Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.105 n.5-6 Pretoria May./Jun. 2009
REVIEW ARTICLES
Imaging spectroscopy (hyperspectral remote sensing) in southern Africa: an overview
O. MutangaI, *; J. van AardtII; L. KumarIII
IDepartment of Geography, School of Applied Environmental Sciences, University of KwaZulu-Natal, P.O. Box X01, Scottsville, Pietermaritzburg 3209, South Africa
IICenter for Imaging Science, Rochester Institute of Technology, Rochester, NY, 14623, U.S.A
IIISchool of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia
ABSTRACT
Recent developments in imaging spectroscopy have seen a dramatic improvement in the characterisation of terrestrial features due to the high spectral resolution of the sensors used. For example vegetation species discrimination, stress detection and foliar chemistry mapping can now be achieved using these high spectral resolution sensors, a task that was almost impossible with coarse resolution satellite sensors. In spite of its capabilities, imaging spectroscopy is still in its early stages of development and application in southern Africa. This overview will attempt to briefly describe the science and analysis techniques, as well as review trends and challenges in the South African imaging spectroscopy landscape. It therefore is not intended as a pure research paper, but merely to illustrate the potential of and developments in imaging spectroscopy. This is pertinent to the South African scientific community where the technology is still in its infancy, especially given that the first-ever spaceborne South African imaging spectrometer, the Multi-Sensor Micro-Satellite Imager Satellite (MSMISat) is being developed for launch in the near future.
Key words: hyperspectral sensors, vegetation analysis, sensor characteristics, trends, spectral resolution
Introduction
Assessment and monitoring of the environment have become increasingly reliant on remote-sensing technologies, especially given the availability of historical data as well as the ability to provide data covering large spatial extents. These remote-sensing sensors typically are defined in terms of their specifications related to resolution.1 Spectral resolution has recently received immense attention as research has proven the capability of sensors with narrow channels (bandwidths of less than 2 nm) to detect subtle variations in surface features that might otherwise be masked by broader bands of multi-spectral scanner systems. Research has shown that subtle variations in features such as vegetation species, foliar chemistry and stress can be detected and mapped using high spectral resolution sensors.2 In this regard, a review and illustration of the developments of high spectral resolution technology is critical for a better understanding of its application in terrestrial ecosystems.
Spectral resolution refers to the division of the spectral space in terms of wavelength range, number and contiguous nature of sampled wavelengths, and spectral breadth of each wavelength sample. High spectral resolution data therefore imply both a large number of wavelength bands and contiguous coverage.3 Figure 1 demonstrates the concept through visualisation of a hyperspectral curve versus Landsat TM bands.
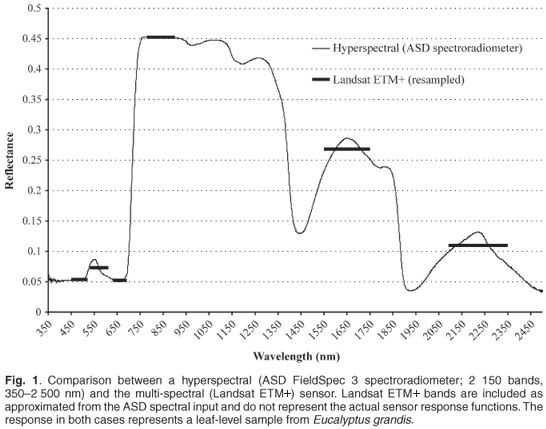
High spectral resolution data make discernment of a target, for example vegetation, more effective through spectral response discrimination than is possible with the broadband multispectral sensors.4 The limited number of channels and wider bandwidths of sensors such as Landsat TM and NOAA AVHRR (National Oceanic and Atmospheric Administration's Advanced Very High Resolution Radiometer), imply a loss of information on vegetation reflectance due to averaging5 and design specifications that do not address measurement of certain portions of the spectrum. High spectral resolution (spectroscopy) data are collected using either field spectrometers under laboratory and field conditions (field spectroscopy) or spectrometers mounted on aircrafts or satellites (imaging spectroscopy).
Imaging spectroscopy allows for the collection of high spectral resolution data on a per-pixel basis, typically using whiskbroom scanning devices. These pixels are then collated to represent an image for an area of interest, with the spectral dimension making the image truly unique.6 Figure 2 shows a 3D imaging spectroscopy cube of a HyMap MK1 image taken over the northern plains of the Kruger National Park, South Africa. The x–y plane forms the spatial domain where each slice represents a spectral band, made up of several pixels. The z dimension represents the reflectance characteristics of a feature, at a particular point (pixel) in different wavelengths.7 The strength of imaging spectroscopy lies in the availability of a large number of narrow and contiguous spectral bands in the z dimension that can reveal subtle differences in the reflectance properties of surface features in each pixel.

This makes it possible to discriminate and map features of relatively similar characteristics, such as vegetation species. Subtle features such as the red-edge position (point of maximum slope between the red and near infrared portions of the electromagnetic spectrum) amplitudes and shape of spectral reflectance curves are usually masked by broadband satellite data. There is therefore a need to obtain contiguous spectral information from imaging spectrometers that can resolve these subtle but important variations in surface features. Sensors such as the Airborne Visible and Infrared Imaging Spectrometer (AVIRIS) have prepared the way for a new set of applications to be explored by providing data in high enough quantity and high spectral resolution to resolve the natural variability in features such as minerals, vegetation and atmospheric gases.4
A comprehensive approach that involves the use of imaging spectroscopy data and its analysis has been taken of late8–12 rather than the traditional 'broader wave range and fewer classes' approach13–16 that was utilised in natural resources research for so long. Several examples serve to illustrate the use of imaging spectroscopy for two broad approaches, namely (i) for basic research to study processes and energy-target interactions and (ii) to move from an 'oversampled' spectral space to define the exact wavelengths and/or spectral characteristics needed to address specific problems or applications.
The first relates to research and applications that require the full width of spectral response to derive indicators, e.g. curve derivatives, slopes and integrals. These types of spectral curve characteristics have been used by many researchers to describe natural system properties.8 Application-specific research, on the other hand, requires that only those spectral indicators that apply to a specific question be identified from the hyperspectral curve. The goal in this case is to identify, from this oversampled imaging spectroscopy data source, exactly which spectral features are required to address an application of interest. The research question thus becomes an issue of moving from a situation of 'more data than we need', to 'exactly the data we need'. This effectively allows the development of operational sensors that are multi-spectral in design, cheaper and tailor- made for a specific set of applications. Research by Curran17 and Yoder and Pettigrew-Crosby,18 for instance, serve as examples where specific features were identified to describe chlorophyll and other leaf components. Such research allows design and development of operational multi-spectral sensors that incorporate application-specific wavelengths.
The main objective of this paper is to describe the science, analysis techniques and broad South African imaging spectroscopy landscape. The paper briefly reviews the imaging spectrometers available, the utility of imaging spectroscopy in vegetation analysis and its potential in southern Africa, with reference to the scheduled MSMISat imaging spectrometer in South Africa.
Selected imaging spectrometers
This section provides an overview of a selection of available high-profile imaging spectrometers and their characteristics. The utility of the various sensors in remote sensing vegetation characteristics within the South African context are discussed. Table 1 highlights the main sensors available and their potential applications.
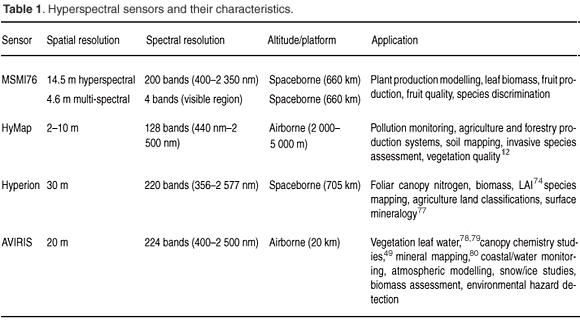
Airborne imaging spectrometers include HyMap and AVIRIS, both of which measure electromagnetic radiation from 400–2 500 nm. The AVIRIS, developed by the Jet Propulsion Laboratory in 1983, arguably is the benchmark for airborne imaging spectroscopy. AVIRIS first started operating in 1987 and has the distinction of being the first imaging spectrometer to measure the electromagnetic spectrum from 400–2 500 nm. This range of the electromagnetic spectrum has seen a wide application of AVIRIS in surface features characterisation (Table 1). The HyMap sensor includes two thermal bands (3–5 µm and 8–10 µm) in addition to the 400–2 500 nm spectral range. These types of spectral characteristics have expanded the utility of HyMap to applications such as pollution monitoring and soil mapping.19
Spaceborne sensors with a synoptic view, such as Hyperion, allow coverage, and therefore monitoring, of large areas. However, the use of Hyperion is limited to large spatial objects due to its relatively coarse spatial resolution.
The proposed launch of the MSMIsat, developed by Sunspace in Stellenbosch, South Africa, has substantial advantages over the existing sensors since it has both multi-spectral and hyperspectral sensors that could be used to capture variations in surface features at different scales. The pointing ability of the platform allows sensors to acquire imagery of the same target area at different viewing angles which, in turn, allow for the assessment of bidirectional reflectance distribution (BRDF) effects.20 The BRDF describes the hemispherical angular distribution of outgoing radiation relative to incoming radiation (irradiance).21 This measurement is important especially in mountainous areas such as the Drakensberg of South Africa where variations in the anisotropic reflectance signature of vegetation are controlled by topographic elements such as slope and aspect, thus rendering nadir sensing less efficient in capturing vegetation structure. The multi-angular viewing capabilities of the MSMIsat enable capturing of such off-nadir variations. In addition, the high temporal resolution of the microsatellites as compared to the current spaceborne sensors provides high multi-temporal data that can facilitate constant monitoring of dynamic variables, such as crop growth and climate change effects on vegetation.
However, the traditional trade-offs of the proposed MSMIsat in terms of spectral, spatial and temporal resolutions1 will apply, given the 14.9-km swath width at an altitude of 660 km. While a revisit time of less than four days is foreseen when using off-nadir data acquisition, this mode of data collection has implications for data use in terms of BRDF effects and atmospheric correction. The nominal revisit time of more than 100 days at nadir, in turn, has implications for applications that require monitoring capabilities, e.g. crop yield estimation and stress detection. These types of applications demand higher temporal resolution, often greater than two days. However, MSMISat should reasonably be regarded as an experimental platform that can address most of these issues when used in conjunction with three similar satellites, as is envisaged with the African Resource Management constellation. Other opportunities for MSMISat research and use include food security applications, invasive species mapping, and mineral mapping, while geometric and atmospheric corrections, tasking, and sensor calibration are challenges that face operators and users of MSMISat data.
In summary the sensors described in this section are capable of imaging at least 100 bands ranging from 350 nm to 2 500 nm. This region of the electromagnetic spectrum is critical for vegetation assessment and monitoring, with certain wavelengths sensitive to biochemical absorption while others, such as the near infrared region, reflect radiation. Data users should be guided by image resolution (spectral, spatial, temporal) and availability, as well as the cost involved. Although the spectral resolution is generally similar in the sensors highlighted above, cost and revisit time play a crucial role. Airborne sensors, such as HyMap, can be made available at any time; however, they are quite expensive compared to satellite-borne sensors such as Hyperion. Nevertheless, the spatial resolution of Hyperion is coarser (30 m) than that of HyMap (between 1–4 m). If one is therefore assessing objects with a relatively coarse spatial resolution of about 30 m (e.g. agricultural land classifications), then Hyperion should suffice. Landcover studies in mountainous regions would require multi-angular image sensors, such as the Compact High Resolution Imaging Spectrometer (CHRIS) Proba or the proposed MSMI sensor for South Africa that can cater for the effects of aspect and slope.
It is imperative to note that the success of hyperspectral data for characterising vegetation biophysical parameters is also dependent on the analytical techniques followed. These analytical techniques can be broadly divided into physically-based models and empirical methods. While most physically-based radiative transfer models, such as the PROSPECT and SAIL, have been developed and widely applied in homogenous vegetation, their application in southern Africa is not common. This is partly because of difficulties related to model parameterisation and also because of the heterogeneous nature of vegetation. Owing to the heterogeneous nature of vegetation in South Africa, which is rather site specific, most studies have followed the empirical approach using statistical tools, such as multiple regression,22 discriminant analysis,10 transforms (e.g. principal component analysis)11 and support vector machines. Research in the physical modelling approach is moving towards the 3D radiative transfer models23 that can cater for the heterogeneous nature of the vegetation in southern Africa.
Analysis of imaging spectroscopy data furthermore presents various challenges, especially in terms of ensuring statistical validity of an approach, given on the large number of potential independent variables in a modelling scenario. This is referred to as 'reduction of data dimensionality'24 and hints at the types of applications that imaging spectroscopy data and analysis are suited for. Many applications, e.g. mineral mapping, require a contiguous set of wavelengths to define a spectral feature that differentiates between minerals, while other applications, e.g. foliar chemistry assessment, require a defined selection or combination of wavelengths. In the first instance, the hyperspectral data curve can be subset to include only that spectral range of interest. However, in the second case, robust methods for sub-selecting only those wavelengths that are pertinent to the application need to be developed. A review of hyperspectral vegetation studies in southern Africa follows.
The South African vegetation and imaging spectroscopy
The nature of heterogeneity that exists in southern Africa poses many challenges to the remote-sensing techniques that are applied in characterising vegetation variables, e.g. species identification, biochemical concentration, stress and biomass, in this region. The South African vegetation distribution closely correlates with the level of annual precipitation. Where rainfall is high and frequent all year round (above 400 mm per year), moist and tropical rain forests are common. Forests are also widespread across the tropical dry regions where miombo, mopane and Acacia woodlands are dominant. Along the eastern coast down to the Cape of Good Hope, woody vegetation is characterised by coastal forests with different floristic, structural, and physiognomic properties from the woodland types.25 Dry montane forest occurs in small patches at higher elevations and mangroves are very common along the coast of the tropical regions.
Plants and/or animals occurring together with some degree of permanence have been classified into biomes in South Africa. These biomes broadly correspond to climatic regions, although other environmental controls are sometimes important. Each biome has a characteristic set of plant and animal species, as well as an overall physiognomy. Rutherford and Westfall26 mapped seven biomes in South Africa: Grassland, Savanna, Succulent Karoo, Nama Karoo, Forest, Fynbos and Desert, while Low and Rebelo27 included a Thicket biome. Acocks28 described vegetation patterns at a scale that is still smaller than the biome and provided descriptions of 70 veld types in South Africa, Lesotho, and Swaziland. Cowling et al.29 give an updated description of vegetation in southern Africa.
Although these vegetation types have been described and mapped, detailed features such as species distribution and finer physiognomic and biochemical characteristics are still outstanding. Variations in leaf structure and orientation due to the different vegetation types,30 plant composition and phenology,31 different soil background effects,32 as well as the highly variable effects of standing litter, which often dominates the total fraction of aboveground biomass,33 complicate the remote sensing of vegetation variables such as biochemicals and species distribution in such heterogeneous environments. Imaging spectroscopy of vegetation is a relatively new field of study in southern Africa, yet research has revealed that the approach is critical in characterising various properties of southern African vegetation.12 A brief overview of the application of imaging spectroscopy for vegetation analysis is presented with particular reference to southern Africa.
Foliar chemistry
Laboratory near-infrared spectroscopy methods34,35 triggered the remote sensing of foliar chemistry, mainly predicting protein, amino acids, lignin and cellulose concentrations contained in dried, ground forage.34 This technique has replaced wet chemistry as the standard analytical procedure for assessing plant biochemicals in many laboratories.18 The premise behind the detection and mapping of foliar biochemicals is that plants absorb electromagnetic radiation through the molecular vibration (rotation, bending and stretching) of bonds (C-H, N-H, O-H, C-N and C-C) which form the primary constituents of organic compounds.36 Therefore, the amount and composition of biochemicals in plants determine the amount of energy reflected per wavelength.17,37 Curran17 produced a list of absorption features that are related to particular plant compounds. The list was modified by Kumar et al.5 to comprise 45 absorption features that are related to particular biochemical compounds between 400 nm and 2 500 nm.
Techniques to estimate foliar biochemicals using imaging spectroscopy have gradually developed over the years.17,38–46 Attempts were made during the late 1980s to estimate forest biochemical composition using first difference-at-sensor radiance measured by the Airborne Imaging Spectrometer (AIS).47 Strong correlations were found between AIS data and total canopy lignin and nitrogen content in deciduous and coniferous forests. Biochemical concentrations have also been estimated using AVIRIS spectra in mixed species forest canopies using first derivative reflectance and stepwise linear regression.46,49 Attempts to estimate foliar chemistry in sparsely vegetated canopies have been made using wavelengths related to known biochemical absorption features,50 a data reduction technique that minimises over-fitting and the effect of spectral variability that is independent of the biochemical concentration.30,41,50,51
With reference to southern Africa, research carried out in the Kruger National Park was aimed at predicting the quality of grass for herbivores as determined by the concentration of biochemicals (N, P, Na, K, Ca, Mg) using field spectroscopy30 and airborne imaging spectroscopy data.12 Owing to the heterogeneous nature of the environment in southern Africa, techniques that minimise the effect of spectral variability that is independent of the biochemical concentration were developed and applied as an alternative to the traditional laboratory near infrared spectroscopy methods.2
Continuum-removed band depths of selected absorption features41,52 were correlated via stepwise regression to the biochemical concentration of sampled grass, as measured in a laboratory. Results indicated that the continuum-removed absorption features could explain up to 80% of the variation of biochemicals in grass.30 In a related study, researchers established a relationship between reflectance and nitrogen content as well as condensed tannin concentration in mopane (Colophospermum mopane) trees.53 The study confirmed that key wavelengths located in the shortwave infrared region as well as the red edge position, are linked to the concentration of foliar nitrogen and tannins.54 The technique was successfully scaled to canopy level for the estimation of foliar biochemicals using airborne HyMap imagery with an artificial neural network.12,54
Studies such as these have proven the potential of imaging spectroscopy in mapping detailed vegetation characteristics in the Savanna biomes of southern Africa at both field and airborne levels. The challenge is to upscale the techniques to spaceborne imaging spectrometers, such as Hyperion. To date, only a few studies have tested the potential of Hyperion data in estimating vegetation parameters.55,56
Plant stress and damage
Pests and diseases cause mortality in plantation forests and natural vegetation. Advances in imaging spectroscopy have offered opportunities to timely assess and delineate a range of forest health conditions.57 Leaf reflectance and shifts in the red edge position have been associated with insect infestation through damage of the waxy cuticle, destruction of cell walls and reduction in plant moisture.57–61 Zhang et al.62 investigated the utility of imaging spectroscopy for crop disease detection using tomatoes infected by P. infestans (late blight) as an example. A minimum noise fraction (MNF) transformation was applied to AVIRIS imaging spectroscopy data (224 bands; 400–2 500 nm), which reduced the dimensions to 28 MNF components. The MNF components were subjected to end-member spectra selection and spectral angle mapper classification. Results indicated that the blight-diseased tomatoes could be effectively separated from the healthy plants.62
A series of spectral indices were computed from airborne CASI imaging spectroscopy data to detect the severity of plantation damage caused by Dothistroma needle blight in the New South Wales region of Australia.57 Results from independent validation data showed that hyperspectral data could discriminate between three levels of blight infection with accuracies above 70%. In South Africa, on the other hand, plantation forests are under threat from the wood-boring pest Sirex noctilio Fabricius (Hymenoptera: Siricidae; Sirex wood wasp). Sirex noctilio affects all commercial pine species in South Africa with none of the species showing a high resistance to attack.63 Recent reports have indicated that mortality might be as high as 30% in some forestry compartments in KwaZulu-Natal.64 A recent study was aimed at identifying diagnostic spectral features of Pinus patula needles under varying degrees of attack by S. noctilio. The authors used data collected from a field spectrometer in the plantation forests of KwaZulu-Natal. Results of the Jeffries Matusita distance analysis indicated that an acceptable separability of 99.2% for all the classes of different levels of Sirex infestation was reached when using a four-band combination comprising bands located in the visible and red edge portions of the electromagnetic spectrum.65
Other applications
Species discrimination, biomass assessment, leaf area index (LAI) estimation, foliar water content measurement, crop growth modelling and net primary productivity estimation are other areas where imaging spectroscopy has been applied effectively.66–72 Serrano et al.68 used AVIRIS data to estimate water content in Chaparral vegetation. A substantial review of imaging spectroscopy as applied to water content estimation is provided by Govender.73 In South Africa, Hyperion imagery was used to estimate LAI of Eucalyptus in the coastal Zululand of KwaZulu-Natal Province.74 A LI-COR 2000 was used to measure LAI on seven plots in the study area. Reflectance measurements and indices from Hyperion Level 1R data were regressed against LAI measurements. Results indicated that all relationships between LAI and the computed vegetation indices were significant (P < 0.05) with relatively high R2 values (R2 > 0.80). Another study assessed the utility of hyperspectral remote sensing to discriminate between site qualities in E. grandis plantation in KwaZulu-Natal, South Africa. The relationships between physiology-based hyperspectral indicators and site quality, as defined by total available water (TAW), were assessed for E. grandis using one-way analysis of variance (ANOVA). These results show that differences in site quality, based on total available water, could be detected using imaging spectroscopy of canopy water or chlorophyll content.75
Conclusions
This review paper has highlighted the development of imaging spectroscopy (hyperspectral) applications in southern Africa, with particular reference to vegetation analysis and monitoring. A number of airborne imaging spectrometers, with largely similar characteristics are now operational. The paper has shown that there is a wide range of techniques, ranging from empirical to physically-based modelling approaches that have proven useful for analysing imaging spectroscopy data for vegetation analysis and monitoring. The development of a South African spaceborne imaging spectrometer presents new opportunities for detailed environmental assessment and monitoring. However, since imaging spectroscopy research is still in its infancy in South Africa, these new developments come with their own challenges in terms of human, financial and physical resources. Preprocessing and analysis of imaging spectroscopy data from the proposed MSMISat satellite, in particular, can only be achieved through collaboration between research institutions and application specialists before developed applications can be viewed as truly operational in the context of a constellation of satellites.
1. Lillesand T.M. and Kiefer R.W. (2003). Remote Sensing and Image Interpretation. John Wiley and Sons, New York. [ Links ]
2. Curran P.J., Dungan J.L. and Peterson D.L. (2001). Estimating the foliar biochemical concentration of leaves with reflectance spectrometry: testing the Kokaly and Clark methodologies. Remote Sens. Environ. 76, 349–359. [ Links ]
3. Niemann K.O. (1995). Remote sensing of forest stand age using airborne spectrometer data. Photogramm. Eng. Remote Sens. 61(9), 1119–1127. [ Links ]
4. Birk R.J. and McCord T.B. (1994). Airborne hyperspectral sensor systems. In Proceedings, Institute of Electrical and Electronics Engineers, Inc. (IEEE) National Aerospace and Electronics Conference, Dayton, Ohio, pp. 309–318. [ Links ]
5. Kumar L., Schmidt K.S., Dury S. and Skidmore A.K. (2001). Imaging spectrometry and vegetation science. In Imaging Spectrometry, eds F. van der Meer and S.M. de Jong, pp. 111–155. Kluwer Academic Publishers, Dordrecht. [ Links ]
6. Clark A.F. (1999). Spectroscopy of rocks and minerals and principles of spectroscopy. In Manual of Remote Sensing: Remote Sensing for the Earth Sciences, ed. A.N. Rencz, pp. 3–58. John Wiley and Sons, New York. [ Links ]
7. Mather P.M. (2005). Computer Processing of Remotely-Sensed Images: an Introduction. John Wiley & Sons, Chichester. [ Links ]
8. Gong P., Pu R. and Yu B. (1997). Conifer species recognition: an exploratory analysis of in situ hyperspectral data. Remote Sens. Environ. 62, 189–200. [ Links ]
9. Martin M.E., Newman S.D., Aber J.D. and Congalton R.G. (1998). Determining forest species composition using high spectral resolution remote sensing data. Remote Sens. Environ. 65, 249–254. [ Links ]
10. Van Aardt J.A.N. and Wynne R.H. (2001). Spectral separability among six southern tree species. Photogramm. Eng. Remote Sens. 67, 1367–1375. [ Links ]
11. Van Aardt J.A.N. and Wynne R.H. (2007). Examining pine spectral separability using hyperspectral data from an airborne sensor: an extension of field-based results. Int. J. Remote Sens. 28(2), 431–436. [ Links ]
12. Mutanga O. and Skidmore A.K. (2004). Integrating imaging spectroscopy and neural networks to map tropical grass quality in the Kruger National Park, South Africa. Remote Sens. Environ. 90(1), 104–115. [ Links ]
13. Nelson R.F., Latty R.S. and Mott G. (1984). Classifying northern forests using thematic mapper simulator data. Photogramm. Eng. Remote Sens. 50(5), 607–617. [ Links ]
14. Shen S.S., Badwar G.D. and Carnes J.G. (1985). Separability of boreal forest species in the Lake Jennette area, Minnesota. Photogramm. Eng. Remote Sens. 51(11), 1775–1783. [ Links ]
15. White J.D., Glenn C.K. and Pinder J.E. (1995). Forest mapping at Lassen Volcanic National Park, California, using Landsat TM data and a geographical information system. Photogramm. Eng. Remote Sens. 61(3), 299–305. [ Links ]
16. Franklin S.E. (1994). Discrimination of subalpine forest species and canopy density using digital CASI, SPOT PLA, and Landsat TM data. Photogramm. Eng. Remote Sens. 60(10), 1233–1241. [ Links ]
17. Curran P.J. (1989). Remote sensing of foliar chemistry. Remote Sens. Environ. 30(3), 271–278. [ Links ]
18. Yoder B.J. and Pettigrew-Crosby R.E. (1995). Predicting nitrogen and chlorophyll content and concentrations from reflectance spectra (400–2500 nm) at leaf and canopy scales. Remote Sens. Environ. 53(3), 199–211. [ Links ]
19. Cocks T., Jensen J.R., Stewart A., Wilson I. and Shields T. (1998). The HyMap airborne hyperspectral sensor: the system, calibration, and performance. In Proceedings, 1st EarSeL Workshop on Imaging Spectroscopy, Zurich, p. 6. [ Links ]
20. ESA (2007). ESA Missions. Online from: http://envisat.esa.int [ Links ]
21. Sandmeier S., Müller C., Hosgood B. and Andreoli G. (1998). Physical mechanisms in hyperspectral BRDF data of grass and watercress. Remote Sens. Environ. 66, 222–233. [ Links ]
22. Skidmore A.K. (2002). Taxonomy of environmental models in the spatial sciences. In Environmental Models with GIS and Remote Sensing, ed. A.K. Skidmore, pp. 8–25. Taylor & Francis, London. [ Links ]
23. Darvishzadeha R., Skidmore A., Schlerf M. and Atzbergerb C. (2008). Inversion of a radiative transfer model for estimating vegetation LAI and chlorophyll in a heterogeneous grassland. Remote Sens. Environ. 112(5), 2592–2604. [ Links ]
24. Hsieh P. and Landgrebe D.A. (1998). Lowpass filter for increasing class separability. In Proceedings, 1998 Institute of Electrical and Electronics Engineers, Inc. (IEEE) International Geoscience and Remote Sensing Symposium, Part 5, Seattle, pp. 2691–2693. [ Links ]
25. White F. (1983). Vegetation of Africa – a Descriptive Memoir to Accompany the Unesco/AETFAT/UNSO Vegetation Map of Africa. UNESCO, Paris. [ Links ]
26. Rutherford M.C. and Westfall R.H. (1994). Biomes of southern Africa: an objective characterisation. Mem. Bot. Surv. S. Afr. 63, 1–94. [ Links ]
27. Low A.B. and Rebelo A.G. (1996). Vegetation of South Africa, Lesotho and Swaziland. DEAT, Pretoria. [ Links ]
28. Acocks J.P.H. (1988). Veld types of South Africa. Mem. Bot. Surv. S. Afr. 57, 1–146. [ Links ]
29. Cowling R.M., Richardson D.M. and Pierce S.M. (1997). Vegetation of Southern Africa. Cambridge University Press, Cambridge. [ Links ]
30. Mutanga O., Skidmore A.K. and Prins H.H.T. (2004). Predicting in situ pasture quality in the Kruger National Park, South Africa using continuum removed absorption features. Remote Sens. Environ. 89(3), 393–408. [ Links ]
31. Asner G.P., Wessman C.A., Bateson C.A. and Privette J.L. (2000). Impact of tissue, canopy and landscape factors on the hyperspectral reflectance variability of arid zone ecosystems. Remote Sens. Environ. 74(1), 69–84. [ Links ]
32. Huete A.R. and Jackson R.D. (1988). Soil and atmosphere influences on the spectra of partial canopies. Remote Sens. Environ. 25, 89–105. [ Links ]
33. Asner G.P., Wessman C.A. and Bateson C.A. (1998). Sources of Variability in Plant Canopy Hyperspectral Data in a Savanna Ecosystem. Seventh Annual JPL Airborne Earth Science Workshop, Pasadena, pp. 23–32. [ Links ]
34. Norris K.H., Barnes R.F., Moore J.E. and Shenk J.S. (1976). Predicting forage quality by infrared reflectance spectroscopy. J. Anim. Sci. 43(4), 889–897. [ Links ]
35. Marten G.C., Shenk J.S. and Barton F.E. (1989). Near Infrared Reflectance Spectroscopy (NIRS): Analysis of Forage Quality. USDA Handbook 643, pp. 1–96. US Department of Agriculture, Washington D.C. [ Links ]
36. Elvidge C.D. (1990). Visible and near infrared reflectance characteristics of dry plant materials. Int. J. Remote Sens. 11(10), 1775–1795. [ Links ]
37. Foley B., Mcllwee A., Lawler I., Agragones L., Woolnough A.P. and Berding N. (1998). Ecological applications of near infrared spectroscopy – a tool for rapid, cost effective prediction of the composition of plant and animal tissues and aspects of animal performance. Oecologia 116, 293–305. [ Links ]
38. Gong P. (2002). Analysis of in situ hyperspectral data for nutrient estimation of giant sequoia. Int. J. Remote Sens. 23(9), 1827–1850. [ Links ]
39. Milton N.M., Eiswerth B.A. and Ager C.M. (1991). Effect of phosphorous deficiency on spectral reflectance and morphology of soybean plants. Remote Sens. Environ. 36, 121–127. [ Links ]
40. Ponzoni F.J. and Goncalves J.L. (1999). Spectral features associated with nitrogen, phosphorous, and potassium deficiencies in Eucalyptus saligna seedling leaves. Int. J. Remote Sens. 20(11), 2249–2264. [ Links ]
41. Kokaly R.F. and Clark R.N. (1999). Spectroscopic determination of leaf biochemistry using band-depth analysis of absorption features and stepwise multiple linear regression. Remote Sens. Environ. 67(3), 267–287. [ Links ]
42. Mutanga O., Skidmore A.K. and Van Wieren S. (2003). Discriminating tropical grass canopies (Cenchrus ciliaris) grown under different nitrogen treatments using spectroradiometry. ISPRS J. Photogramm. Remote Sens. 57(4), 263–272. [ Links ]
43. Curran P.J., Windham W.R. and Gholz H.L. (1995). Exploring the relationship between reflectance red edge and chlorophyll concentration in slash pine leaves. Tree Physiol. 15, 203–206. [ Links ]
44. Cho M.A. and Skidmore A.K. (2006). A new technique for extracting the red edge position from hyperspectral data: the linear interpolation method. Remote Sens. Environ. 101(2), 181–193. [ Links ]
45. Mutanga O. and Skidmore A.K. (2007). Red edge shift and the quality of pasture canopies. ISPRS J. Photogramm. Remote Sens. 62, 34–42. [ Links ]
46. Mutanga O., Skidmore A.K., Kumar L. and Ferwerda J. (2005). Estimating pasture quality at canopy level using band depth analysis with continuum removal in the visible domain. Int. J. Remote Sens. 26(6), 1093–1108. [ Links ]
47. Wessman C.A., Aber J.D. and Peterson D.L. (1989). An evaluation of imaging spectroscopy for estimating forest canopy chemistry. Int. J. Remote Sens. 10(8), 1293–1316. [ Links ]
48. Johnson L.F., Hlavka C.A. and Peterson D.L. (1994). Multivariate analysis of AVIRIS data for canopy biochemical estimation along the Oregon transect. Remote Sens. Environ. 47, 216–230. [ Links ]
49. Martin M.E. and Aber J.D. (1997). High resolution remote sensing of forest canopy lignin, nitrogen and ecosystem process. Ecol. Appl. 7, 431–443. [ Links ]
50. Serrano L., Penuelas J. and Ustin S. (2002). Remote sensing of nitrogen and lignin in Mediterranean vegetation from AVIRIS data: decomposing biochemical from structural signals. Remote Sens. Environ. 81, 355–364. [ Links ]
51. Kokaly R.F., Despain D.G., Clark R.N. and Livo K.E. (2003). Mapping vegetation in Yellowstone National Park using spectral feature analysis of AVIRIS data. Remote Sens. Environ. 84(3), 437–456. [ Links ]
52. Clark R.N. and Roush T.L. (1984). Reflectance spectroscopy: quantitative analysis techniques for remote sensing applications. J. Geophys. Res. 89(B7), 6329– 6340. [ Links ]
53. Ferwerda J.G., Skidmore A.K. and Mutanga O. (2005). Nitrogen detection with hyperspectral normalised ratio indices across multiple plant species. Int. J. Remote Sens. 26(18), 4083–4095. [ Links ]
54. Ferwerda J.G. (2005). Charting to quality of forage. Mapping and measuring the chemical composition of foliage using hyperspectral remote sensing. Ph.D. thesis, ITC-Wageningen, Enschede, Netherlands. [ Links ]
55. Gong P., Ruiliang P., Biging G.S. and Larrieu M.R. (2003). Estimation of forest leaf area index using vegetation indices derived from Hyperion hyperspectral data. IEEE Trans. Geosci. Remote Sens. 44(6), 1355–1362. [ Links ]
56. Apan A., Held A., Phinn S. and Markley J. (2003). Detecting sugarcane 'orange rust' disease using EO-1 Hyperion hyperspectral imagery. IEEE Trans. Geosci. Remote Sens. 25(2), 489–498. [ Links ]
57. Coops N.C., Stanford M., Old K., Dudzinski M., Calvenor D. and Stone C. (2003). Assessment of Dothistroma needle blight of Pinus radiata using airborne hyperspectral imagery. Phytopathology 33(12), 1524–1532. [ Links ]
58. Wilson B.A., Luther J.E. and Stuart T.D.T. (1998). Spectral reflectance characteristics of Dutch Elm Disease. Can. J. Remote Sens. 24, 200–205. [ Links ]
59. Dawson T.P. and Curran P.J. (1998). A new technique for interpolating the reflectance red edge position. Int. J. Remote Sens. 19(11), 2133–2139. [ Links ]
60. Gitelson A. and Merzlyak M.N. (1994). Quantitative estimation of chlorophyll a using reflectance spectra: experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. A 22, 247–252. [ Links ]
61. Lichtenthaler H.K., Gitelson A. and Lang M. (1996). Non-destructive determination of chlorophyll content of leaves of a green and an aurea mutant tobacco by reflectance measurements. J. Plant Physiol. 148, 483–493. [ Links ]
62. Zhang M., Qin Z., Liu X. and Ustin S.L. (2003). Detection of stress in tomatoes induced by late blight disease in California, U.S.A., using hyperspectral remote sensing. Int. J. Appl. Earth Obs. Geoinformation 4, 295–310. [ Links ]
63. Anon. (2004). Sirex Woodwasp. FABI: Tree Protection Co-Operative Programme, Pretoria. [ Links ]
64. Slippers B. (2006). The sirex epidemic in KwaZulu-Natal and its control. Wood and Timber Times Southern Africa 31(7), 24–25. [ Links ]
65. Ismail R., Mutanga O. and Ahmed F. (2008). Discriminating Sirex noctilio attack in pine forest plantations in South Africa using high spectral resolution data. In Hyperspectral Remote Sensing of Tropical and Sub-Tropical Forests, pp. 161–174. CRC Press, Boca Raton. [ Links ]
66. Schmidt K.S. and Skidmore A.K. (2003). Spectral discrimination of vegetation types in a coastal wetland. Remote Sens. Environ. 85, 92–108. [ Links ]
67. Schmidt K.S. and Skidmore A.K. (2001). Exploring spectral discrimination of grass species in African rangelands. Int. J. Remote Sens. 22 (17), 3421–3434. [ Links ]
68. Serrano L., Ustin S.L., Roberts D.A., Gamon J.A. and Peñuelas J. (2000). Deriving water content of Chaparral vegetation from AVIRIS data. Remote Sens. Environ. 74, 570–581. [ Links ]
69. Knapp A.K. and Carter G.A. (1998). Variability in leaf optical properties among 26 species from a broad range of habitats. Am. J. Bot. 85(7), 940–946. [ Links ]
70. Kimes D.S., Nelson R.F., Manry M.T. and Fung A.K. (1998). Attributes of neural networks for extracting continuous vegetation variables from optical and radar measurements. Int. J. Remote Sens. 19(14), 2639–2663. [ Links ]
71. Mutanga O. and Skidmore A.K. (2004). Hyperspectral band depth analysis for a better estimation of pasture biomass. Int. J. Appl. Earth Obs. Geoinformation 5, 87–96. [ Links ]
72. Hurcom S.J. and Harrison A.R. (1998). The NDVI and spectral decomposition for semi-arid vegetation abundance estimation. Int. J. Remote Sens. 19(16), 3109–3125. [ Links ]
73. Govender M., Chetty K. and Bulcock H. (2007). A review of hyperspectral remote sensing and its application in vegetation and water resource studies. Water SA, 33(2), 1–8. [ Links ]
74. Ahmed F. and Mthembu I. (2006). Assessing the utility of Hyperion in extrafting vegetation indices (VI) and leaf area index (LAI). In Proceedings, 6th International Conference on Earth Observation & Geoinformation Sciences in Support of Africa's Development, Cairo, Egypt, pp. 1–10. [ Links ]
75. Cho M.A., Aardt J.V., Main R. and Majeke B. (in press). Evaluating variations of physiology-based hyperspectral features along a soil-water gradient in Eucalyptus grandis plantation. Int. J. Remote Sens. [ Links ]
76. Schoonwinkel A.H., Burger H. and Mostert S. (2005). Integrated hyperspectral, multispectral and video imager for microsatellites. Proceedings, 19th Annual AIAA/USU Conference on Small Satellites, August 8–11, Logan, Utah [ Links ]
77. Kruse F.A., Boardman J.W. and Huntington J.F. (2002 ). Comparison of EO-1 Hyperion and airborne hyperspectral remote sensing data for geologic applications. Aerospace Conference Proceedings 3, 1501–1513. [ Links ]
78. Chappelle E.W., Kim M.S. and McMurtrey III J.E. (1992). Ratio analysis of reflectance spectra (RARS): an algorithm for the remote estimation of the concentrations of chlorophyll A, chlorophyll B, and carotenoids in soybean leaves. Remote Sens. Environ. 39, 239–247. [ Links ]
79. Green R.O., Eastwood M., Sarture C.M., Chrien T.G., Aronsson M., Chippendale B.J., Faust J.A., Pavri B.E., Chovit C.J., Solis M., Olah M.R. and Williams O. (1998). Imaging Spectroscopy and the Airborne Visible/Infrared Imaging Spectrometer (AVIRIS). Remote Sens. Environ. 65, 227–248. [ Links ]
80. Baugh W.M., Kruise F.A. and Atkinson W.W. (1998). Quantitative geochemical mapping of ammonium minerals in the southern Cedar Mountains, Nevada, using the Airborne Visible/Infrared Imaging Spectrometer (AVIRIS). Remote Sens. Environ. 65, 292–308. [ Links ]
Received 30 April 2008. Accepted 8 June 2009.
* Author for correspondence E-mail: mutangao@ukzn.ac.za














