, 800–807. [ Links ]
42. Ralph M.R., Foster R.G., Davis F.C. and Menaker M. (1990) Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978. [ Links ]
43. Stephan F.K. and Zucker I. (1972). Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. U.S.A. 69, 1583–1586. [ Links ]
44. Oishi K., Sakamoto K., Okada T., Nagase T. and Ishida N. (1998). Humoral signals mediate the circadian expression of rat period homologue (rPer2) mRNA in peripheral tissues. Neurosci. Lett. 256, 117–119. [ Links ]
45. Allen G., Rappe J., Earnest D.J. and Cassone V.M. (2001). Oscillating on borrowed time: diffusible signals from immortalized suprachiasmatic nucleus cells regulate circadian rhythmicity in cultured fibroblasts. J. Neurosci. 21, 7937–7943. [ Links ]
46. Balsalobre A., Brown S.A., Marcacci L., Tronche F., Kellendonk C., Reichardt H.M., Schütz G. and Schibler U. (2000). Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347. [ Links ]
47. Buxton O.M., Lee C.W., L'Hermite-Baleriaux M., Turek F.W. and van Cauter E. (2003). Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am. J. Physiol.Regul. Integr. Comp. Physiol. 284, R714–R724. [ Links ]
48. Hirota T. and Fukada Y. (2004). Resetting mechanism of central and peripheral circadian clocks in mammals. Zool. Sci. 21, 359–368. [ Links ]
49. Lockley S.W., Brainard G.C. and Czeisler C.A. (2003). High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 88, 4502–4505. [ Links ]
50. Smith M.R. and Eastman C.I. (2009). Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol. Int. 26, 709–725. [ Links ]
51. Campbell S.S. and Murphy P.J. (1998). Extraocular circadian phototransduction in humans. Science 279, 396–399. [ Links ]
52. Wright K.P. Jr and Czeisler C.A. (2002). Absence of circadian phase resetting in response to bright light behind the knees. Science 297, 571. [ Links ]
53. Kavakli I.H. and Sancar A. (2002). Circadian photoreception in humans and mice. Mol. Interv. 2, 484–492. [ Links ]
54. Provencio I., Jiang G., De Grip W.J., Hayes W.P. and Rollag M.D. (1998). Melanopsin: an opsin in melanophores, brain and eye. Proc. Natl. Acad. Sci. U.S.A. 95, 340–345. [ Links ]
55. Provencio I., Rodriguez I.R., Jiang G., Hayes W.P. and Rollag M.D. (2000). A novel human opsin in the human retina. J. Neurosci. 20, 600–605. [ Links ]
56. Golombek D.A., Ferreyra G.A., Katz M.A., Marpegan L., Fernandez Alfonso T. and Yannieli P.C. (2000). The neurochemical basis of photoentrainment in mammals. Biol. Rhythm Res. 31, 56–70. [ Links ]
57. Golombek D.A., Agostino P.V., Plano S.A. and Ferreyra G.A. (2004). Signaling in the mammalian circadian clock: the NO/cGMP pathway. Neurochem. Int. 45, 929–936. [ Links ]
58. Ehlen J.C. and Paul K.N. (2009). Regulation of light's action in the mammalian circadian clock: role of extrasynaptic GABAA receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1606–R1612. [ Links ]
59. Albrecht U., Sun Z.S., Eichele G. and Lee C.C. (1997). A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 1, 1055–1064. [ Links ]
60. Shearman L.P., Zylka M.J., Weaver D.R., Kolakowski L.F. Jr. and Reppert S.M. (1997). Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19, 1261–1269. [ Links ]
61. Albrecht U., Zheng B., Larkin D., Sun Z.S. and Lee C.C. (2001). MPer1 and MPer2 are essential for normal resetting of the circadian clock. J. Biol. Rhythms. 16, 100–104. [ Links ]
62. Skene D. and Arendt J. (2006). Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann. Clin. Biochem. 43, 344–353. [ Links ]
63. Williams L.M., Hannah L.T., Hastings H. and Maywood E.S. (1995). Melatonin receptors in the rat brain and pituitary. J. Pineal Res. 19, 173–177. [ Links ]
64. Shin S.Y.W., Ng N. and Pang S.F. (1996). A molecular perspective of the genetic relationship of G-protein coupled melatonin receptor subtypes. J. Pineal Res. 20, 198–204. [ Links ]
65. Liu C., Weaver D.R., Jin X., Shearman L.P., Pieschl R.L., Gribkoff V.K. and Reppert S.M. (1997). Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19, 91–102. [ Links ]
66. Shochat T., Luboshitzky R. and Lavie P. (1997). Nocturnal melatonin onset is phase locked to the primary sleep gate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 273, R364–R370. [ Links ]
67. Dijk D.J. and Cajochen C. (1997). Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J. Biol. Rhythms 12, 627–635. [ Links ]
68. Lewy A.J., Wehr T.A., Goodwin F.K., Newsome D.A. and Markey S.P. (1980). Light suppresses melatonin secretion in humans. Science 210, 1267–1269. [ Links ]
69. Kramer A., Yang F.C., Snodgrass P., Li X., Scammell T.E., Davis F.C. and Weitz C.J. (2001). Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294, 2511–2515. [ Links ]
70. McNamara P., Seo S.B., Rudic R.D., Sehgal A., Chakravarti D. and FitzGerald G.A. (2001) Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105, 877–889. [ Links ]
71. Waterhouse J., Reilly T., Atkinson G. and Edwards B. (2007). Jet lag: trends and coping strategies. Lancet 369, 1117–1129. [ Links ]
72. Haus E. and Smolensky M. (2006). Biological clocks and shift work: circadian dysregulation and potential long term effects. Cancer Causes Control 17, 489–500. [ Links ]
73. Yamazaki S., Numano R., Abe M., Hida A., Takahashi R., Ueda M., Block G.D., Sakaki Y., Menaker M. and Tei H. (2000). Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685. [ Links ]
74. Touitou Y., Motohashi Y., Reinberg A., Touitou C., Bourdeleau P., Bogdan A. and Auzéby A. (1990). Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol and testosterone. Eur. J. Appl. Physiol. Occup. Physiol. 60, 288–292. [ Links ]
75. Karlsson B.H., Knutsson A.K., Lindahl B.O. and Alfredsson L.S. (2003). Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int. Arch. Occup. Environ. Health 76, 424–430. [ Links ]
76. Burch J.B., Yost M.G., Johnson W. and Allen E. (2005). Melatonin, sleep, and shift work adaptation. J. Occup. Environ. Med. 47, 893–901. [ Links ]
77. James F.O., Cermakian N. and Boivin D.B. (2007). Circadian rhythms of melatonin, cortisol, and glock gene expression during simulated night shift work. Sleep 30, 1427–1436. [ Links ]
78. Piccoli B., Parazzoli S., Zaniboni A., Dermartini G. and Frashini F. (1991). Non-visual effects of light mediated via the optical route: review of the literature and implications for occupational medicine. Med. Lav. 82, 213–232. [ Links ]
79. Shechter A., James F.O. and Boivin D.B. (2008). Circadian rhythms and shift working women. Sleep Med. Clin. 3, 13–24. [ Links ]
80. Arendt J. and Skene D.J. (2005). Melatonin as a chronobiotic. Sleep Med. Rev. 9, 25–39. [ Links ]
81. Maestroni G.J.M. (1993).The immunoneuroendocrine role of melatonin. J. Pineal Res. 14, 1–10. [ Links ]
82. Bandyopadhyay D., Biswas K., Bhattacharyya M., Reiter R.J. and Banerjee R.K. (2001). Gastric toxicity and mucosal ulceration induced by oxygen-derived reactive species: protection by melatonin. Curr. Mol. Med. 1, 501–513. [ Links ]
83. Karbownick M., Lemuniski A. and Reiter R.J. (2001). Anticarcinogenic actions of melatonin which involve antioxidative processes: comparison with other antioxidants. Int. J. Biochem. Cell Biol. 33, 735–753. [ Links ]
84. Cuzzocrea S. and Reiter R.J. (2001). Pharmacological action of melatonin in shocks, inflammation and ischemia/reperfusion injury. Eur. J. Phamacol. 426, 1–10. [ Links ]
85. León J., Acuña-Castroviejo D., Escames G., Tan D.X. and Reiter R.J. (2005). Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 38, 1–9. [ Links ]
86. Brzezinski A. (1997). Melatonin in humans. N. Engl. J. Med. 336, 186–195. [ Links ]
87. Tamarkin L., Cohen M., Roselle D., Reichert C., Lippman M. and Chabner B. (1981). Melatonin inhibition and pinealectomy enhancement of 7,12- demethylbenz(a)anthracene-induced mammary tumors in the rat. Cancer Res. 41, 4432–4436. [ Links ]
88. Blask D.E. (2009). Melatonin, sleep disturbance and cancer risk. Sleep Med. Rev. 13, 257–264. [ Links ]
89. Reiter R.J. (2004). Mechanisms of cancer inhibition by melatonin. J. Pineal Res. 37, 213–214. [ Links ]
90. Davis S. and Mirick D.K. (2006). Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control 17, 539–545. [ Links ]
91. Blask D.E., Sauer L.A. and Dauchy R.T. (2002). Melatonin as a chronobiotic/ anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr. Top. Med. Chem. 2, 113–132. [ Links ]
92. Flynn-Evans E.E., Stevens R.G., TabandehH., Schernhammer E.S. and Lockley S. (2009). Total visual blindness is protective against cancer. Cancer Causes Control 20(9), 1753–1756. [ Links ]
93. Iliakis G., Wang Y., Guan J. and Wang H. (2003). DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 22, 5834–5847. [ Links ]
94. Sancar A., Lindsey-Boltz L.A., Unsal-Kacmaz K. and Linn S. (2004). Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 73, 39–85. [ Links ]
95. Gery S., Komatsu N., Baldjyan L., Yu A., Koo D. and Koeffler H.P. (2006). The circadian gene Per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 22, 375–382. [ Links ]
96. Yang X.,Wood P.A., Oh E-Y., Du-Quiton J., Ansell C.M. and HrusheskyW.J.M. (2008). Down regulation of circadian clock gene Period 2 accelerates breast cancer growth by altering its daily growth rhythm. Breast Cancer Res. Treat., doi 10.1007/s10549-008-0133-z [ Links ]
97. Winter S.L., Bosnoyan-Collins L., Pinnaduwage D. and Andrulis I.L. (2007). Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast cancers. Neoplasia 9, 797–800. [ Links ]
98. Kuo S-J., Chen S-T., Yeh K-T., How M-F., Chang Y-S., Hsu N.C. and Chang J-G (2009). Disturbance of circadian gene expression in breast cancer. Virchows Arch 454, 467–474. [ Links ]
99. Viswanathan A.N. and Schernhammer E.S. (2009). Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 281, 1–9. [ Links ]
100.Tynes T., Hannevik M., Andersen A., Vistnes A. and Haldorsen T. (1996). Incidence of breast cancers in Norwegian female radio and telegraph operators. Cancer Causes Control 7, 197–204. [ Links ]
101. Cajochen C. (2007). Alerting effects of light. Sleep Med. Rev. 11, 453–464. [ Links ]
102.Lockley S.W. (2007). Safety considerations for the use of blue-light blocking glasses in shift workers. J. Pineal Res. 42, 210–211. [ Links ]
Received 7 July.
Accepted 16 October 2009.
* Author for correspondence E-mail: laura.roden@uct.ac.za














