Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.105 n.5-6 Pretoria May./Jun. 2009
NEWS & VIEWS
Where are we going with HIV vaccines?
Lynn MorrisI; Carolyn WilliamsonII; Koleka MlisanaIII; Glenda GrayIV
INational Institute for Communicable Diseases, Private Bag X4, Sandringham, Johannesburg 2131. E-mail: lynnm@nicd.ac.za
IIDivision of Medical Virology, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory 7925. E-mail: carolyn.williamson@uct.ac.za
IIICentre for the AIDS Programme of Research in South Africa (CAPRISA), Doris Duke Medical Research Institute, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Private Bag X7, Congella 4013. E-mail: mlisanak@ukzn.ac.za
IVPerinatal HIV Research Unit, University of the Witwatersrand, PO Box 114, Diepkloof 1864. E-mail: gray@pixie.co.za
The disappointing results from the HIV Vaccine Trials Network (HVTN) 502 phase IIB vaccine trial (also know as the STEP trial) has caused the scientific community to pause and wonder 'What went wrong?' and even worse 'Is a vaccine against HIV possible?' This trial demonstrated no potential benefit of the Merck Adenovirus 5 (Ad5) HIV vaccine, and in some participants with pre-existing immunity to Ad5, increased susceptibility to HIV infection.1 Some of the answers to the first question will come from a full analysis of this trial as well as the South African equivalent trial (HVTN 503/Phambili) that was halted last year. Attempting to answer the second question has brought about a re-think of current paradigms. This has resulted in a call for a back-to-basics approach, requiring a better understanding of both the virus and human immune responses to HIV.
History of vaccination
The history of vaccination is a good-news story—one from which we need to draw encouragement and inspiration in the wake of the failed Merck Ad5 vaccine. Smallpox was eradicated by vaccination with vaccinia, a virus related to smallpox; and polio should be eradicated in the near future. We have successful vaccines against measles, hepatitis A and B and many other viral infections—we know that vaccines work and in many cases we have a good idea of how they work. Part of the difficulty in making a vaccine against HIV is that we do not know the type of immune responses to stimulate in order to provide protection against either HIV infection or to control viremia. This is because unlike other viral infections, there is no spontaneous clearance of HIV from the body: once infection is established a person is infected for life. Thus, an HIV vaccine needs to do better than nature, which is no small task. Nevertheless, the immune response against HIV is extremely potent at keeping the virus in check for many years, although as a result of its remarkable ability to mutate, HIV evades and erodes these defences, eventually causing complete immune collapse. This exceptional variability, too, presents an enormous obstacle to vaccine developers, who are finding it difficult to design a vaccine which will protect against such enormous diversity.
How do vaccines work?
Most vaccines work by laying down a network of antigen-specific memory B and T cells that on exposure to the pathogen rapidly expand and control the infection. Neutralising antibodies, produced by B cells, are able to bind virus particles and probably restrict the initial burst of viremia; while cytotoxic T cells (CTL) kill virus-infected infected cells. Unfortunately, it has not yet been possible to stimulate broadly neutralising antibodies to HIV through vaccination, and indeed such antibodies are rarely ever made in HIV-infected humans. Many early vaccine efforts have focused on eliciting T-cell responses, as these responses have been associated with the initial control of HIV replication following infection, and some CTL responses have been shown to control virus replication. The Merck Ad5 vaccine was designed to stimulate CTLs, and while this did occur in a large proportion of the vaccinees, an early analysis of data has shown that these CTL responses failed to control the levels of virus in participants who became infected during the trial.2 This has caused much debate as to whether the failure of the Merck Ad5 vaccine was due to the vaccine per se or whether CTL are indeed a useful correlate of immune protection. Experimental evidence in monkeys has shown that CTL can control HIV replication in vivo and so the concept of inducing these immune responses remains solid.
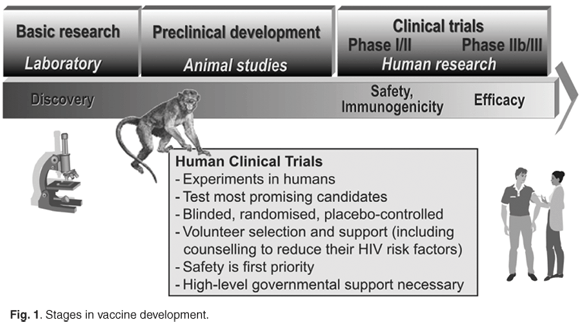
What have we learned from HIV vaccine efficacy trials?
There is no doubt that the HIV vaccine field has suffered a number of major set-backs in recent years. Nonetheless, we have learned a great deal from conducting such trials. The VaxGen efficacy trial reported in 2003, involving roughly 5000 volunteers, showed conclusively that a recombinant form of the viral envelope protein (so called monomeric gp120) was not able to stimulate protective antibodies.3 We now know that this is because the antibodies induced by this vaccine failed to recognise the complex trimeric envelope glycoprotein on the surface of the virus which is required for neutralisation. This not only inspired a large research effort to understand the native structure of the envelope trimer, but also encouraged structural biologists to design immunogens that mimic these complex epitopes. The clinical testing of these novel vaccines is still a long way off, as none has yet shown promise in animals. The STEP trial has given us an indication of the properties of CTL that are non-protective. The challenge for any new vaccine will be to show that the resulting CTLs are quantitatively and/or qualitatively better than those induced by the Merck vaccine. However, the nature of protective CTLs remains unidentified, and this will only be revealed by doing further clinical testing. An unexpected, and still not yet understood, outcome of the STEP trial was that vaccinated individuals with pre-existing Ad5 antibody titres were at increased risk of HIV infection. This highlighted the need to understand the role of anti-vector immunity and to design better vectors to obviate these interfering responses.
Where to from here?
Despite these set-backs there is still optimism in the field that a vaccine against HIV is possible.4–6 The lessons learned from developing the polio vaccine are useful reminders that making a vaccine is no easy task, and we still face many obstacles. Following the release of the STEP trial results in September 2007, and the subsequent discontinuation of vaccination in the Phambili trial in South Africa, there have been calls to scale down and even halt HIV vaccine trials. Some have argued that funding for AIDS vaccine research should be redirected towards treatment or other useful interventions. However, history tells us that the most effective way to curb viral epidemics is through vaccination.
So, rather than cut-back our efforts we need to double them. But, we need to do it smartly and differently. There are many interesting approaches and novel ideas being discussed in basic research laboratories. We need to find a way to get these funded and fast-tracked in order to speed up the testing of potential efficacious vaccine products. This includes devising better ways to assess candidate vaccines and a robust go/no-go strategy. For example, there was considerable debate as to whether a large scale efficacy (Phase III) trial (called the PAVE 100 trial) of a DNA prime in combination with a different Ad5-vectored vaccine boost should go ahead. In the end a decision was made not to conduct this trial and in its place the HVTN 505, a smaller Phase II trial will start later this year in the U.S.A. among men who have sex with men. This is a small focused trial aimed to assess if the vaccine lowers viral load in the blood among individuals who subsequently become infected with HIV despite vaccination (as there was some hint of this in a subgroup analysis of the Merck Ad5 trial). It will be conducted on individuals who do not have pre-existing immunity to Ad5 and who are circumcised (uncircumcised men were at even higher risk in the STEP trial) to maximise safety. This vaccine is not expected to prevent HIV infection or be licensed, but it will help to inform us about the role of CTL and guide the next generation of vaccines. It is highly unlikely, given our current knowledge, that Ad5-vectored vaccines will be tested in South Africa, which has high rates of Ad5 antibodies and low rates of circumcision.
What role can South Africa play?
As the country with the most HIV infections in the world, South Africa has the greatest need for an HIV vaccine. The importance of starting a vaccine programme was recognised over 10 years ago7 and the South African AIDS Vaccine Initiative (SAAVI) is now an internationally respected contributor to the global effort. Two locally designed vaccines, a DNA vaccine and a Modified Vaccinia Ankara (MVA)-vectored vaccine containing genes from HIV strains circulating within South Africa8–9 are currently being tested in a Phase I safety trial in the U.S.A., with vaccinations due to start in South Africa later this year. Furthermore, South Africa has the infrastructure to run large-scale efficacy trials, as recently demonstrated by the Phambili trial. It is imperative that South Africa is not deterred by the recent setbacks and continues to build on the years of investment and hard work to make a major contribution towards this global health crisis.
1. Buchbinder S.P., Mehrotra D.V., Duerr A., Fitzgerald D.W., Mogg R., Li D., Gilbert P.B., Lama J.R., Marmor M., Del Rio C., McElrath M.J., Casimiro D.R., Gottesdiener K.M., Chodakewitz J.A., Corey L., Robertson M.N., Step Study Protocol Team. (2008). Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372, 1881–1893. [ Links ]
2. McElrath M.J., De Rosa S.C., Moodie Z., Dubey S., Kierstead L., Janes H., Defawe O.D., Carter D.K., Hural J., Akondy R., Buchbinder S.P., Robertson M.N., Mehrotra D.V., Self S.G., Corey L., Shiver J.W., Casimiro D.R., Step Study Protocol Team (2008). HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372, 1894–1905. [ Links ]
3. Cohen J. (2003). AIDS Vaccine trial produces disappointment and confusion. Science 299, 1290–1291. [ Links ]
4. Johnston M.I. and Fauci A.S. (2007). An HIV vaccine – evolving concepts. N. Engl. J. Med. 356, 2073–2081. [ Links ]
5. Walker B.D. and Burton D.R. (2008). Towards an AIDS vaccine. Science 320, 760–764. [ Links ]
6. Bernstein A. (2008). AIDS and the next 25 years. Science 320, 717. [ Links ]
7. Morris L., van der Ryst E., Gray C.M. and Williamson C. (1997). Should South Africa be preparing for HIV-1 vaccine efficacy trials? S. Afr. Med. J. 87, 285–290. [ Links ]
8. Burgers W.A., van Harmelen J.H., Shephard E., Adams C., Mgwebi T., Bourn W., Hanke T., Williamson A.L. and Williamson C. (2006). Design and preclinical evaluation of a multigene human immunodeficiency virus type 1 subtype C DNA vaccine for clinical trial. J. Gen. Virol. 87, 399–410. [ Links ]
9. Burgers W.A., Shephard E., Monroe J.E., Greenhalgh T., Binder A., Hurter E., Van Harmelen J.H., Williamson C. and Williamson A.L. (2008). Construction, characterization, and immunogenicity of a multigene modified vaccinia Ankara (MVA) vaccine based on HIV type 1 subtype C. AIDS Res. Hum. Retro. 24, 195–206. [ Links ]














