Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.105 no.7-8 Pretoria jul./ago. 2009
RESEARCH ARTICLES
Carbon-nanostructured materials for energy generation and storage applications
P. Ndungu*; A. Nechaev; L. Khotseng; N. Onyegebule; W. Davids; R. Mohammed; G. Vaivars; V. Linkov
South African Institute for Advanced Materials Chemistry, University of the Western Cape, Private Bag X17, Bellville 7535, Cape Town, South Africa
ABSTRACT
We have developed and refined a chemical vapour deposition method to synthesise nanotubes using liquid petroleum gas as the carbon source. The nanotubes were thoroughly characterised by scanning electron microscopy, transmission electron microscopy, X-ray diffraction and thermogravimetric analysis. The protocol to grow nanotubes was then adapted to deposit nanotubes on the surface of different substrates, which were chosen based upon how the substrates could be applied in various hydrogen energy conversion systems. Carbon nanotubes are a nanostructured material with an extremely wide range of applications in various energy applications. The methods outlined demonstrate the complete development of carbon nanotube composite materials with direct applications in hydrogen energy generation, storage and conversion.
Key words: chemical vapour deposition, carbon nanotubes, consolidated nanomaterials, LPG, iron–titanium alloys, hydrogen storage
Introduction
An area of research that may produce a viable replacement to fossil fuels is the use of hydrogen. Compared to fossil fuels, hydrogen has a higher energy density (142 MJ kg–1 versus an average 42 MJ kg–1) and, when used in a simple combustion system with lean air mixtures, water is the only byproduct.1 When hydrogen is used in a fuel cell, the overall efficiency can be upwards of 50% compared to 25% in a simple combustion system.1 When the hydrogen is produced from a non-hydrocarbon source it has no polluting effects.1 Systems that utilise, store and produce hydrogen are key to implementing a future hydrogen-based economy.
Carbon nanotubes (CNTs) are one of the most versatile materials in current nanotechnological research. In the field of energy generation and storage, which includes research in solar cells, fuel cells and batteries (specifically lithium-ion), CNTs have shown a marked improvement over conventional systems.2 In addition, there are several reports of CNTs utilised in a variety of disciplines, including biomedicine,3 polymer science,2 catalysis,4 chemical and biological analytical sciences, as well as nano-electronic device applications.2–4
We synthesised nanostructured materials, elucidated their structure, and studied their properties and applications. This paper summarises our past and ongoing efforts in the use of CNTs in hydrogen-based energy systems.
Methods
Carbon nanotubes can be synthesised using a wide variety of methods; however, there are three general methods employed. These are electric arc discharge, laser vaporisation and chemical vapour deposition (CVD).2 Chemical vapour deposition is the most flexible of the three methods as it allows for the growth of CNTs over a range of temperature regimes and on a wide variety of substrates. These are the key reasons we have chosen to exploit this method.
Synthesis of carbon nanotubes
Carbon nanotubes were grown in a horizontally-aligned tube furnace. The catalysts used were nickel (Ni) foils, cobalt (Co) foils or nickel porous membrane measuring 0.5 cm × 0.5 cm. In some cases the nickel or cobalt foils were covered with an electroless deposit of the respective metal. The carbon source was liquid petroleum gas (LPG), and the deposition temperatures used were 600°C, 800°C and 1 000°C. In a typical experiment, the catalyst was loaded into a quartz tube located inside the tube furnace and then the system was flushed with nitrogen. After 10 min the furnace was ramped to the desired temperature and, once the temperature had stabilised, the nitrogen flow was stopped and the LPG flow was initiated. After 30 min the LPG flow was terminated and the system was flushed with nitrogen and then cooled to ambient temperature under a flow of nitrogen gas. The nitrogen gas flow rate was 500 ml min–1 and the LPG flow rate was maintained at 300 ml min–1. Samples were collected, weighed and characterised by scanning electron microscopy (SEM), transmission electron microscopy (TEM), thermal gravimetric analysis (TGA) and X-ray diffraction (XRD).
Synthesis of consolidated carbon nanotube architectures
The consolidation of different types of nanomaterials using one or more techniques, such as mechanical or chemical milling, extrusion, high or low temperature vapour deposition, self assembly and electrodeposition, is an intensive area of research for the production of novel nanocomposites.5 Introducing certain nano-architectures to the surface of CNTs or, conversely, adding nanotubes as a 'dopant' to a desired material, produces a new composite that combines and changes the properties of the parent structure, i.e. a new consolidated nanomaterial is synthesised.
Carbon vapour deposition modification of carbon cloth with CNTs
Nickel was deposited onto 2 cm × 2 cm pieces of carbon cloth. The nickel was deposited using a magnetron sputter coater or an electroless deposition method. The carbon cloth was then placed in the CVD system and CNTs were grown using a deposition temperature of 800°C, a deposition time of 10 min and LPG as the carbon source. Samples were characterised using SEM.
Synthesis of carbon nanotube paper
Carbon nanotubes were oxidised in a solution consisting of 98% sulphuric acid and 55% nitric acid. The acid mixture, in a ratio of 3:2 (sulfuric acid:nitric acid), had a total volume of 50 ml and was mixed with 1.5 g of CNTs and refluxed at 130°C for 180 min. Once the solution had cooled to ambient temperature, the solution was diluted with deionised water and filtered onto a nylon membrane filter with an average pore size of 0.45 µm. The collected nanotubes were washed with deionised water until a pH of 7.0 and then dried in an oven at 120°C for 30 min. The mat was then peeled off the nylon membrane to produce the CNT paper. Deposition of platinum onto the nanotube paper was carried out using one of two electroless deposition methods. Samples were characterised using SEM and energy dispersive spectrometry (EDS).
Carbon nanotube growth on an intermetallic alloy and subsequent doping with nanoparticles
Iron-titanium (TiFe) powders were pre-treated by mechanical ball milling. The ball:powder ratio was 20:1, the jar used was a 250 ml tungsten carbide jar, the diameter of the tungsten carbide balls was 3 cm, the ball milling time was 60 min, the milling speed was 350 rpm and the mill used was a Retsch PM100 planetary ball mill. Chemical vapour deposition was performed at 800°C for 60 min using LPG as the carbon source. The final step was the deposition of palladium or magnesium nanoparticles on the nanotube + TiFe composite. For deposition of magnesium, 125 ml of a 1 mM magnesium bis(2,2,6,6-tetramethyl-3,5-heptanedionate) hydrate in toluene was mixed with 1 g of powder in a round-bottom flask. The flask was placed in a bath sonicator, 10% hydrogen (balance argon) was bubbled through the mixture and then the mixture was sonicated for 3 h. The bath temperature was maintained at 65°C. Palladium deposition was performed using a similar method, but 165 ml of 1 mM palladium (II) acetylacetonate in toluene was used. Samples were characterised using TGA, SEM and EDS.
Chemical vapour deposition modification of nickel nanowire arrays
Nickel nanowires were grown using previously reported methods.6 Briefly, polyethylene terephthalate (PET) membranes with a thickness of 10–30 µm (trade name Hostaphan or Lavsan) and 200-nm pores, were seeded with a layer of nickel on one side using a magnetron sputter coater. The seed layer was used to grow a 10–20-µm nickel layer via electroless plating using a nickel electroless plating bath at a temperature of 20–30°C for 5–30 min. The nickel wires were then grown in the pores using galvanostatic deposition from a nickel sulphate hard bath (Watts). After deposition, the samples were thoroughly washed with deionised water and the PET template was removed by immersion in 2 M KOH solution for 30 min. Chemical vapour deposition was done using LPG as the carbon source, a deposition temperature of 800°C and a 5-min deposition time. The samples were characterised using SEM, EDS, XRD, scanning electrochemical microscopy (SECM) and impedance electrochemical spectroscopy (EIS).
Results and discussion
The carbon source for the production of CNTs was LPG. Thus the first step in the development of a protocol for nanotube synthesis was to determine and optimise the conditions necessary for the CVD growth of CNTs, using LPG.
Optimisation of CNT growth using LPG
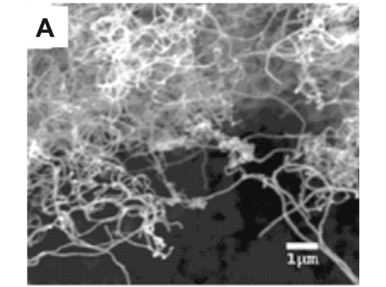
Weighing the carbon deposits produced after CVD was a quick and effective way to determine the effects of temperature on the production of carbon deposit. At 800°C, the maximum amount of carbon deposit was obtained on the nickel foils without electroless-deposited nickel, cobalt foils without electroless-deposited cobalt and the nickel porous membranes. At 600°C, on both the nickel and cobalt foils, the amount of carbon deposit was less than 1 mg; however, visual inspection of the foils after deposition revealed a small amount of discolouration. By contrast, the nickel porous membranes at 600°C produced tens of milligrams of carbon deposit. Scanning electron microscopy examination showed no CNTs nor amorphous carbons present on the metal foils, but the deposit on the nickel porous membranes was a mixture of nanotubes and amorphous carbons. At 1 000°C, the deposits on both the nickel and cobalt foils were a few milligrams more than at 600°C; however, visual inspection revealed the deposits to consist of grey 'flakes' which differed significantly from those at 600°C. Scanning electron microscopy examination revealed no CNTs, only amorphous carbons. The maximum amount of carbon deposit was seen at 800°C. Scanning electron microscopy examination of the samples (Fig. 1A) produced at 800°C revealed that the carbon deposits consisted of CNTs. The CNTs varied in diameter from 50–300 nm. The lengths of the CNTs varied from tens to hundreds of micrometres. There were no significant amounts of amorphous carbons seen in the deposits at 800°C. From these preliminary experiments, we determined that 800EC was the optimum growth temperature for CNTs, using LPG in our CVD system.
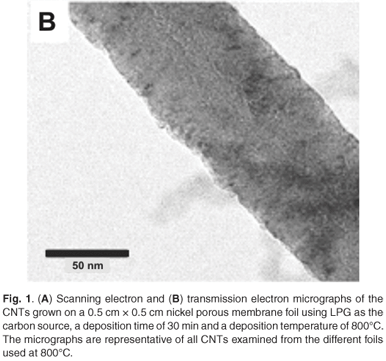
When samples were characterised using TEM (Fig. 1B), very little difference in the morphology was seen between the nanotubes grown with the different catalysts. The nanotubes varied in diameter and type; nanotubes with bamboo structure also were seen.
From the TGA data presented in Fig. 2A, the CNTs grown off the nickel porous membrane began to oxidise at 542°C, while the CNTs grown off the nickel foil with electroless nickel deposits began to oxidise at 505°C and the CNTs grown off the cobalt foil with electroless cobalt deposit began to oxidise at 380°C. From the TGA data presented, the nanotubes grown off the nickel porous membranes had a higher percentage of residual metal than the nanotubes grown off the nickel or cobalt foils. The amount of metal catalyst in the sample of nanotubes has been shown to affect the combustion rate and the onset oxidation temperature;7 however, in the samples prepared using LPG, this did not seem to be a factor. The lower the number of defect sites, and the greater the degree of graphitisation, the more stable nanotubes are to thermal oxidation; this has been shown for CVD-grown nanotubes.7 The higher oxidation onset temperature for the nanotubes grown off nickel does suggest that these nanotubes contain fewer defect sites, a higher degree of graphitisation and less non-nanotube material than the nanotubes grown with cobalt.
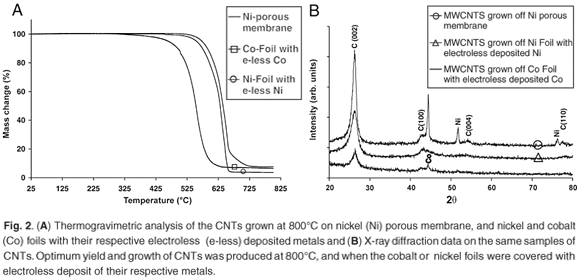
From the XRD data in Fig. 2B, the C (002) reflection is identified at 2θ = 26.42° for nanotubes grown off the nickel porous membrane, and at 2θ = 26.36° for the metal foils. These values are similar to those of crystalline graphite. The C (100) peak (at 2θ = 43.24°), is present on all three samples examined by XRD; however, the two extra carbon peaks are seen only on the sample grown off the nickel porous membrane. These peaks were identified as the C(004) at 2θ = 54.36° and the C (110) at 2θ = 77.68°. The presence of the C(004) and C(110) peaks for the nanotubes grown on the nickel-porous membrane indicate that these nanotubes are highly crystalline or contain less non-nanotube material compared to the nanotubes grown on the metal foils. It is interesting to note that the C(004) and C(110) peaks have been previously observed after nanotube samples were annealed at high temperature.8 Thus these two peaks do show that the nanotubes grown on the nickel-porous membrane at 800°C were highly crystalline when compared to the nanotubes grown on the metal foils. This result also explains the higher onset oxidation temperature seen in the TGA results for the nanotubes grown on the nickel-porous membrane. Thus, based on the TGA and XRD data, nickel catalysts in the LPG-CVD system for the growth of CNTs produced nanotubes with higher crystalline features than those produced by cobalt.
Consolidated CNT architectures
Chemical vapour deposition growth of CNT has the advantage of allowing one to grow CNTs on a multitude of substrates, which avoids the lengthy and sometimes complicated chemical processing methods needed to purify and eventually attach the CNTs to the substrate of interest. The examples presented below are specific to hydrogen energy based systems.
Carbon cloth modified with CNTs
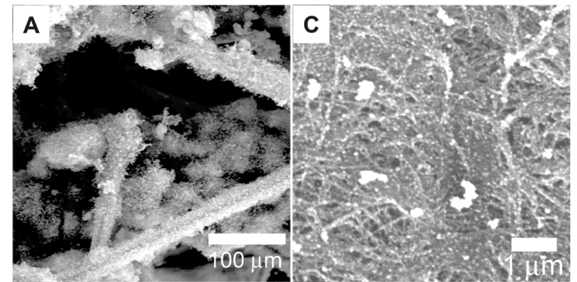
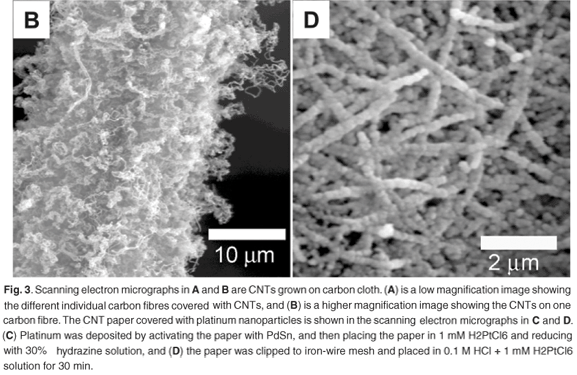
Scanning electron micrographs (Figs 3A and 3B) of the carbon cloth after CVD revealed the presence of CNTs. The electroless deposition of nickel on the carbon cloth resulted in a total surface coverage of every individual carbon fibre that makes up the carbon cloth. After CVD, CNTs grew all around the surface of the individual carbon fibres. This in turn led to the CNTs pushing individual fibres apart as they grew, which gave a bulky and brittle carbon cloth after deposition. In comparison, carbon cloth with nickel sputtered on the surface did not increase in size significantly, was not bulky and maintained some flexibility. This was due to the placement of the nickel onto the carbon fibres located near or at the surface of the carbon cloth. Thus, the growth of CNTs did not push apart individual fibres, and as a result the carbon cloths were not as brittle as those with the nickel electroless deposits.
Carbon nanotube paper
After metal deposition, the carbon nanotube paper was characterised using SEM (Figs 3C and 3D) and EDS. Energy dispersive spectrometry analysis (not shown) revealed that the respective metals did not have a significant amount of contaminants. Depending on the method used, the surface coverage could be controlled to produce discrete nanoparticles or an extensive metal coverage on the surface of the individual CNTs on the nanotube paper.
Both of these structures may be applied to fuel cell applications as part of the electrode system for the conversion of chemical to electrical energy. However, it is interesting to note that the structures may have a dual use and be applied in electrochemical sensor technologies. This potential is based upon the observation that, in both structures, there is an increase in the surface area; further characterisation is needed to confirm this potential.
Iron–titanium alloy substrate for CVD growth of CNTs
Iron–titanium is a hydrogen storage alloy with a low storage capacity (1.5%) and high activation temperature (450°C), and requires several cycles for activating the material.9 Ball milling the alloy increases the storage capacity slightly (1.6–1.8%) and reduces the number of activation cycles, but the activation temperature remains high.9 The growth of CNTs on the surface of the alloy powder has two main purposes. The first is to provide a stable, high surface area scaffold for the deposition of nanoparticles and the second is to provide an area for fast transfer of heat to, and from, the alloy particles.
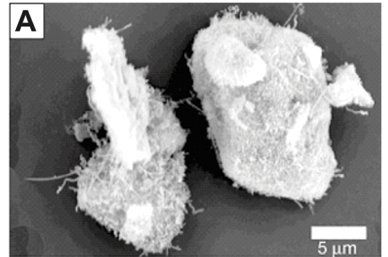
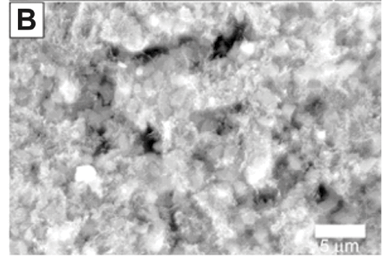
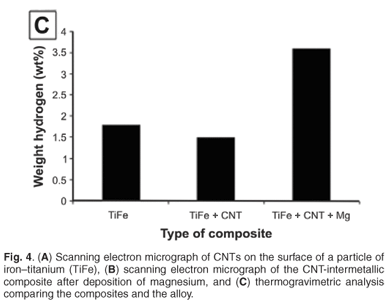
Carbon nanotubes on the surface of the intermetallic alloy are shown in Fig. 4A. The addition of magnesium to the nanotube alloy composite was confirmed by EDS and SEM analysis (Fig. 4B). The magnesium was clearly identified with non-stoichiometric amounts of oxygen, which indicates that magnesium, and not magnesium oxide, was deposited. This is expected because the deposition was done under dry gases with HPLC-grade dry toluene as the solvent. The TGA analysis, summarised in Fig. 4C, showed the addition of magnesium had a significant effect on the hydrogen storage capacity. The capacity increased to 3.6% weight, an increase of 2.0% compared to the alloy alone. Future work will report on the kinetic sorption properties of this novel composite.
Chemical vapour deposition of CNTs on nickel nanowire arrays
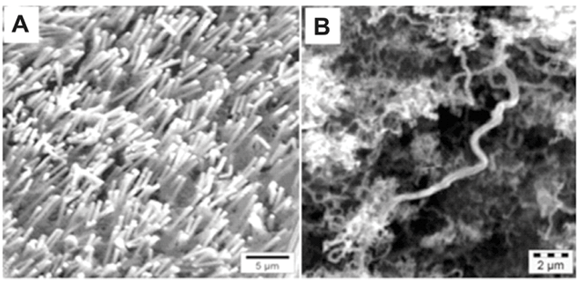
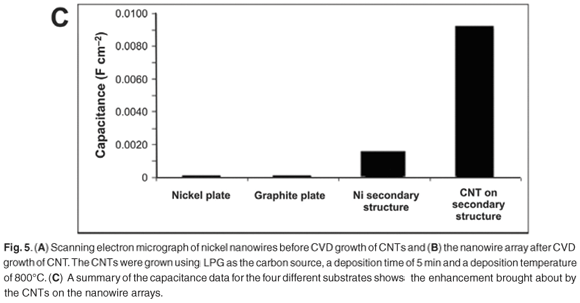
Before CVD on the nickel nanowires, SEM examination of the samples (Fig. 5A) revealed separate nickel wires arranged in a vertical array off a nickel-base structure. The XRD patterns of the nickel nanowires showed that the Ni (111) peak was the most intense peak, indicative of a face cubic centre structure. A peak was observed at 2θ = 26.3° on the X-ray diffractogram of the nickel nanowires after CVD growth. The peak was characteristic of crystalline carbon structures, such as CNTs; these structures were confirmed by the SEM micrographs (Fig. 5B), which showed an extensive coverage of the surface by nanotubes.
Cyclic voltammetry was done on the SECM in an alkaline solution. The electrolyte used was 0.1 M potassium hydroxide, the counter electrode was platinum, the reference electrode was a silver–silver chloride electrode and the working electrode was a flat nickel plate, nickel nanowires, or nickel nanowires with CNT deposits. The sweep rate for all experiments was 50 mV s–1 and the potential was swept from –1.0 V to 0.6 V. An increase in the measured current was seen as the working electrode was changed from the nickel plate to the nickel nanowires to the nickel nanowires with CNTs. This significant increase clearly shows that the composite would be ideally suited to use in an alkaline electrolysis system; however, the composite will have to be compared to commercial electrodes.
Electrochemical impedance spectroscopy was also done in alkaline solution. Electrochemical impedance spectroscopy can easily measure the electrochemical capacitance of an electrode and at the same time indirectly give a measurement of the electrochemically-accessible surface area. Electrochemical impedance spectroscopy measurements showed that the novel composite carbon material had a much higher capacitance compared to a flat nickel surface, the nickel nanowires, a graphite surface and a commercially-available anode for a lithium-ion battery (Fig. 5C). The higher capacitance shows that this is a promising electrode material for lithium-ion battery applications, and as a high surface area electrode.
Conclusions
Carbon nanotube growth was successfully achieved with LPG as the carbon source, and the optimum deposition temperature was determined to be 800°C. Bulk production of CNT powders allows us to extend the CNT protocols developed, and apply the CNTs as supports in electrocatalysis applications, either as nanotube paper or in conventional methods similar to those reported in the literature. The versatility in the CVD technique was clearly demonstrated by producing consolidated CNT architectures, specifically CNTs on carbon cloth, on an intermetallic alloy and on nickel nanowire arrays. Carbon nanotubes on the intermetallic alloy are a novel structure and serve as a model for the synthesis of new, exciting nanocomposite materials for hydrogen storage, or even for electrochemical systems. The nickel nanowire CNT composite shows great promise as a high surface area electrode that may find use in lithium-ion batteries or electrochemical-based sensors.
1. Schlapbach L. and Zuttel A. (2001). Hydrogen-storage materials for mobile applications. Nature 414, 353–358. [ Links ]
2. Baughman R.H., Zakhidov A.A. and de Heer W.A. (2002). Carbon nanotubes – the route toward applications. Science 297, 787–792. [ Links ]
3. Martin C.R. and Kohli P. (2003). The emerging field of nanotube biotechnology. Nat. Rev. Drug Discov. 2, 29–37. [ Links ]
4. Serp P., Corrias M. and Kalck P. (2003). Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A 253, 337–358. [ Links ]
5. Viswanathana V., Lahab T., Balanib K., Agarwalb A. and Seala S. (2006). Challenges and advances in nanocomposite processing techniques. Mater. Sci. Eng. R 54, 121–285. [ Links ]
6. Nkosi M.M. (2006). Preparation and physico-chemical properties of nickel nanostructured materials deposited in etched ion-track membrane, pp. 30–45. Ph.D. thesis, University of the Western Cape, South Africa. [ Links ]
7. Bom D., Andrews R., Jacques D., Anthony J., Chen B., Meier M.S. and Selegue J.P. (2002). Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: evidence for the role of defect sites in carbon nanotube chemistry. Nano. Lett. 2, 615–619. [ Links ]
8. Wu X.B., Chen P., Lin J. and Tan K.L. (2000). Hydrogen uptake by carbon nanotubes. Int. J. Hydrogen Energy 25, 261–265. [ Links ]
9. Sandrock G. (1999). A panoramic overview of hydrogen storage alloys from a gas reaction point of view. J. Alloys Compd. 293–295, 877–888. [ Links ]
Received 18 May. Accepted 3 August 2009.
* Author for correspondence. Present address: Environmental and Nanoscience Research Group, Chemistry Department, Faculty of Natural Science, University of the Western Cape, Private Bag X17, Bellville 7535, Cape Town, South Africa. E-mail: pndungu@uwc.ac.za