Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.105 n.5-6 Pretoria May./Jun. 2009
RESEARCH LETTERS
Evidence for opal phytolith preservation in the Langebaanweg 'E' Quarry Varswater Formation and its potential for palaeohabitat reconstruction
L. RossouwI, II, *; D.D. StynderIII; P. HaarhofIV
IFlorisbad Quaternary Research, National Museum, P.O. Box 266, Bloemfontein 9300, South Africa
IIDepartment of Plant Sciences, University of the Free State, P.O. Box 339, Bloemfontein 9300, South Africa
IIICenozoic Palaeontology, Natural History Department, Iziko South African Museum, P.O. Box 61, Cape Town 8000, South Africa
IVWest Coast Fossil Park, P.O. Box 42, Langebaanweg 7375, South Africa
ABSTRACT
At the end of the Miocene epoch, C4 grasslands began to expand at the expense of tree-, shrub- and forb-dominated C3 ecosystems. While C4 grasses were spreading throughout most regions of the world, C3 grasses may have been spreading along South Africa's southwest coast. Stable isotope analyses of hypsodont fossil ungulates from 'E' Quarry, a well-known Late Miocene/Early Pliocene fossil locality near the town of Langebaanweg, suggest that the local environment might have included a substantial C3 grass component. Besides this indirect evidence, little is known about the evolution, nature and importance of grass in the 'E' Quarry biome. As a preliminary step towards addressing these questions, we initiated a trial investigation to assess whether sediments at the site are conducive to the preservation of phytoliths, an important tool in the reconstruction of palaeohabitats. Results indicate that fossil phytoliths are sufficiently well preserved to allow a comprehensive analysis of the 'E' Quarry phytolith assemblage.
Key words: phytoliths, grassland, habitat reconstruction, Langebaanweg, fynbos, hypsodonty
Introduction
The distribution of savannas and grasslands are associated with a range of climatic parameters and provide a link to past climate changes.1 More specifically, grasses are potentially good indicators of past climates since the taxonomic composition of grass-dominated ecosystems indirectly reflect local climatic conditions.2,3 This is because grasses are likely to adapt relatively quickly to environmental changes, including variation in atmospheric carbon dioxide, temperature and moisture availability.4
During the Late Miocene to Early Pliocene (7–5 Myr), a major vegetational shift occurred when C4 grasslands expanded globally at the expense of tree-, shrub- and forb-dominated C3 ecosystems.5–8 The reason for this shift is not overtly clear. However, there is a suggestion that a drop in CO2 concentrations at the time would have favoured plants using the C4 photosynthetic pathway.7,9–11 The shift towards grass-dominated biomes may in turn have driven many of the evolutionary changes seen in terminal Miocene faunal communities around the globe.7 Faunal changes were particularly evident in Africa, where there was a proliferation in grazing ungulates and open-country adapted carnivores. Most significantly, some of the earliest known possible human ancestors also made their appearance.12–14
At a time when C4 grasses were spreading through most of Africa, C3 grasses may have been spreading along South Africa's southwest coast. Stable isotope analyses of a variety of ungulate specimens from 'E' Quarry (32°58'S, 18°7'E), a well-known Late Miocene to Early Pliocene fossil locality near the town of Langebaanweg (Fig. 1), indicate that the local environment remained dominated by C3 taxa.15 While this might indicate a closed environment dominated by trees, shrubs and forbs, the occurrence of several species of high-crowned (hypsodont) ungulates in the 'E' Quarry faunal assemblage, hint at the presence of a substantial grass component. Despite this evidence, very little is known about the evolution, nature and importance of grass in the Langebaanweg 'E' Quarry biome. As a first step towards answering these questions, we initiated a trial investigation to assess whether sediments at the site are conducive to the preservation of phytoliths, an important tool in palaeohabitat reconstruction. Here we present the results of this preliminary study and elaborate on how we plan to take the research further.

Langebaanweg fossil deposits and geological sequence
Fossil deposits were first discovered close to the town of Langebaanweg during phosphate mining operations which initially began at Baard's Quarry in 1943 and later moved to 'C' and 'E' Quarries (Fig. 1). However, the importance of these and other fossil localities in the area was only recognised in the late 1950s and 1960s, once scientists commenced with formal fossil descriptions. The prolific bone beds of 'E' Quarry in particular, soon established this locality as a fossil site of great importance. From the late 1950s until the late 1980s, 'E' Quarry was a major focus of research at the South African Museum (now Iziko South African Museum). Museum palaeontologist, Brett Hendey, in particular, was a productive researcher and publisher on the geology and fauna of the Langebaanweg localities.16–21
All the 'E' Quarry fossils originate from the phosphatic sediments of the Mio-Pliocene Varswater Formation.17,21,22 Four members are recognised in the most recent lithostratigraphic review of this formation—the Middle/Late Miocene Langeenheid Sandy Clay Member (LSCM) and Konings Vlei Gravel Member (KVGM), and the Late Miocene/Early Pliocene Langeberg Quartzose Sand Member (LQSM) and Muishond Fontein Pelletal Phosphate Member (MPPM)22 (Fig. 2). The LQSM and the MPPM produced the majority of fossils (more than 230 vertebrate and invertebrate taxa). The former is construed as primarily a floodplain and salt marsh deposit, while the latter is interpreted as primarily a river channel deposit.22,23 Two river channels, beds 3aS and 3aN, have been identified. Although the temporal relationship between these two beds remains unclarified, bed 3aS appears to be the earlier of the two.24,25 In any event, both river channels reflect a progressive northward shift of the lower course of the proto-Berg River.

The 'E' Quarry palaeoenvironment
Pollen sequences from the LSCM point towards the presence of subtropical forests, palms and marshy vegetation in the region during the early Late Miocene. However, Podocarpus and grass pollens tend to dominate in subsequent members of the Varswater Formation.26,27 There is also an increase in taxa which are characteristic of fynbos shrublands.28,29
As previously mentioned, stable carbon isotope analyses of tooth enamel carbonate of several ungulates from LQSM and MPPM, some with hypsodont dentition, provided values that remain within the C3 range. The degree of hypsodonty in ungulates is used in palaeoenvironmental reconstruction as an indicator of feeding preferences and habitat selection.30–33 The feeding classification of living ungulates ranges from grazers to mixed browsers/grazers to browsers, with grazers usually having more hypsodont teeth compared to species not specialised in grass consumption.34–37 An increase in tooth crown height represents an adaptation against tooth wear resulting from an abrasive diet consisting primarily of grasses with abundant phytoliths, and from the airborne grit and dust accumulated on the herbaceous plants of open environments.38–40 The presence of hypsodont ungulates with C3 stable carbon isotope values suggests that C3 grasses were the prevalent grass type and also implies that the modern wet winter/dry summer climate regime was established by Late Miocene to Early Pliocene times.15 Whereas fynbos vegetation today is characterised by an insignificant C3 grass component,41 the 'E' Quarry Late Miocene/Early Pliocene habitat may have included a substantial C3 grass component along with riverine forests and fynbos shrublands.42
Phytolith analysis
Opal phytoliths are particles of hydrated silica that are produced in various parts of living plants.43 Herbaceous plants, especially monocotyledonous plants, are prolific producers of phytoliths, particularly in the epidermal tissue of stems and leaves.44 The morphology and rate of production of phytoliths are largely under genetic control and there is evidence for metabolic exclusion or preferential concentration of silica in some plants.44 The grass family (Poaceae) in particular, produce abundant opaline silica bodies with diagnostic morphological features that permit identification to subfamily, or in some instances, lower taxonomic levels.45–47
Phytoliths preserve well in oxidising environments and have the potential to yield a record of past vegetation cover where there is no other information available.43,48 The abundant production of opaline silica in grasses, for example, is reflected in post-depositional contexts, which facilitate quantitative applications of fossil grass phytolith assemblages.49 This suggests that a predominance of any particular phytolith type in the soil is evidently a reflection of a locally-dominant group of plants. The predictive power of grass phytoliths also yields additional potential because the distribution of grasses in southern Africa is primarily linked to growing season temperature, which also accounts for the geographic distribution of C3 and C4 grasses.2 Recently, a few preliminary studies of fossil grass phytolith assemblages from Pretoria Saltpan, the Free State Province and Lesotho, drew attention to the potential for grass phytolith analysis in palaeograssland research in South Africa.50–52
Methods
Eleven soil samples were collected from three different localities in 'E' Quarry. Sampling of the LSCM, the top of the KVGM and the LQSM was conducted in an old test pit, approximately half a kilometre southwest of the current fossil exhibition tunnel. Three samples were collected from the MPPM—two from previously excavated areas in the exhibition tunnel and one from a newly excavated area in the adjacent tunnel, which currently is closed to the public. An additional two samples were collected from fine-grained sandy deposits of the LQSM, in the latter tunnel.
The procedure for recovering phytoliths from sediment samples is based on published techniques.53,54 Approximately 50 g of each sample was used for each phytolith extraction. Essential steps included deflocculation, removal of clays by means of sedimentation, and the elimination of carbonates using HCl in low concentration (10%). Phytolith extraction also involved mineral separation with a heavy liquid solution of sodium polytungstate (s.d. = 2.3). Fractions were mounted on microscope slides in glycerin jelly and scanned under a Nikon 50i polarising microscope at ×400 magnification. Morphological descriptions follow the guidelines provided by the International Code for Phytolith Nomenclature 1.0.55 A modern phytolith comparative collection aided the identification of fossil phytoliths.
Results and conclusion
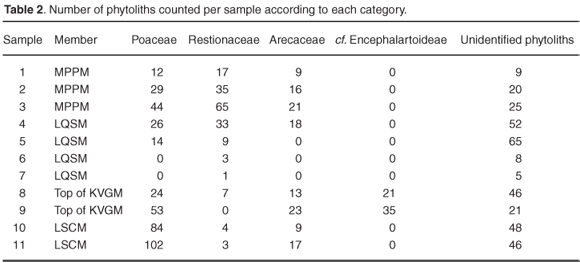
Preservational conditions for biogenic silica are generally favourable throughout the sequence despite comparatively low and more variable phytolith counts in the LQSM and MPPM samples (Table 1 and Fig. 3). The predominantly sandy matrix of the estuarine LQSM and fluviatile MPPM deposits most likely facilitated leaching of biogenic silica during waterlogged conditions, but more detailed analyses of phytoliths from the various depositional environments are needed to adequately interpret post-depositional processes.


Diagnostic grass phytolith morphotypes, as well as morphotypes produced by the monocot families Restionaceae (Cape reed grasses) (Figs 4e and 4r) and Arecaceae (palms) (Fig. 4f), were identified. Globular cavate phytoliths, analogous to silica bodies produced by the gymnospermous Encephalartoideae (cycad subfamily), were identified in the KVGM (Figs 4l and 4m). In addition, pennate diatoms, with surface markings at right angles to the long axis, are abundant in the LSCM (Fig. 4i).
Four grass short-cell phytolith types were recognised, namely bilobates, saddles, rondels and trapeziform morphotypes43,46–48 (Figs 4a–d, 4k, 4o and 4p). Saddle-shaped grass phytoliths were absent in the KVGM, LQSM and MPPM samples. This is noteworthy and seems to support the suggestion that C3 grasses prevailed during Late Miocene/Early Pliocene times at Langebaanweg. In contrast, saddle-shaped grass phytoliths were identified in the LSCM (Figs 4b and 4c), which is significant given that in the modern environment these phytoliths are almost exclusively found in the Chloridoideae, a grass subfamily that utilises the C4 photosynthetic pathway.3,56
The results are very promising and show that fossil phytoliths are sufficiently preserved in terms of both quantity and variety (Table 2). The occurrence of palm and cycad-type phytoliths in the LSCM and KVGM supports previous work based on palynological indicators.26 Except for the LQSM, grasses are well represented in the Varswater Formation—more than 50% of the total phytolith count in the LSCM and about 30% of the total phytolith count in the KVGM and MPPM (Fig. 5). A comparatively large proportion of restoid phytoliths in the LQSM and MPPM compliments earlier and more recent reconstructions, suggesting that South Africa's southwest coast was becoming drier and more open during the Early Pliocene.57,58 The presence of chloridoid-type grass phytoliths in the LSCM and subsequent lack in the KVGM, LQSM and MPPM further suggest that radical changes in grassland composition may have occurred towards the end of the Miocene when climate changed from subtropical to more temperate.27,59 While intrigued, we are nevertheless reluctant to make firm inferences regarding the presence of C3 or C4 grasses in the 'E' Quarry Varswater sediments at this preliminary stage. Rather, we intend to proceed with a comprehensive quantitative study of grass phytoliths from the Varswater Formation to ascertain the nature of Mio-Pliocene grass expansion in the southwestern Cape. This study will involve referencing modern plant material from the region, systematic soil sampling of the geological succession and evaluation of the fossil phytolith data through standard laboratory and analytical techniques.

Heritage Western Cape provided the permit to excavate and sample sediments for phytoliths at 'E' Quarry. This project benefited from a National Research Foundation grant (Roger Smith, Iziko Museums of Cape Town) and an African Origins Platform grant (Roger Smith, Iziko Museums of Cape Town). We would like to thank Albrecht Manegold, Natashe Kotze, Joan Modinger, Werner Modinger and Rhasieda Bester for assistance during the 2008 field season.
1. Jacobs B.F., Kingston J.D. and Jacobs L.L. (1999). The origin of grass-dominated ecosystems. Ann. Missouri Bot. Gard. 86, 590–643. [ Links ]
2. Vogel J.C., Fuls A. and Ellis R.P. (1978). The geographical distribution of Krantz grasses in South Africa. S. Afr. J. Sci. 74, 209–215. [ Links ]
3. Gibbs Russell G.E. (1988). Distribution of subfamilies and tribes of Poaceae in southern Africa. Monogr. Syst. Bot. Missouri Bot. Gard. 25, 555–566. [ Links ]
4. Wooller M.J. and Beuning K.R. (2002). Introduction to the reconstruction and modelling of grass-dominated ecosystems. Palaeogeogr. Palaeoclimatol. Palaeoecol. 177, 1–3. [ Links ]
5. Quade J., Cerling T.E., Andrews P. and Alpagut B. (1995). Paleodietary reconstruction of Miocene faunas from Pasalar, Turkey using stable carbon and oxygen isotopes of fossil tooth enamel. J. Hum. Evol. 28, 373–384. [ Links ]
6. Cerling T.E. (1992). Development of grasslands and savannas in East Africa during the Neogene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 97, 241–247. [ Links ]
7. Cerling T.E. (1993). Expansion of C4 ecosystems as an indicator of global ecological change in the late Miocene. Nature 361, 344–345. [ Links ]
8. Cerling T.E., Harris J.M., MacFadden B.J., Leakey M.G., Quade J., Eisenmann V. and Ehleringer J.R. (1997). Global vegetation change through the Miocene/ Pliocene boundary. Nature 389, 152–157. [ Links ]
9. Ehleringer J.R., Sage R.F., Flanagan L.B. and Pearcy R.W. (1991). Climate change and the evolution of C4 photosynthesis. Trends Ecol. Evol. 6, 95–99. [ Links ]
10. Ehleringer J.R., Cerling T.E. and Helliker B.R. (1997). C4 photosynthesis, atmosphere CO2, and climate. Oecologia 112, 285–299. [ Links ]
11. Pagani M., Zachos J.C., Freeman K.H., Tipple B. and Bohaty S. (2006). Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science 309, 600–603. [ Links ]
12. Senut B., Pickford M., Gommery D., Mein P., Cheboi K. and Coppens Y. (2001). First hominid from the Miocene (Lukeino Formation, Kenya). C.R. Acad. Sci., Paris, Ser. IIa 332, 137–144. [ Links ]
13. Brunet M., Guy F., Pilbeam D., Mackaye H.T., Likius A., Ahounta D., Beauvilain A., Blondel C., Bocherens H., Boisserie J.R., De Bonis L., Coppens Y., Dejax J., Denys C., Duringer P., Eisenmann V., Fanone G., Fronty P., Geraads D., Lehmann T., Lihoreau F., Louchart A., Mahamat A., Merceron G., Mouchelin G., Otero O., Pelaez Campomanes P., Ponce De Leon M., Rage J.C., Sapanet M., Schuster M., Sudre J., Tassy P., Valentin X., Vignaud P., Viriot L., Zazzo A. and Zollikofer C. (2002). A new hominid from the Upper Miocene of Chad, Central Africa. Nature 418, 145–151. [ Links ]
14. Pickford M., Senut B., Gommery D. and Treil J. (2002). Bipedalism in Orrorin tugenensis by its femora. C.R. Paléo. 1, 191–203. [ Links ]
15. Franz-Odendaal T.A., Lee-Thorp J.A. and Chinsamy A. (2002). New evidence for the lack of C4 grassland expansions during the early Pliocene at Langebaanweg, South Africa. Paleobiol. 28, 378–388. [ Links ]
16. Hendey Q.B. (1970). A review of the geology and palaeontology of the Plio/Pleistocene deposits at Langebaanweg, Cape Province. Ann. S. Afr. Mus. 56, 75–117. [ Links ]
17. Hendey Q.B. (1970). The age of fossiliferous deposits at Langebaanweg, Cape Province. Ann. S. Afr. Mus. 56, 119–131. [ Links ]
18. Hendey Q.B. (1972). Further observations on the age of the mammalian fauna from Langebaanweg, Cape Province. Palaeoecol. 6, 172–175. [ Links ]
19. Hendey Q.B. (1973). Fossil occurrences at Langebaanweg, Cape Province. Nature 244, 13–14. [ Links ]
20. Hendey Q.B. (1974). The late Cenozoic carnivora of the southwestern Cape Province. Ann. S. Afr. Mus. 63, 1–369. [ Links ]
21. Hendey Q.B. (1976). The Pliocene occurrences in 'E' Quarry, Langebaanweg, South Africa. Ann. S. Afr. Mus. 69, 215–247. [ Links ]
22. Roberts D.L. (2006). Lithostratigraphy of the Varswater Formation. S. Afr. Com. Stratigraphy Lithostratigraphic Series 9, 27–31. [ Links ]
23. Hendey Q.B. (1982). Langebaanweg. A Record of Past Life, pp. 1–72. South African Museum, Cape Town. [ Links ]
24. Hendey Q.B. (1981). Geological succession at Langebaanweg, Cape Province, and global events of the late Tertiary. S. Afr. J. Sci. 77, 33–38. [ Links ]
25. Hendey Q.B. (1984). Southern African late Tertiary vertebrates. In Southern African Prehistory and Palaeoenvironments, ed. R.G. Klein, pp. 81–106. A.A. Balkema, Rotterdam. [ Links ]
26. Coetzee J.A. and Rogers R. (1982). Palynological and lithological evidence for the Miocene palaeoenvironment in the Saldanha region (South Africa). Palaeogeogr. Palaeoclimatol. Palaeoecol. 39, 71–85. [ Links ]
27. Scott L. (1995). Pollen evidence for vegetational and climatic change in southern Africa during the Neogene and Quaternary. In Paleoclimate and Evolution with Emphasis on Human Origins, eds E.S. Vrba, G.H. Denton, T.C. Partridge and L.H. Burckle, pp. 65–76. Yale University Press, New Haven. [ Links ]
26. Tankard A.J. and Rogers J. (1978). Late Cenozoic palaeoenvironments on the west coast of southern Africa. J. Biogeogr. 5, 319–337. [ Links ]
29. Scott L., Anderson H.M. and Anderson J.M. (1997). Vegetation history. In Vegetation of Southern Africa, eds R.M. Cowling, D.M. Richardson and S.M. Pierce, pp. 62–90. Cambridge University Press, Cambridge. [ Links ]
30. Janis C.M., Damuth J. and Theodor J.M. (2000). Miocene ungulates and terrestrial primary productivity: where have all the browsers gone? Proc. Natl. Acad. Sci. USA 14, 7899–7904. [ Links ]
31. Janis C.M., Damuth J. and Theodor J.M. (2002). The origins and evolution of the North American grassland biome: the story from the hoofed mammals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 177, 183–198. [ Links ]
32. Stromberg C.A.E. (2002). The origin and spread of grass-dominated ecosystems in the late Tertiary of North America: preliminary results concerning the evolution of hypsodonty. Palaeogeogr. Palaeoclimatol. Palaeoecol. 177, 59–75. [ Links ]
33. Stromberg C.A.E. (2006). Evolution of hypsodonty in equids: testing a hypothesis of adaptation. Paleobiology 32, 236–258. [ Links ]
34. McNaughton S.J. and Georgiadis N.J. (1986). Ecology of African grazing and browsing mammals. Ann. Rev. Ecol. Syst. 17, 39–65. [ Links ]
35. Bodmer R.E. (1990). Ungulate frugivores and the browser–grazer continuum. Oikos 57(3), 319–325. [ Links ]
36. Mendoza M. and Palmqvist P. (2008). Hypsodonty in ungulates: an adaptation for grass consumption or for foraging in open habitat? J. Zool. 274, 134–142. [ Links ]
37. Fortelius M. (1985). Ungulate cheek teeth: developmental, functional and evolutionary interrelations. Acta Zool. Fenn. 180, 1–76. [ Links ]
38. Brizuela M.A., Detling J.K. and Cid M.S. (1986). Silicon concentration of grasses growing in sites with different grazing histories. Ecology 67, 1098–1101. [ Links ]
39. Janis C.M. and Fortelius M. (1988). On the means whereby mammals achieve increased functional durability of their dentitions, with especial reference to limiting factors. Biol. Rev. 63, 197–230. [ Links ]
40. Lucas P.W., Turner I.M., Dominy N.J. and Yamashita N. (2000). Mechanical defences to herbivory. Ann. Bot. 86, 913–920. [ Links ]
41. Rutherford M.C. and Westphall R.H. (1994). Biomes of southern Africa: an objective categorization. Mem. Bot. Surv. S. Afr. 63, 1–94. [ Links ]
42. Hendey Q.B. (1981). Palaeoecology of the Late Tertiary fossil occurrences in 'E' Quarry, Langebaanweg, South Africa, and a reinterpretation of their geological context. Ann. S. Afr. Mus. 84(1), 1–104. [ Links ]
43. Piperno D.R. (2006). Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists, pp. 1–238. Altamira Press, New York. [ Links ]
44. Piperno D.R. (1988). Phytolith Analysis: An Archaeological and Geological Perspective, pp. 1–279. Academic Press, London. [ Links ]
45. Hodson M.J., White P.J., Mead A. and Broadley M.R. (2005). Phylogenetic variation in the silicon composition of plants. Ann. Bot. 96(6), 1027–1046. [ Links ]
46. Mulholland S.C. and Rapp G.J. (1992). A morphological classification of grass silica-bodies. In Phytolith Systematics: Emerging Issues. Advances in Archaeological and Museum Science, eds G.J. Rapp and S.C. Mulholland, pp. 65–81. Plenum Press, New York. [ Links ]
47. Stromberg C.A.E. (2004). Using phytolith assemblages to reconstruct the origin and spread of grass-dominated habitats in the great plans of North America during the late Eocene to early Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 207, 275–339. [ Links ]
48. Fredlund G.G. and Tieszen L.T. (1994). Modern phytolith assemblages from the North American Great Plains. J. Biogeogr. 21, 321–335. [ Links ]
49. Fredlund G.G. and Tieszen L.T. (1997). Calibrating grass phytolith assemblages in climatic terms: application to Late Pleistocene assemblages from Kansas and Nebraska. Palaeogeogr. Palaeoclimatol. Palaeoecol. 136, 199–211. [ Links ]
50. McLean B. and Scott L. (1999). Phytoliths in sediments of the Pretoria Saltpan and their potential as indicators of environmental history at the site. In Tswaing-investigations into the Origin, Age and Palaeoenvironments of the Pretoria Saltpan, ed. T.C. Partridge, pp. 167–171. Council for Geosciences, Pretoria. [ Links ]
51. Grab S., Scott L., Rossouw L. and Meyer S. (2005). Holocene palaeoenvironments inferred from a sedimentary sequence in the Tsoaing River Basin, western Lesotho. Catena 61, 49–62. [ Links ]
52. Scott L. and Rossouw L. (2005). Reassessment of botanical evidence for palaeoenvironments at Florisbad, South Africa. S. Afr. Archaeol. Bull. 60, 96–102. [ Links ]
53. Lentfer C.J. and Boyd W.E. (2000). Simultaneous extraction of phytoliths, pollen and spores from sediments. J. Archaeol. Sci. 27, 363–372. [ Links ]
54. Horrocks M. (2005). A combined procedure for recovering phytoliths and starch residues from soils, sedimentary deposits and similar soils. J. Archaeol. Sci. 32, 1169–1175. [ Links ]
55. Madella M., Alexandre A. and Ball T. (2005). International code for phytolithnomenclature 1.0. Ann. Bot. 96, 253–260. [ Links ]
56. Ellis R.P. (1987). A review of comparative leaf blade anatomy in the systematics of the Poaceae: the past twenty five years. In Grass Systematics and Evolution, eds T.R. Soderstrom, K.W. Hilu, C.S. Campbell and M.E. Barkworth, pp. 3–10. Smithsonian Institution Press, Washington D.C. [ Links ]
57. Franz-Odendaal T.A., Kaiser T.M. and Bernor R.L. (2003). Systematics and dietary evaluation of a fossil equid from South Africa. S. Afr. J. Sci. 99, 453–459. [ Links ]
58. Unger P.S., Mercaron G. and Scott R.S. (2006) Dental microwear of bovids from Langebaanweg: evidence for diet and palaeoecology. Langebaanweg 2006: Mini-symposium and Workshop Proceedings. Afr. Nat. Hist. 2, 199–200. [ Links ]
59. Coetzee J.A., Scholtz A. and Deacon H.J. (1983). Palynological studies and vegetation history of the fynbos. In Fynbos Palaeoecology: A Preliminary Synthesis, eds H.J. Deacon, Q.B. Hendey and J.J.N. Lambrechts, pp. 156–173. SANSP, Foundation for Research Development, Pretoria. [ Links ]
Received 19 February. Accepted 15 May 2009.
* Author for correspondence E-mail: lloyd@nasmus.co.za


















