Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.105 n.7-8 Pretoria Jul./Aug. 2009
RESEARCH LETTERS
Synthesis and study of carbon microspheres for use as catalyst support for cobalt
S.D. MhlangaI, II; K.C. MondalI, II; N. NaidooI; N. KunjuzwaI; M.J. WitcombII, III; N.J. CovilleI, II, *
IMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Private Bag 3, WITS 2050, Johannesburg, South Africa
IIDST/NRF Centre of Excellence in Strong Materials, University of the Witwatersrand, Private Bag 3, WITS 2050, Johannesburg, South Africa
IIIElectron Microscopy and Microanalysis Unit, University of the Witwatersrand, Private Bag 3, WITS 2050, Johannesburg, South Africa
ABSTRACT
The production of pure carbon spheres was achieved in the absence of a catalyst through the direct pyrolysis of two hydrocarbon sources, acetylene and ethylene. Systematic studies using acetylene as the feedstock indicated that the size distribution of the resulting carbon microspheres can be controlled by pyrolysis temperature, time and feedstock flow rate. The resulting spheres were fully characterised by transmission electron microscopy (TEM) and thermogravimetric analysis. The TEM examination showed that these spheres have a ball-like and chain-like morphology, and the balls have smooth surfaces with a variation in diameter size and distribution determined by the reaction conditions. Carbon microsphere-supported cobalt catalysts were synthesised and have shown good activity in the ethylene hydrogenation reaction.
Key words: carbon microspheres, supports, ethylene hydrogenation, electron microscopy, cobalt
Introduction
Carbon is a versatile element because it can be a useful building block to form a wide range of structures. The unique ability of carbon to form complexes in which the carbon atom has sp, sp2 and sp3 electronic configurations results in a wide range of structures and morphologies with widespread applications.1 The successful growth of diamond films, the fullerene molecule C60 and its family members Cn e.g. C70 and C80, and carbon nanotubes (CNTs) have attracted much attention.2 In particular, an enormous amount of research has to date focused on the synthesis and applications of CNTs. Another spherical form of carbon, which is graphitic in nature, has recently been emphasised in the literature. Earlier studies referred to this spherical material as nanosized carbon spheres2 or carbon nanobeads, while more recent studies have called them carbon spherules,3–5 nanoballs,6 carbon pearls7 or carbon microspheres.8 This form of carbon has not received as much interest to date by those working with CNTs because in most cases it was discovered accidentally as a byproduct in the synthesis of CNTs by chemical vapour deposition methods. However, since then, some work has been carried out on these carbon microspheres (CMSs), focusing on their fabrication using catalytic synthesis1,2 as well as direct pyrolysis of hydrocar-bons.9,10 Interestingly, carbon spheres have been known for many decades and have been made by procedures similar to the methods described herein. These materials, referred to as carbon black particles,11,12 have been produced from a carbon source such as oil or natural gas, typically in the presence of small amounts of oxygen. For example, when acetylene is used under a reduced presence of oxygen the process is called the acetylene black process.13–15
Different processes have been used to synthesise the sphere-like forms of carbon. Carbon onions were observed to be formed from soot that contained polyhedral carbon particles under electron-beam irradiation in a high-resolution transmission electron microscope.16 These highly graphitic structures were found to be stable under irradiation conditions. Monodispersed nanosized carbon spheres (~210 nm in diameter) were synthesised by Wang and Kang,17 using a mixed-valent oxide catalytic carbonisation process in which methane was decomposed at 1 100°C in the presence of a metal oxide catalyst. When a temperature of 950°C was used, carbon tubes were produced.17 Solid and hollow nanospheres with diameters in the range 250–850 nm have also been obtained by a catalytic process by Sharon et al.18 from camphor vapour at 1000°C using ferrocene as the catalyst precursor. All the above processes are reported to require a catalyst. More recently, large-scale production of pure carbon spheres, with diameters from 50 nm to 1 µm, has also been achieved through direct pyrolysis of a wide range of hydrocarbons in the absence of a catalyst. These include styrene, toluene, benzene, hexane, cyclohexane and ethane.9,10 Wang et al.19 have also reported the production of carbon microbeads (2 mm) from a synthetic naphthalene isotropic pitch by simple heating at 420°C under nitrogen.
In this study we report on the synthesis of CMSs, using ethylene or acetylene as a carbon source, in the absence of a catalyst.
The search for new synthetic strategies for generating nanostructured carbon or carbon-hybrid materials is topical in materials chemistry, motivated by the natural abundance of carbon and therefore the cost effectiveness of carbon precursors and the promising applications of the resulting materials. Due to the intrinsic properties of the carbon materials, such as their high strength, high thermal resistance and light weight, CMSs can be used as high-strength composites, catalyst supports, lubricants and as wear-resistant materials.10
Catalytic hydrogenation is a common and straightforward application of a heterogeneous catalyst. The usefulness of this reaction has been well covered by texts and reviews.20 Such work, however, has been done on amorphous carbon as a catalyst support in hydrogenation reactions, and similar work on new forms of carbon, such as CMSs, remains to be explored. We recently reported that carbon microspheres could be used as a catalyst support for palladium (Pd) for the hydrogenation reaction of ethylene to ethane. The study showed that better ethylene hydrogenation activity and mechanical stability against deactivation were achieved when compared to an activated amorphous carbon-supported Pd catalyst. This was suggested to be due to the stronger metal-support interaction between the Pd and the carbon, even though some sintering was noted.21 The use of CMSs as support for cobalt (Co) catalysts for the hydrogenation reaction of ethylene to ethane, has not previously been reported and fewer sintering episodes are expected. Herein we report our results on the use of Co/CMS for the hydrogenation reaction of ethylene to ethane.
Methods
Carbon microsphere synthesis
Direct pyrolysis of a pure hydrocarbon source was carried out to synthesise carbon microspheres. The experiment was carried out at atmospheric pressure in a flow reactor made of a quartz tube (having dimensions 51 × 1.9 cm). The reactor was placed horizontally into an electronically-controlled furnace. The front end of the tube was connected to a glass manifold which allowed for the free flow of gases (i.e. hydrocarbon feedstock gas, argon inert gas) into the reactor. The rear end of the tube was closed with a quartz glass plug, thus ensuring that the reaction took place in the absence of oxygen.
The temperature in the furnace was then raised at a constant heating rate of 10°C min–1 under a constant flow rate of 40 ml min–1 of 5% H2/Ar gas. Once the desired temperature was reached the inert gas was replaced with the hydrocarbon source (i.e. acetylene or ethylene) at a total constant flow rate of 100 ml min–1. The carbon source was converted into the desired activated carbon species which diffused into the quartz tube while the temperature was held constant. After a certain time, the carbon source was replaced by the inert gas (5% H2/Ar, 40 ml min–1), and the system was allowed to cool to room temperature before the product was collected. The soot was collected, weighed and characterised using TEM (JEOL 100S Electron Microscope) and thermogravimetric analysis (TGA) (Perkin Elmer Pyris 1 TGA Analyzer). Since no catalyst was required in the synthesis of the CMSs, the materials produced were very pure and no removal of catalyst was required in the post-synthesis process. The type of feedstock gas, reaction temperature, reaction time and flow rate of the carbon source were varied in order to study their effects on the structure and yield of CMSs obtained.
Preparation of carbon microsphere supported cobalt catalysts (Co/CMS)
The CMS supported Co catalysts were prepared by the wet impregnation method. Cobalt nitrate [Co(NO3)2.6H2O] was used as a source of cobalt. For the synthesis of 5 g of 5 wt% Co/CMS, 1.24 g of cobalt nitrate was dissolved in 20 ml distilled water and the solution stirred. Other catalysts containing 10 wt% and 20 wt% Co were also prepared as described above. Each of the support-catalyst mixtures was stirred for 15 min and dried in an oven at 120°C for 30 min. The catalysts were then calcined in a static air calcination oven for 4 h at 400°C (heating rate = 5°C min–1) and rapidly ground to a fine powder and sieved (powder < 150 µm). Temperature-programmed reduction (TPR) studies were done to determine the reduction temperature of cobalt oxide species under hydrogen and indicated that a temperature of 400°C would be sufficient for catalyst reduction (data not shown). The catalysts were then reduced under a continuous flow of 5% H2/Ar at 30 ml min–1 for 1 h.
Catalytic hydrogenation reaction
The catalytic hydrogenation reactions were performed, at atmospheric pressure, by passing a gaseous feed containing pure ethylene and hydrogen (H2/C2H4 mol ratio = 3.05, total flow of feed gas = 77 ml min–1) continuously over the catalyst (50 mg), which was packed in a fixed-bed quartz microreactor with a thermocouple. After attaining reaction steady state, the reaction products were analysed by a gas chromatograph with a flame ionisation detector (FID) using a Porapak Q column. The catalyst was studied in its prepared state. Only hydrocarbon products are detected by the FID and hence selectivity and conversion data were based only on analysis of the hydrocarbon species. The gas hourly space velocity was measured at 0°C and 1 atm pressure. The experimental setup is shown in Fig. 1.
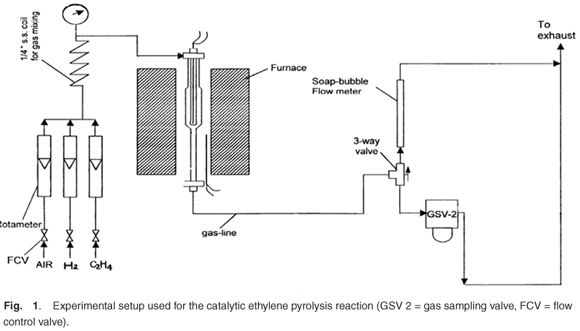
Results and discussion
Effect of carbon source
The direct hydrocarbon pyrolysis experiments using acetylene and ethylene as the carbon source were performed over a wide temperature range (600–950°C). Ethylene gave no CMS formation at 600–850°C. Low yields were obtained at 900°C (0.2 g) while the maximum yield attainable was 1.4 g at 950°C and the CMSs formed had a broad size distribution range (350–650 nm).
With acetylene, CMSs were observed at all temperatures used and very high yields were obtained at 900°C (>10 g). The size distribution was also much narrower than that observed with ethylene. As a result, acetylene was used as the source of carbon to make CMSs in this study. It may be noted that the yields reported above and others to follow, relate to the mass of the microspheres. No other carbonaceous materials were observed in the products. Our method of carbon microsphere production resulted in the synthesis of a clean material with purity >99%, as determined by TEM analysis.
Effect of temperature
The effect of temperature on the yield, size and morphology of the carbon spheres was investigated. Experiments were carried out in a temperature range of 600–1000°C. At each experimental temperature, the carbon deposit was removed from the reactor and weighed. Transmission electron microscopy studies of the products enabled the measurement of their diameters as well as their distribution. The acetylene gas flow rate was kept at 100 ml min–1 and a reaction time of 2 h was used unless otherwise stated. The effect of temperature on the yield is represented graphically in Fig. 2.
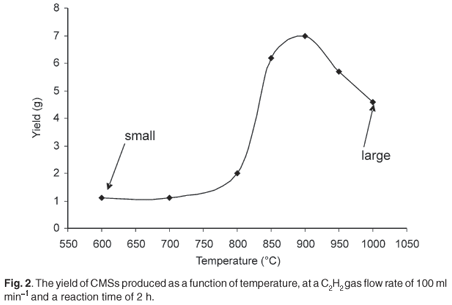
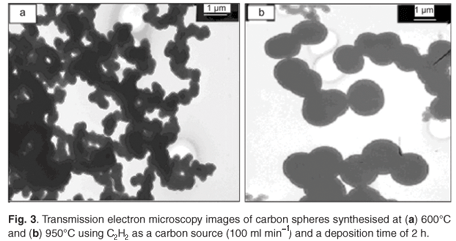
The yield of CMSs produced when acetylene was the feedstock gas increased with temperature from 600°C (<0.2 g) to 900°C (>7.0 g). Thereafter, the yield decreased with further increase in temperature from 900°C to 1 000°C. After further experiments (not shown) we could conclude that the optimum temperature for the production of carbon microspheres under the reaction conditions was 900°C. The size and diameter distribution of the carbon spheres became larger with an increase in temperature as shown in Fig. 3. At 600°C, the average diameter of the spheres was 250 nm with uniform distribution (Fig. 3a). At 950°C (Fig. 3b) and 1 000°C (not shown), the average diameter size increased to 1 000 nm and 1 300 nm respectively, with a broader diameter distribution. The higher temperature produced a larger size CMS.
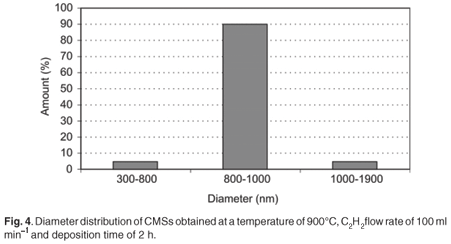
The diameter distribution of CMSs obtained at a temperature of 900°C with a C2H2 flow rate of 100 ml min–1 is shown in Fig. 4. It can be seen that the diameters of carbon spheres obtained were in the range of 300–1 900 nm, with about 90% in the range of 800–1 000 nm. This implies that the carbon spheres obtained at 900°C were quite uniform in size.
Effect of reaction time
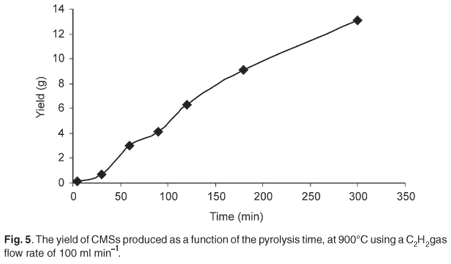
The acetylene pyrolysis time was varied from 5 min to 300 min at a set temperature. The yield of CMSs increased almost linearly with an increase in pyrolysis time (Fig. 5). The products however changed from being soft and powdery to hard solid products at reaction times >50 min. It appears that the hardness was related to a core-shell structure;21 with increased time in the hot reactor the shell formed, and this made the spheres 'harder'.
A diameter distribution was observed within each sample that was studied under TEM. The observed variation of the diameters of the carbon microspheres within a unique experiment prompted the investigation of the effect of the temperature gradient inside the reactor. A series of experiments were thus performed at an acetylene flow rate of 100 ml min–1, a pyrolysis time of 2 h and a temperature of 900°C (Fig. 6).
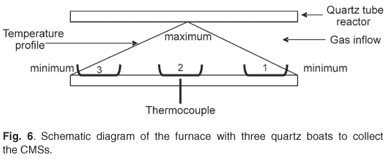
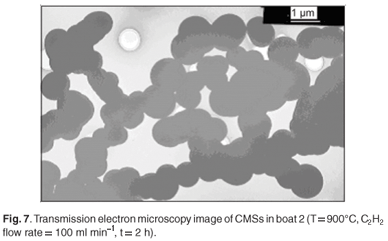
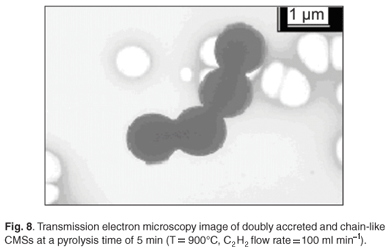
By analysing samples collected in the three different boats used in each experiment, it was observed that the microspheres in each boat contained material with uniform diameters. However, the boats contained different sizes of microspheres. Generally, the boat placed at the centre of the tube closest to the thermocouple i.e. boat 2, contained spheres with larger diameters (Fig. 7). This observation was attributed to the fact that the temperature in the reactor is highest at this point. These results agree with those obtained earlier, that showed that the diameters of the microspheres increased at higher temperatures. Multiple conglomerates of the carbon spheres were abundant in some cases, even with experiments conducted at a pyrolysis time of 5 min (Fig. 8).
Characterisation of CMSs
A growth mechanism for the graphitic CMSs has been proposed based on the microstructural information obtained by high resolution transmission electron microscopy (HRTEM).2 Our study is in agreement with previous studies and reveals that the carbon spheres are perfectly round, clean, solid and do not contain any large porosity (surface area 10 m2 g–1). The structure is composed of graphitic flakes and the flakes are in the size range of 1–30 nm, with a partial order similar to earlier reports.21 The flake-like nature of the CMSs implies that the surface structure is defective.1,2 This suggests that functionalisation of the spheres should be possible and that if metals are attached to the surface they should bind with ease. Indeed it is this feature that suggested that the Co/CMS materials should be excellent catalysts.
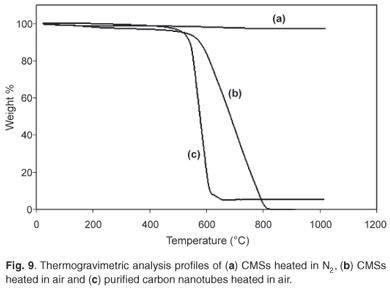
Thermogravimetric analysis was used to study the thermal stability of the carbon microspheres. The TGA profile was compared to that of carbon nanotubes (CNTs) that were synthesised in our laboratory. It was anticipated that if the spheres were graphitic, they should be stable up to about 600°C, after which they would begin to gradually lose weight in air to about 800°C. The TGA profiles are shown in Fig. 9. The differences observed between the two profiles are as shown. It may be noted that the TGA profile of the CNTs contained residual metal oxide (3%) associated with the catalyst used to make the CNTs. This can be contrasted with CMSs that contained only carbon and no deposit was left at temperatures greater than 800°C.
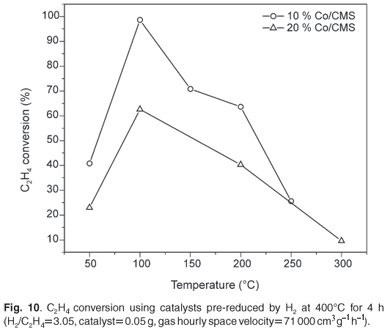
The effect of temperature on the performance of Co/CMS as a catalyst
The activity of as-synthesised (without any pre-treatment) as well as pre-reduced 5 wt%, 10 wt% and 20 wt% Co/CMS catalysts was investigated for the ethylene hydrogenation reaction. As expected, the unreduced catalysts were less active and no further information on these unreduced materials will be reported here.
The order of activity for the reduced catalysts was 10 wt% > 20 wt% > 5 wt%. This effect relates to the exposed surface Co atoms and suggests that if the metal particles are too large, then catalytic activity is lowered. This issue will need to be explored further in later studies. The optimum temperature for the conversion of the ethylene was found to be 100°C for all three catalysts. The conversion of C2H4 with the 10 wt% Co/CMS catalyst was approximately 100% at 100°C. However, at higher temperatures (T >100°C), the activity of all three catalysts decreased significantly (Fig. 10). This decrease could have arisen from catalyst deactivation by sintering or by deposition of carbonaceous deposits on the Co. The latter explanation is discussed below.
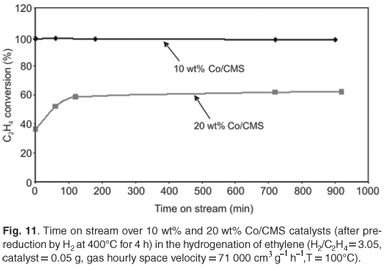
Time on stream studies using pre-reduced catalysts
The pre-reduced catalysts were tested for activity by running reactions for 16 h. The amount of C2H4 conversion was monitored at regular time intervals. It was observed that the catalysts maintained a markedly high percentage conversion throughout the reaction (Fig. 11). Such activity is not always observed with many commonly used hydrogenation catalysts (e.g. Pt, Pd and Ni), which are usually supported on silica, activated carbon and γ-alumina.22–24 The data suggest that sintering of the catalyst particles and poisoning due to coverage of the Co by carbonaceous material, are not taking place at this temperature. Further, it is believed that catalyst sintering is highly unlikely under these mild reaction conditions and preliminary HRTEM results suggest no change in catalyst particle size after reaction.
Conclusion
We have demonstrated that direct pyrolysis of C2H2 in the absence of a catalyst is an efficient method to produce carbon microspheres. Different pyrolysis conditions were used to study the size and morphology as well as the distribution of the resulting microspheres. The effect of pyrolysis temperature played an important role and influenced both the size and yield of the product. The experiments performed showed that at 900°C, the resulting spheres were prepared in high yield, were of large size (~1 µm) and had uniform distribution (not less than 90%). The detailed experiments conducted with C2H2 as a precursor indicate that the diameters of the carbon spheres can be controlled by varying the pyrolysis conditions (e.g. temperature and deposition time) and that the process could readily be scaled up for commercial production.
Carbon microspheres have shown to be a promising support for metal catalysts. The percentage of catalyst loading greatly affects the activity of the CMS-supported catalysts. The CMS-supported catalysts showed remarkable stable activity for a long period of time on stream. The abundance of defects in the CMSs makes them highly reactive and is consistent with their catalytic support applications. The possibilities for further surface chemical modification of the CMSs to produce modified catalyst supports is to be explored.
The authors are grateful for financial support from DST/NRF Centre of Excellence in Strong Materials, the University of the Witwatersrand, the Mellon Postgraduate Mentoring Programme and the CSIR (Pretoria). Services from the Electron Microscopy and Microanalysis Unit are much appreciated.
1. Kang Z.C. and Wang Z.L. (1997). Chemical activities of graphitic carbon spheres. J. Mol. Catal. A: Chemical 118, 215–222. [ Links ]
2. Kang Z.C. and Wang Z.L. (1996). On accretion of nanosize carbon spheres. J. Phys. Chem. 100, 5163–5165. [ Links ]
3. Wang Q., Li H., Chen L. and Huang X. (2001). Monodispersed hard carbon spherules with uniform nanopores. Carbon 39, 2211–2214. [ Links ]
4. Pol V.G., Motiei M., Gedanken A., Calderon-Moreno J. and Yoshimura M. (2004). Carbon spherules: synthesis, properties and mechanistic elucidation. Carbon 42, 111–116. [ Links ]
5. Li F., Qian Q., Yan F. and Yuan G. (2006). Nitrogen-doped porous carbon micro-spherules as supports for preparing monodisperse nickel nanoparticles. Carbon 44, 128–132. [ Links ]
6. Liu X., Huang B. and Coville N.J. (2002). The Fe(CO)5 catalyzed pyrolysis of pentane: carbon nanotube and carbon nanoball formation. Carbon 40, 2791–2799. [ Links ]
7. Biró L.P., Márk G.I., Horváth Z.E., Kertész K., Gyulai J., Nagy J.B. and Lambin Ph. (2004). Carbon nano architectures containing non-hexagonal rings: 'necklaces of pearls'. Carbon 42, 2561–2566. [ Links ]
8. Ma A., Wang X., Li T., Liu X. and Xu B. (2007). Characteristics of carbon microspheres and study on its adsorption isotherms. Mat. Sci. Eng. A 443, 54–59. [ Links ]
9. Qian H., Han F., Zhang B., Guo Y., Yue J. and Peng B. (2004). Non-catalytic CVD preparation of carbon spheres with a specific size. Carbon 42, 761–766. [ Links ]
10. Jin Y.Z., Gao C., Hsu W.K., Zhu Y., Huczko A., Bystrzejewski M., Roe M., Lee C.Y., Acquah S., Kroto H. and Walton D.R.M. (2005). Large-scale synthesis and characterization of carbon spheres prepared by direct pyrolysis of hydrocarbons. Carbon 43, 1944–1953. [ Links ]
11. Wissler M. (2006). Graphite and carbon powders for electrochemical applications. J. Power Sources 156, 142–150. [ Links ]
12. Mitsubishi Chemical Corporation (2006). Application examples of carbon black. Online at: http://www.carbonblack.jp/en/cb/youto.html#top [ Links ]
13. Lahaye J. (1992). Particulate carbon from gas phase. Carbon 30, 309–314. [ Links ]
14. Fallcheri F. and Schwob Y. (1995). From methane to hydrogen, carbon black and water. J. Hydrogen Energy 20, 197–202. [ Links ]
15. Scully D.B. and Davies R.A. (1965). Carbon formation from aromatic hydrocarbons. Combust. Flame 9, 185–191. [ Links ]
16. Ugarte D. (1992). Curling and closure of graphitic networks under electron beam irradiation. Nature 359, 707–709. [ Links ]
17. Wang Z.L. and Kang Z.C. (1996). Pairing of pentagonal and heptagonal carbon rings in the growth of nanosize carbon spheres synthesized by a mixed-valent oxide-catalytic carbonization process. J. Phys. Chem. 100, 17725–17731. [ Links ]
18. Sharon M., Mukhopadhyay K., Yase K., Ijima S., Ando Y. and Zhao X. (1998). Spongy carbon nanobeads – a new material. Carbon 36, 507–511. [ Links ]
19. Wang Y-G., Chang Y-C., Ishida S., Korai Y. and Mochida I. (1999). Stabilization and carbonization properties of mesocarbon microbeads (MCMB) prepared from a synthetic naphthalene isotropic pitch. Carbon 37, 969–976. [ Links ]
20. Gates B.C. (1992). Catalytic Chemistry. John Wiley and Sons Inc., New York. [ Links ]
21. Mondal K.C., Cele L.M., Witcomb M.J. and Coville N.J. (2008). Carbon microsphere supported Pd catalyst for the hydrogenation of ethylene. Cat. Comm. 9, 494–498. [ Links ]
22. Park C. and Baker R.T.K. (1999). Catalytic behavior of graphite nanofiber supported nickel particles. 3. The effect of chemical blocking on the performance of the system. J. Phys. Chem. B 103, 2453–2459. [ Links ]
23. Chambers A., Nemes T., Rodriguez N.M. and Baker R.T.K. (1998). Catalytic behavior of graphite nanofiber supported nickel particles. 1. Comparison with other support media. J. Phys. Chem. B 102, 2251–2258. [ Links ]
24. Park C. and Baker R.T.K. (1998). Catalytic behavior of graphite nanofiber supported nickel particles. 2. The influence of the nanofiber structure. J. Phys. Chem. B 102, 5168–5177. [ Links ]
Received 18 May. Accepted 26 May 2009.
* Author for correspondence E-mail: neil.coville@wits.ac.za














