Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.105 no.5-6 Pretoria Mai./Jun. 2009
RESEARCH ARTICLES
The ultrastructural effects and immunolocalisation of fumonisin B1 on cultured oesophageal cancer cells (SNO)
R.B. Myburg; N. Needhi; A.A. Chuturgoon*
Discipline of Medical Biochemistry, School of Medical Sciences, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Private Bag 7, Congella, Durban 4013, South Africa
ABSTRACT
Numerous investigations have shown that fumonisin B1 (FB1) is the causal agent in a range of animal toxicities, including leucoencephalomalacia, pulmonary oedema and renal and hepatic cancer in rats and mice. Fumonisin B1 has also been implicated in the aetiology of oesophageal cancer in South Africa. Human data are lacking, however, and the International Agency for Research on Cancer has accordingly classified this mycotoxin as a Type 2B carcinogen. This study investigated the ultrastructural effects of FB1 cytotoxicity on a human oesophageal carcinoma cell line (SNO). The pathological changes induced by FB1 were determined using transmission and scanning electron microscopy. Immunocytochemistry was used to immunolocalise FB1 (monoclonal anti-FB1) within the cells. The results showed marked pathological changes that included enlargement or microsegregation of the nucleus, microsegregation of the nucleolus, and swelling and elongation of mitochondria, as well as signs of membrane damage. These cytotoxic effects were associated with the action of FB1, since the toxin was internalised in nuclei, mitochondria and the cytoplasm of affected cells. This study shows that FB1 may exert its biological effects in SNO cells through binding to cellular macromolecules or membrane components within the affected organelles.
Key words: oesophageal cancer, fumonisin B1, cell culture, immunocytochemistry
Introduction
Cancer of the oesophagus (OC) follows the increasing incidence of cancer worldwide. There is a high incidence in the black population in certain parts of Transkei, South Africa and in parts of China;1 both have increased in recent times.1,2
Maize is the staple food of the population of Transkei.3 Fumonisins are mycotoxins produced by Fusarium verticillioides and other Fusarium fungi, found worldwide on maize and maize-based foods.4,5 Maize from an area of high OC incidence in the Transkei contained higher levels of fumonisin B1 (FB1) (44 ppm) than did commercial maize meal (<10 ppm).6
Fumonisin B1, a strongly polar compound,5 is the most prevalent of the fumonisin mycotoxins.7–10 The polarity of the toxin determines its level of carcinogenicity11 i.e. the more polar the molecule, the greater the cytotoxic response. In addition to polarity, other determinants, such as the presence of a free amino group, carboxyl groups and the location of the hydroxyl group, could also affect the biological activity of these compounds. Thus, both the amino group and the intact molecule play an important role in the toxic and cancer-promoting activity of fumonisins.8,12 This would be compatible with the association of FB1 with both soluble and insoluble portions of the cell.13
One of the established characteristics of fumonisin toxicity is its species specificity. A causal role of FB1 in equine leucoencephalomalacia,14 porcine pulmonary oedema,15 and liver and kidney carcinoma in rats16 has been reported. An initial study by Marasas et al.17 showed that BD IX rats chronically exposed to F. verticillioides developed oesophageal hyperplasia, forestomach papillomas and carcinomas, hepatocellular carcinomas and cholangiocarcinomas. Several subsequent studies with laboratory animals in which cultures of F. verticillioides or fumonisins were fed found no signs of cancerous or precancerous lesions of the oesophagus,16,18,19 however. Thus, there is no consistent animal model to support the theory that FB1 may be related to human OC.
In human health, the role of fumonisins is still unclear,12 but the consumption of Fusarium-contaminated maize has been correlated with human OC in areas of South Africa, China and other countries.20,21 The high incidence of OC in the Transkei has been demographically associated with a prevalence of FB1-contaminated corn.22,23 Although other factors, such as smoking, alcohol consumption and certain dietary and environmental components could be involved in the aetiology of the disease, several recent studies have implicated fumonisins as a possible contributing factor. Nitrosamines or other carcinogenic agents may be responsible for the increased incidence of OC in humans, with fumonisins contributing to the problem through their potent tumour-promoting activity.24 In a previous study,25 we showed that FB1 (2–34 µM), a type 2B carcinogen, was cytotoxic to cultured SNO oesophageal cancer cells. We speculate that FB1 would alter organelle ultrastucture in cultured SNO human oesophageal cells.
In this study, the SNO epithelial cell line (cells that retain most of the functions associated with primary cells), derived from a well-differentiated squamous cell carcinoma explanted from a 62-year old indigenous black male,26 was used to determine the effects of FB1 on cellular ultrastructure. The pathological changes induced by FB1 were determined using transmission electron microscopy. The cells were then immunocytochemically probed for the presence of FB1.
Materials and methods
Reagents
Fumonisin B1 (98%) was purchased from Sigma (Johannesburg, South Africa). A monoclonal FB1 antibody was purchased from Neogen (Michigan, U.S.A.). All other immunochemicals were purchased from Sigma (Johannesburg, South Africa). Cell culture media and disposables were purchased from Adcock Scientific (Durban, South Africa). All HPLC and other chemicals were purchased from Merck (Johannesburg, South Africa).
Maintenance of the SNO cell line
The SNO epithelial cells26 (a well-differentiated squamous carcinoma line) were grown and maintained in Eagle's minimum essential medium (EMEM) containing 0.25 mM Hepes buffer supplemented with 5–10% foetal calf serum (FCS), 1% L-glutamine and 1% penstrep fungizone (complete culture media). The cells were maintained in a 37°C incubator and grown to confluency (25 cm2 flasks) before use.
Transmission electron microscopy
Confluent 25 cm2 flasks of SNO cells were treated with FB1 at concentrations of 1 µM, 2 µM, 4 µM, 8 µM, 16 µM or 32 µM for 24 h; these concentrations were in keeping with a previous in vitro study.25 Flasks containing untreated cells served as controls. After a 24-h incubation period, the treatment medium was removed and stored for subsequent HPLC analysis. The cells were washed twice with Hanks' balanced salt solution (HBSS) (3 ml), and the cells were then fixed with 1% glutaraldehyde in HBSS for transmission electron microscopy (TEM) and immunocytochemistry (ICC). After a 30-min fixation period, the epithelial cells in flasks were washed twice with HBSS (5 ml), and then processed for microscopy. Briefly, cells were post-fixed in 1% osmium tetroxide in distilled water (4°C) (omitted for flasks used for immunocytochemistry) and then dehydrated with increasing strengths of alcohol (70%, 90%, 100%) for 15 min each.
Flasks containing SNO cells were infiltrated with Spurr's resin (2 × 60 min) and then left to polymerise at 60°C for 24 h. The blocks, which comprised cells sandwiched between the resin and plastic flasks, were trimmed and vertically sectioned on a Reichert Ultracut microtome. Sections (0.5 µm) were picked up on 200 mesh copper grids, stained with uranyl acetate in alcohol:water (1:1), counterstained with Reynold's lead citrate, and viewed and photographed using a JEOL-JEM 100S transmission electron microscope. Sections (0.5 µm) were also picked up on 200 mesh nickel grids and probed immunochemically for FB1.
Immunocytochemistry
Non-osmicated sections on 200 mesh nickel grids were etched to block endogenous peroxidase activity by placing in 5% H2O2 (20 µl, 5 min) and then washed in distilled water (10 ml), and finally drained on fibre-free paper. The sections were incubated in normal goat serum diluted 1:20 in 50 mM Tris (pH 7.2) for 30 min, in order to block non-specific binding sites, before placing them in the primary antibody (monoclonal mouse anti-FB1 diluted 1:100 in 50 mM Tris with 0.2% bovine serum albumin (BSA), pH 7.2) for 3 h. Grids were washed in 50 mM Tris (pH 7.2), 50 mM Tris containing 0.2% BSA (pH 7.2), and finally in 50 mM Tris containing 1% BSA (pH 7.2). The secondary antibody used was goat anti-mouse IgG conjugated to a 10 nm gold probe (1:15 in 50 mM Tris with 1% BSA, pH 7.2) to localise FB1. The grids were thoroughly washed in 50 mM Tris 0.2% BSA (pH 7.2), 50 mM Tris (pH 7.2) and finally in distilled water. Untreated cultured cells served as the negative controls. The grids were stained with 1% uranyl acetate, counterstained using Reynold's lead citrate and then viewed using the Joel JEM 100 TEM.
Method controls (in which the primary antibody was omitted and replaced with PBS, pH 7.4) were used to determine the method specificity. This results in the exclusion of staining caused by mechanisms other than the immunological interactions between the primary antibody and antigens(s).
Scanning electron microscopy
The SNO cells were grown on 20 mm2 coverslips in six-well plates until confluency. The cells were then treated with FB1 (at the same concentrations as those used for TEM), fixed, post-fixed and dehydrated as described for TEM. Coverslips were then critical point-dried, coated to 10 nm thickness with gold particles, and viewed using a Hitachi S520 scanning electron microscope.
Results and discussion
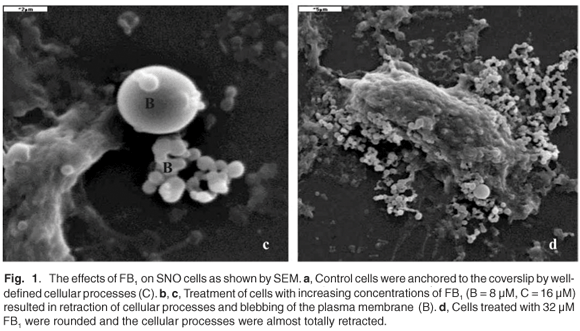
SNO epithelial cells in culture have to be firmly attached to the substrate on which they are grown. Such anchorage-dependence was evident in untreated control cells where the cellular processes were well defined (Fig. 1a). In FB1-treated cells (Figs 1b–d), there was a retraction of cellular processes that was more pronounced with increasing concentrations of the toxin; this may have been associated with the progression towards cell death. Treatment of SNO cells with 4 µM and 8 µM FB1 resulted in blebbing or vesiculation of the plasma membrane (Figs 1b and 1c). The treated cells broke up into smaller bodies with no swelling (Figs 1b and 1c). Blebbing of the plasma membrane into smaller membrane-bound like apoptotic bodies is a common structural feature of apoptotic cells.28,29 Thus, at the lower concentrations of FB1, apoptosis was the likely mechanism of cell death, since the integrity of the plasma membrane was maintained. At higher concentrations of FB1 (16 µM), the SNO cells exhibited gross pathology such as swelling (Fig. 1d); the plasma membrane is a target of FB1 and may therefore have been the major site of damage.30 Loss of membrane integrity prevents cells from regulating osmotic pressure, causing them to swell and rupture (Fig. 1d). Since necrosis is the death of cells through external damage, usually mediated via the destruction of the plasma membrane or the biochemical supports of its integrity,31 it is likely that necrosis was the mechanism of death at higher concentrations of FB1. Loss of cells due to cell death mechanisms (apoptosis or necrosis) may initiate a compensatory mitosis that could contribute to the onset of cancer.32,33
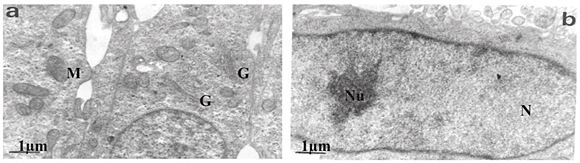
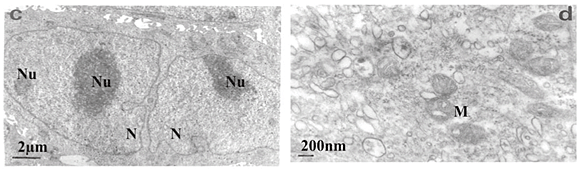
Transmission electron microscopy revealed that untreated SNO cells contained several Golgi bodies and mitochondria (Fig. 2a); a single large prominent nucleolus was present in the nucleus (Fig. 2b). Nuclear and nucleolar alterations were some of the toxic effects in FB1-treated SNO cells. Nuclear alterations of FB1-treated cells included enlargement, irregular profiles, and invagination of the nuclear membrane that often led to microsegregated nuclei (Fig. 2c). Nucleolar effects included an increase in the size and number of nucleoli (Fig. 2c). Mitochondria were elongated (Fig. 2d) and/or had swollen cristae (Fig. 2e). Endoplasmic reticuli (ER) were more abundant and some cisternae of ER were vesiculated (Fig. 2e) or swollen (Fig. 2f). Other cellular alterations of FB1-treated cells included the presence of numerous and occasionally swollen cellular processes (Fig. 3d).
Using ICC, FB1 was identified by the presence of 10 nm gold probes. Both the untreated SNO cells and method controls showed no positivity for FB1, whilst gold-labelled FB1 was immunolocalised in the treated cells (Figs 3a–d). Fumonisin B1 was present in the cytoplasm (Fig. 3a). Within nuclei of FB1-treated cells (Fig. 3b), label was associated with the nuclear membrane, nucleoplasm and nucleolus. There was an abundance of FB1 in mitochondria that showed pathological changes (Fig. 3c), but FB1 label was also localised to a limited extent in mitochondria that appeared normal. Label also was present within swollen processes or membrane blebs of FB1-treated cells (Fig. 3d).
The presence of the gold probes within the cytoplasm, mitochondria and nucleus of treated cells (Figs 3a–c) shows that FB1 gained entrance into the cell. Studies with 14C-labelled FB1 conducted by Cawood et al.13 showed that FB1 bound tightly to microsomes and plasma membranes of rat liver after 1 h incubation, and FB1 remained in the membranes even after extensive washing of these fractions. Fumonisin B1 is a highly polar molecule consisting of four carboxyl groups, one amine group and several hydroxyl groups. Even allowing for complete chelation of the carboxyls with elements such as calcium, it does not seem possible that FB1 could freely permeate membranes. Two possibilities exist for the passage of FB1 through the cell membrane, namely, that a trans-membrane transport system existed or that it was metabolically modified to allow permeation. Because of the unusual structure of FB1, it is tempting to suggest that it entered the cell via some sort of endocytic process, possibly mimicking sphingolipid-type membrane-binding agents. Continuity between the outer membrane of the nuclear envelope and the sacs of the ER were frequent, presumably providing FB1 access to the nucleus and nucleolus. Alternatively, FB1 in the soluble portion of the cell may have diffused into the nucleus via the nucleopore, bearing in mind the strong resemblance of the toxin to membrane lipids.
The higher levels of FB1 found in nuclei and nucleoli in this study correlated well with the ultrastructural observation that FB1 targeted the nucleolus, resulting in enlargement and microsegregation of the nucleolus. In addition, some of the ultrastructural alterations observed, particularly swelling of organelles, were manifestations of membrane damage, which may have been caused by the action of FB1 on these cells. Cellular membranes are postulated to be one of the principal targets for the fumonisins in vivo.30 Fumonisin B1 has been shown to exert its effects through a disruption in sphingolipid metabolism.34,35 Fumonisin B1 is a competitive inhibitor of the enzyme, ceramide synthase, which catalyses the acylation of sphinganine (Sa) in the de novo biosynthesis of sphingolipids and the re-utilisation of sphingosine (So) derived from sphingolipid metabolism.36 As a result, FB1 causes an increase in the amount of free Sa and a decrease in the formation of complex sphingolipids such as So and ceramide.37 Complex sphingolipids have been implicated in cell–cell interactions.34 Therefore, a blockage of de novo sphingolipid synthesis might weaken intercellular interactions and make membranes leaky. This would allow increased penetration of plasma components into underlying tissues.38
Fumonisin B1 has also been implicated in the disruption of a variety of cellular responses including mitogenesis39 and cytotoxicity.40 Other in vitro investigations have demonstrated that FB1 inhibited cell proliferation and induced either cell necrosis or apoptosis in SNO cells,25 LLC-PK1 cells,40 cultured turkey lymphocytes,41 chicken macrophages42 and CV-1 African green monkey kidney cells.43 A 4-h exposure of FB1 caused significant cytoplasmic blebbing and varying degrees of nuclear disintegration in vitro.42
Long-term studies on FB1 exposure in rodents32 and nonhuman primates33 showed no oesophageal lesions in either animal model. In our indigenous population, however, maize is the staple diet and often heavily contaminated maize (maize not suitable to be milled into flour) is brewed into a traditional beer. This coupled to chronic FB1 exposure, alcohol consumption, smoking, nitrosamines and scalding hot food or drinks may all be aetiological agents in OC in South Africa.
Conclusion
The cellular pathology observed suggests that FB1 specifically targeted mitochondria, the nucleus and nucleolus in SNO cells. The high levels of label (gold probes) found in these organelles further suggests that ultrastructural alterations occurred as a result of FB1 toxicity. The known mechanism of FB1-induced toxicity is through disruption of sphingolipid metabolism. It may, however, also be possible that FB1 exerts its biological effects through binding to macromolecules in these organelles.
The authors wish to thank the National Research Foundation of South Africa, Kennedy Potts Foundation and the Cancer Association of South Africa (CANSA) for funding.
1. McCabe M.L. and Dlamini Z. (2005). The molecular mechanisms of oesophageal cancer. Int. Immunopharm. 5, 1113–1130. [ Links ]
2. Pickens A. and Orringer M. (2003). Geographical distribution and racial disparity in oesophageal cancer. Ann. Thorac. Surg. 76, S1367–S1369. [ Links ]
3. Van Rensburg S.J. (1985). Recent studies on the aetiology of oesophageal cancer. S. Afr. Cancer Bull. 29, 22–31. [ Links ]
4. Dutton M.F. (1996). Fumonisins, mycotoxins of increasing importance: their nature and their effects. Pharmacol. Ther. 70, 137–161. [ Links ]
5. Diaz G.J. and Boermans H.G. (1994). Fumonisin-induced pulmonary oedema and hydrothorax in swine. Mycopathologia 117, 79–82. [ Links ]
6. Sydenham E.W., Gelderblom W.C.A., Thiel P.G. and Marasas W.F.O. (1990). Evidence for the natural occurrence of fumonisin B1, a mycotoxin produced by Fusarium moniliforme, in corn. J. Agric. Food Chem. 38, 285–290. [ Links ]
7. Humpf H-U. and Voss K.A. (2004). Effects of food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol. Nutr. Food Res. 48, 255–269. [ Links ]
8. Lemke S.L., Ottinger S.E., Ake C.L., Mayura K. and Phillips T.D. (2001). Deamination of fumonisin B1 and biological assessment of reaction product toxicity. Chem. Res. Toxicol. 14, 11–15. [ Links ]
9. Norred W.P. (1993). Fumonisins – mycotoxins produced by Fusarium verticillioides. J. Toxicol. Environ. Health 38, 309–328. [ Links ]
10. Thiel P.G., Shephard G.S., Sydenham E.W., Marasas W.F.O., Nelson P.E. and Wilson T.M. (1991). Levels of fumonisins B1 and B2 in feeds associated with confirmed cases of equine leucoencephalomalacia. J. Agric. Food Chem. 39, 109–111. [ Links ]
11. Gelderblom W.C.A., Cawood M.E., Snyman S.D., Vleggaar R. and Marasas W.F.O. (1993). Structure–activity relationships of fumonisins in short-term carcinogenesis and cytotoxicity assays. Food Chem. Toxicol. 31(6), 407–414. [ Links ]
12. Voss K.A., Smith G.W. and Haschek W.M. (2007). Fumonisins: toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci.Technol. 137, 299–325. [ Links ]
13. Cawood M.E., Gelderblom W.C.A., Alberts I.F. and Snyman S.D. (1994). Interaction of 14C-labeled fumonisin B mycotoxins with primary rat hepatocyte cultures. Food Chem. Toxicol. 32(7), 627–632. [ Links ]
14. Marasas W.F.O., Kellerman T.S., Gelderblom W.C.A., Coetzer J.A.W., Thiel F.G. and van der Lugt J.J. (1988). Leucoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium verticillioides. Onderstepoort J. Vet. Res. 55, 197–203. [ Links ]
15. Harrison L.R., Colvin B.M., Greene J.T., Newman L.E. and Cole J.R. (1990). Pulmonary oedema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium verticillioides. J. Vet. Diagn. Invest. 2, 217–221. [ Links ]
16. Gelderblom W.C.A., Kriek N.P.J., Marasas W.F.O. and Thiel P.G. (1991). Toxicity and carcinogenicity of the Fusanum monilzforine metabolite, fumonisin B1, in rats. Carcinogenesis 12(7), 1247–1251. [ Links ]
17. Marasas W.F.O., Kriek N.P.J., Fincham J.E. and van Rensburg S.J. (1984). Primary liver cancer and oesophageal basal cell hyperplasia in rats caused by Fusarium moniliforme. Int. J. Cancer 34, 383–387. [ Links ]
18. Jaskiewicz K., van Rensburg S.J., Marasas W.F.O. and Gelderblom W.C.A. (1987). Carcinogenicity of F. verticillioides culture material in rats. J. Natl. Cancer Inst. 78, 321–325. [ Links ]
19. Voss K.A., Chamberlain W.J., Bacon C.W. and Norred W.P. (1993). A preliminary investigation on renal and hepatic toxicity in rats fed purified fumonisin B1. Nat. Toxins 1, 222. [ Links ]
20. Sydenham E.W., Thiel P.G., Marasas W.F.O., Shepard G.S., van Schalkwyk D.J. and Koch K.R. (1990). Natural occurrence of some Fusarium mycotoxins in corn from low and high oesophageal cancer prevalence areas of the Transkei, South Africa. J. Agric. Food Chem. 38, 1900–1903. [ Links ]
21. Chelule P.K., Gqaleni N., Dutton M.F. and Chuturgoon A.A. (2001). Exposure of rural and urban populations in KwaZulu-Natal, South Africa, to fumonisin B1 in maize. Environ. Health Perspect. 109(3), 253–256. [ Links ]
22. Marasas W.F.O., Wehner F.C., van Rensburg S.J. and van Schalkwyk D.J. (1981). Mycoflora of corn produced in human oesophageal cancer areas in Transkei, South Africa. Phytopathology 71, 792–796. [ Links ]
23. Marasas W.F.O. (1982). Mycotoxicological investigations on corn produced in oesophageal cancer areas in Transkei. In Cancer of the Oesophagus, vol. I, ed. C.J. Pfeiffer, pp. 29–40. CRC Press, Boca Raton. [ Links ]
24. Norred W.P. and Voss K.A. (1994). Toxicity and role of fumonisins in animal diseases and human oesophageal cancer. J. Food Prot. 57(6), 522–527. [ Links ]
25. Myburg R.B., Dutton M.F. and Chuturgoon A.A. (2002). Cytotoxicity of fumonisin B1, diethylnitrosamine, and catechol on the SNO esophageal cancer cell line. Environ. Health Perspect. 110, 813–815. [ Links ]
26. Bey A.J., Whitcutt J.M., Hunt J.A. and Gear J.H.S. (1976). Carcinoma of the oesophagus in Africans: establishment of a continuously growing cell line from a tumour specimen. In Vitro 12(2), 107–114. [ Links ]
27. Shephard G.S., Thiel P.G., Sydenham E.W., Vleggaar R. and Alberts J.F. (1994). Determination of the mycotoxin fumonisin B1 and identification of its partially hydrolysed metabolites in the faeces of non-human primates. Food Chem. Toxicol. 32, 23–29. [ Links ]
28. Cohen J.J. (1993). Apoptosis. Immunol. Today 14(3), 126–130. [ Links ]
29. Wyllie A.H. (1997). Apoptosis: an overview. Brit. Med. Bull. 53(3), 451–465. [ Links ]
30. Yin J.J., Smith M.J., Eppley R.M., Troy A.L., Page S.W. and Sphon J.A. (1996). Effects of fumonisin B1 and (hydrolysed) fumonisin backbone AP1 on membranes: a spin-label study. Arch. Biochem. Biophys. 335(1), 13–22. [ Links ]
31. Willingham M.C. (1999). Cytochemical methods for the detection of apoptosis. J. Histochem. Cytochem. 47(9), 1101–1109. [ Links ]
32. Howard P.C., Eppey R.M., Stack M.E., Warbitton A., Voss K.A., Lorentzen R.J., Kovach R.M. and Bucci T.Z. (2001). Fumonisin B1 carcinogenicity in a two-year feeding study using F344 rats and B6C3F mice. Environ. Health Perspect. 109(S2), 277–282. [ Links ]
33. Gelderblom W.C.A., Abel S., Smuts C.M., Marnewick J., Marasas W.F.O., Lemmer E.R. and Ramljak D. (2001). Toxicity of cultured material of Fusarium verticilloides strain MRC 826 to nonhuman primates. Environ. Health Perspect. 109(S2), 291–300. [ Links ]
34. Merrill A.H., Schmelz E., Wang E., Dillehay D.L., Rice L.G., Meredith F. and Riley R.T. (1997). Importance of sphingolipids and inhibitors of sphingolipid metabolism as components of animal diets. J. Nutr. 127(5), 830S–833S. [ Links ]
35. Merrill A.H., Schelmz E., Wang E., Schroeder J.J., Dillehay D.L and Riley R.T. (1995). Role of dietary sphingolipids and inhibitors of sphingolipid metabolism in cancer and other diseases. J. Nutr. 125, 1677S–1682S. [ Links ]
36. Wang E., Norred W.P., Bacon C.W., Riley R.T. and Merrill A. (1991). Inhibition of sphingolipid biosynthesis by fumonisins. J. Biol. Chem. 266, 1486–1490. [ Links ]
37. Merrill A.H., Wang E., Vales R.T., Smith E.R., Schroeder J.J., Menaldino D.S., Alexander C., Crane H.M., Xia J., Liotta D.C., Meredith F.I. and Riley R.T. (1996). Fumonisin toxicity and sphingolipid biosynthesis. In Fumonisins in Food, ed. L. Jackson, pp. 297–306. Plenum Press, New York. [ Links ]
38. Ramasamy S., Wang E., Hennig B. and Merrill A.H. Jr (1995). Fumonisin B1 alters sphingolipid metabolism and disrupts the barrier function of endothelial cells in culture. Toxicol. Appl. Pharmacol. 133, 343–348. [ Links ]
39. Schroeder J.J., Crane H.M., Xia J., Liotta D.C. and Merrill A.H. (1994). Disruption of sphingolipid metabolism and stimulation of DNA synthesis by fumonisin B1: a molecular mechanism for carcinogenesis associated with F. verticillioides. J. Biol. Chem. 269, 3475–3481. [ Links ]
40. Yoo H.S., Shawker J.L. and Riley R.T. (1994). Relationship between fumonisin B1 induced cytotoxicity and the elevation of free sphinganine (Sa) in LLC-PK1 cells. Toxicologist 14, 772. [ Links ]
41. Dombrink-Kurtzman M.A., Bennett G.A. and Richard J.L. (1994). An optimized MTT bioassay for determination of cytotoxicity of fumonisins in turkey lymphocytes. J. AOAC Int. 77, 512–516. [ Links ]
42. Qureshi M.A. and Hagler W.M. (1992). Effect of fumonisin B1 exposure on chicken macrophage functions in vitro. Poult. Sci. 71, 104–112. [ Links ]
43. Jones C., Huang H., Dickman M.M., Henderson G., Wang H. and Gilchrist D. (1995). Analysis of a carcinogen fumonisin, which is a fungal toxin. Proc. Am. Assoc. Cancer Res. 36, 668. [ Links ]
Received 13 February. Accepted 27 May 2009.
* Author for correspondence E-mail: chutur@ukzn.ac.za