Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.105 no.3-4 Pretoria Mar./Abr. 2009
RESEARCH LETTERS
Nutritional and phytochemical evaluation of cultivated Psathyrella atroumbonata Pegler, a Nigerian edible mushroom
S.M. AyodeleI, *; J.A. OkhuoyaII
IDepartment of Biological Sciences, Kogi State University, P.M.B. 1008, Anyigba, Kogi State, Nigeria
IIDepartment of Plant Biology and Biotechnology, University of Benin, P.M.B. 1154, Benin City, Edo State, Nigeria
ABSTRACT
A nutritional and phytochemical evaluation of cultivated Psathyrella atroumbonata Pegler was carried out at the immature and mature stages of the mushroom. The cultivated mushroom is very rich in protein and fibres compared with the wild species, and has a low lipid and sugar content. The nutrient composition is dependent upon the stage of its development and nutrient content was at a maximum at immature stage and decreased during further development. Alkaloids were detected in the mature fruit body, but not in the immature stage. However, saponins and tannins were present in both immature and mature stages. Flavonoids and anthraquinones were absent in the mushroom. The importance of these findings is discussed.
Key words: nutritional, phytochemical, evaluation, Psathyrella atroumbonata
Introduction
The use of mushrooms as food is probably as old as civilisation and mushrooms currently have greater importance in the diet of mankind. Cultivation and production of edible mushrooms are on the increase, particularly in Europe, America and Asia. Their increased nutritional importance is due to the nutritive value of high-grade mushrooms, which almost equals that of milk.1 Mushrooms have been evaluated for their nutritional status on the basis of their chemical composition. Cultivated and wild mushrooms contain reasonable amounts of proteins, carbohydrates, minerals, fibres and vitamins.2–5 Furthermore, mushrooms are low in calories, sodium, fats and cholesterol.6 Edible mushrooms have long been considered to have medicinal value and to be devoid of undesirable effects.7 The United States National Cancer Institute has chosen mushrooms as a source of new drugs for the treatment of cancer8 and the ethno-medicinal value of many edible mushrooms has been reported by many researchers.2,3,9–11
Psathyrella atroumbonata Pegler is one of the most valuable edible mushrooms in Nigeria. The mushroom is a lignicolous fungus commonly found in small clumps on or around rotten wood or on dead roots of trees in the last phases of decomposition. In Nigeria, the mushroom appears around May and persists until the end of the rainy season. The mushroom is similar to Coprinus in appearance but does not undergo auto-digestion, as in the case of Coprinus. According to Wuyep et al.,12 Psathyrella atroumbonata has enzymes with high cellulose digestibility. The mushroom is usually collected in the wild and it grows across different parts of Nigeria. Many researchers have collected wild species of the mushroom and analysed its proximate nutrient contents.2,3,13 In many areas of Nigeria, the mushroom yields substantial income and enhances the nutritional value of meals. Efforts have been made to domesticate the mushroom. The aim of this study was to evaluate the nutritional and phytochemical components of the cultivated strain of Psathyrella atroumbonata, a Nigerian edible mushroom.
Materials and methods
Psathyrella atroumbonata was harvested from the sawdust of Khaya ivorensis used for cultivation of the mushroom. The harvested fruit bodies were divided into young (button stage) and mature (fully opened pileus) stage according to the methods of Hammond and Nichols,14 Gruen and Wong15 and Kadiri and Fasidi.10 The fruit bodies were dried at 80°C for 48 h and powdered in a laboratory mortar with a pestle.
The ash content was determined by incinerating 3 g of powdered sample in a furnace at 550°C for 6 h.16 The ethanol-soluble sugar content was estimated with phenol-sulphuric acid after extracting 1 g of the powdered sample with 80% ethanol for 6 h in a soxhlet extractor.17 The total lipid content was determined by extracting 2 g of powdered sample with 30 ml petroleum ether in a soxhlet extractor for 4 h.18 Gross energy was assayed by using the anthrone method of Carroll et al.19 The moisture content was recorded as the loss in weight after oven-drying fresh mushrooms at 80°C for 48 h. The protein and crude fibre content was determined according to the method of the Association of Official Analytical Chemists.20 The mineral content was determined after wet digestion with a mixture of nitric, sulphuric and perchloric acids using an atomic absorption spectrophotometer (AAS model PV 9100X).
For the phytochemical screening (testing for alkaloids, flavonoids, saponins, tannins and anthraquinones), the following methods were used: Alkaloid determination was done using Mayer's and Dragendoff's reagents following the method of Kapoor et al.21 and Odebiyi and Sofowora22. The methods described by Kapoor et al.21 were used for determining the flavonoids. The persistent frothing test for saponins as described by Kapoor et al.21 and Odebiyi and Sofowora22 was used. Five hundred millilitres of dissolved powdered sample was mixed with 10 ml of distilled water, and the resulting mixture vigorously shaken and filtered. Seven drops of 10% FeCl3 were added to the filtrate. A colour change to blue, black, green or blue-green was taken as evidence of the presence of tannins.22 One gram of powdered sample was shaken with benzene (10 ml) and the mixture filtered. 10% NH4OH (10 ml) was added to the filtrate and the mixture transferred into a separating funnel. The development of pink, red or violet colour in the ammonia (lower) layer indicated the presence of anthraquinones.22
Results
The proximate nutrient composition and gross energy value of cultivated Psathyrella atroumbonata are shown in Table 1. The cultivated mushroom's nutrient composition is comparable to that of wild strains and those of other locally-consumed mushrooms (Table 2). It is very rich in protein, crude fibre and ash. However, it has a low sugar and lipid content. The crude protein represented about 30 g 100 g–1 of the dry matter, energy value 3 g 100 g–1, ash content 67 g 100 g–1, crude fibre 3 g 100 g–1, while the lipid and sugar contents were 0.6 g 100 g–1 and 1 g 100 g–1, respectively. The results also revealed that the nutrient composition was affected by the stage of development (Table 1). The protein content, crude fibres, ethanol-soluble sugar, lipid and gross energy were at a maximum at the immature stage and decreased during further development of the mushroom. However, the ash content showed an opposite trend. The dry matter of both mature and immature stages did not show significant differences. The mineral content of the sporophore also decreased with maturity (Table 3).



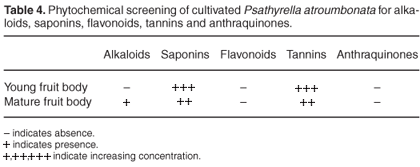
The results of phytochemical screening of the mushroom are shown in Table 4. Alkaloids were detected in the mature fruit body stage but not in the young fruit body stage. Saponins and tannins were also present in both young and mature stages. Flavonoids and anthraquinones were absent in the mushrooms.
Discussion
The result of the nutritional evaluation of cultivated Psathyrella atroumbonata is similar to the wild strains and other common species of edible mushroom studied in Nigeria.2,13,23,24 This mushroom is a richer source of protein than most commonly-consumed vegetables in Nigeria.25 While the protein content is lower than that found in eggs, meat and fish, it is adequate to be used as a substitute in the diet of the general public. These findings are similar to the previous findings of Aletor.3 The mineral content in this mushroom is higher than in several cowpea varieties,26 but lower than in fish, snails and broiler meat. On the basis of the observed nutritional value of cultivated P. atroumbonata, its nutritional quality falls between most legumes and meat.3 The protein content was at a maximum at the immature stage and slightly decreased during further development. However, the protein content continued to be high up to the harvest stage. These findings are in line with the work of Garcha et al.27 who evaluated the nutritional importance of Agaricus bisporus, Pleurotus florida and Pleurotus ostreatus and found that their protein contents were at a maximum at immature primordial stage and decreased as the mushrooms aged.
From the information gathered so far about the nutritional potential of edible mushrooms, it is conceivable that a number of wild and cultivated edible species of mushrooms such as P. atroumbonata hold great promise of reducing the protein and mineral deficiencies prevalent in the diets of humans in several developing countries.
The phytochemical screening shows the presence of alkaloids, saponins and tannins. Alkaloids were detected only in the mature stage of the fruit bodies. Flavonoids and anthraquinones could not be detected. In comparison, Kadiri and Fasidi10 detected alkaloids, tannins and anthraquinones, but not flavonoids, in some edible Nigerian mushrooms. These findings are in line with those of Hammond.28 The medicinal uses of many Nigerian mushrooms have been reported.9 The medicinal value may be due to the presence of the secondary metabolites in these mushrooms, such as Psathyrella atroumbonata.10 However, further studies on its polysaccharide contents, toxicity and medicinal value are highly recommended.
1. Chang S.T. and Miles P.G. (1989). In Edible Mushrooms and Their Cultivation, pp. 293–302. CRC Press, Boca Raton. [ Links ]
2. Alofe F.V. (1991). Amino acids and trace minerals of three edible wild mushrooms from Nigeria. J. Food Compost. Anal. 4, 147–174. [ Links ]
3. Aletor V.A. (1995). Compositional studies on edible tropical species of mushrooms. Food Chem. 54, 205–209. [ Links ]
4. Oei P. (1996). In Mushroom Cultivation with Special Emphasis on Appropriate Techniques for Developing Countries, pp. 3–5. Tool Publications, Leiden. [ Links ]
5. Stamets P. (2000). In Growing Gourmet and Medicinal Mushrooms, pp. 207–430. Ten Speed Press, California. [ Links ]
6. Chang R.Y. (1996). Potential application of Ganoderma polysaccharides in the immune surveillance and chemoprevention of cancer. In Mushroom Biology and Mushroom Products, Proceedings of the Second International Congress, ed. D.J. Royse, pp. 164–173. Penn State University Press, Philadelphia. [ Links ]
7. Sakagami H., Kwazoe Y., Komatsu N., Simpson A., Nonoyama M., Konno K., Yoshida T., Kuroiwa Y. and Yanuma S. (1991). Antitumor, antiviral and immunopotentiating activities of pine cone extracts: potential medicinal efficacy of natural and synthetic lignin-related materials. Anticancer Res. 11, 881–888. [ Links ]
8. Liu G. (1993). Pharmacology and clinical use of Ganoderma. In Mushroom Biology and Mushroom Products, Proceedings of the First International Congress, eds S.T. Chang, J.A. Buswell and S.W. Chiu, pp. 267–271. The Chinese University Press, Hong Kong. [ Links ]
9. Oso B.A. (1977). Mushrooms in Yoruba mythology and medicinal practices. Econ. Bot. 31, 367–371. [ Links ]
10. Kadiri M. and Fasidi I.O. (1992). Secondary plant products in some Nigerian mushrooms. Niger. J. Bot. 5, 187–192. [ Links ]
11. Akpaja E., Isikhuemhen O.S. and Okhunoya J.A. (2003). Ethno-mycology and usage of mushrooms among the Igbo people of Nigeria. Int. J. Med. Mushr. 3, 313–319. [ Links ]
12. Wuyep P.A., Khan A.U. and Nok A.J. (2002). Production regulation of lignin enzymes from Lentinus squarrosulus (Mont) Sing. and Psathyrella atroumbonata Pegler. Afr. J. Biotechnol. 2, 444–447. [ Links ]
13. Nwanze P.I, Jatto W., Oranusi S. and Josaih S.J. (2006). Proximate analysis of Lentinus squarrosulus (Mont.) Singer and Psathyrella atroumbonata Pegler. Afr. J. Biotechnol. 5, 366–368. [ Links ]
14. Hammond W.B.J. and Nichols R. (1992). Glycogen in Agaricus bisporus. Trans. Brit. Mycol. Soc. 66, 325–369. [ Links ]
15. Gruen E.H. and Wong M.W. (1982). Distribution of cellular amino acids, protein and total organic nitrogen during fruit body development in Flammulina velutipes I: growth on sawdust medium. Can. J. Bot. 60, 1330–1341. [ Links ]
16. Parent G. and Thoen D. (1977). Food values of edible mushrooms from the upper Sheba region. Econ. Bot. 31, 436–445. [ Links ]
17. Dubois M., Giles A.K., Hamilton K.J., Rebers A.P. and Smith F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–354. [ Links ]
18. Mukiibi J. (1973). The nutritive value of some edible mushrooms. Acta Hortic. 33, 173. [ Links ]
19. Carrol V.N., Longley W.R. and Roe H.J. (1956). The determination of glycogen in liver and muscle by use of anthrone reagent. J. Biol. Chem. 220, 583–593. [ Links ]
20. Association of Official Analytical Chemists (AOAC) (1984). Official Methods of Analysis, 13th edn, pp. 987–1012. AOAC, Washington D.C. [ Links ]
21. Kapoor D.I., Singh A.L., Kapoor I.S. and Svivaslava N.S. (1969). Survey of Indian plants for saponins, alkaloids and flavonoids. Lloydia 32, 297–304. [ Links ]
22. Odebiyi O.O. and Sofowora E.E. (1978). Phytochemical screening of Nigerian medicinal plants. Lloydia 41, 234–246. [ Links ]
23. Fasidi I.O. and Ekuere V. (1993). Studies on Pleurotus tuberregium (Fr) Sing: cultivation, proximate composition and mineral contents of sclerotia. Food Chem. 48, 255–258. [ Links ]
24. Kuforiji O.O., Fasidi I.O. and Olatunji O. (2000). Production of oyster mushrooms (Pleurotus tuberregium) from agro-industrial wastes. Niger. J. Microbiol. 17, 68–70. [ Links ]
25. Oyenuga V. (1968). In Nigerian Foods and Feeding Stuffs: Their Chemistry and Nutritive Value, pp. 88–96. Ibadan University Press, Ibadan. [ Links ]
26. Aletor V.A. and Aladetimi O.O. (1989). Compositional evaluation of some cowpea varieties and some under-utilized edible legumes in Nigeria. Nahrung 33, 99–107. [ Links ]
27. Garcha H.S., Khanna P.K. and Soni G.L. (1993.) Nutritional importance of mushrooms. In Mushroom Biology and Mushroom Products, Proceedings of the First International Congress, eds S.T. Chang, J.A. Buswell and S.W. Chiu, pp. 227–236. The Chinese University Press, Hong Kong. [ Links ]
28. Hammond W.B.J. (1978). Changes in composition of harvested mushrooms (Agaricus bisporus). Phytochemistry 18, 415–418. [ Links ]
29. Crisan E.V. and Sands A. (1978). Nutritional values of edible mushrooms. In Biology and Cultivation of Edible Mushrooms, eds S.T. Chang and W.A. Hayes, pp. 137–168. Academic Press, New York. [ Links ]
30. Kurtzman R.H. Jr. (1993). Analysis, digestibility and the nutritional value of mushrooms. In Mushroom Biology and Mushroom Products, Proceedings of the First International Congress, eds S.T. Chang, J.A. Buswell and S.W. Chiu, pp. 219–225. The Chinese University Press, Hong Kong. [ Links ]
Received 22 October 2008. Accepted 29 January 2009.
* Author for correspondence E-mail: ayodelemichael2007@yahoo.com














