Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.105 no.3-4 Pretoria Mar./Abr. 2009
RESEARCH LETTERS
Traditional herbal medicines: potential degradation of sterols and sterolins by microbial contaminants
D. du Plessis-Stoman; T.G. Downing; M. van de Venter; S. Govender *
Department of Biochemistry and Microbiology, Nelson Mandela Metropolitan University, Summerstrand South Campus, P.O. Box 77000, Port Elizabeth 6031, South Africa
ABSTRACT
Medicinal plants with a high content of sterols and sterolins, such as Bulbine natalensis (rooiwortel) and Hypoxis hemerocallidea (African potato), are commonly and inappropriately used in South Africa for the treatment of HIV/AIDS due to the inaccessibility of antiretroviral drugs. This study investigated the presence of active compounds, such as sterols and sterolins, in the herbal medicines. The research was carried out in the Nelson Mandela Metropole area. The effect of microbial contaminants isolated from the medicines on sterols and sterolins of rooiwortel extracts was assessed. Sterols and sterolins were detected in rooiwortel, raw African potatoes and one ready-made mixture. Co-incubation of rooiwortel with bacteria (Bacillus spp. and Pseudomonas putida) and fungi (Aspergillus spp., Penicillium spp. and Mucor spp.) that were isolated from these samples increased the rate of degradation of sterols and sterolins over time, with slower degradation at 4°C than at 28°C.
Key words: traditional herbal medicine, biodegradation, microbial contaminants, active compounds
Introduction
Medicinal plants with a high content of sterols and sterolins, such as Hypoxis hemerocallidea (African potato) and Bulbine natalensis (rooiwortel), are commonly and inappropriately used in South Africa for the alleviation of many immune-related ailments,1 and for the treatment of HIV/AIDS, due to the inaccessibility of antiretroviral drugs.2,3 The role of plant sterols and sterolins as immune modulators,4–7 and anti-inflammatory agents8 has been described. Herbal products are sold as either raw plants or extracts of portions of the plant.
Extraction involves boiling or percolating the herb in water, alcohol or other solvents, to release the biologically-active constituents in the plant.9 Both the raw herb and the extract contain complicated mixtures of organic chemicals, which may include fatty acids, sterols, alkaloids, flavonoids, glycosides, saponins, tannins and terpenes. It is often difficult to determine which component, if any, of the herb has biological activity in humans. In addition, the processing of herbs by procedures such as boiling, may alter the pharmacological activity of the organic constituents.10
Quality control of plant medicines is difficult because herbal medicines either presenting as herbs, roots or collections of herbs in composite formulae are extracted with boiling water during the decoction process. Thin layer chromatography (TLC) is frequently used for preliminary phytochemical analysis of medicinal plant extracts.11–13 TLC has also been recommended by various pharmacopoeias for initial screening to provide first characteristic fingerprints of herbs.14
In a previous study that assessed the microbial quality of herbal medicines from shops in the Nelson Mandela Metropole (previously known as Port Elizabeth), significant contamination by bacteria and fungi suggested inadequate storage facilities and poor hygienic practice during preparation of these medicines. A Staphylococcus aureus strain isolated showed resistance to methicillin and vancomycin, while Bacillus diarrhoeal enterotoxin was detected in three of the medicines, directly jeopardising public safety. This has highlighted the importance of introducing standards or guidelines for the microbiological quality control of traditional herbal medicines.15
This study investigated the detection of the putative active compounds in herbal medicines, in this instance sterols and sterolins, and their potential degradation by microbial contaminants.
Materials and methods
Sample collection
Samples consisted of remedies recommended by shop attendants for the treatment of HIV/AIDS. They were collected from four herbal shops (A–D) in the Nelson Mandela Metropole area and comprised ready-made mixtures (five samples), as well as material prepared according to the shop attendant's instructions (ten samples) (Table 1).15 All samples were processed under aseptic conditions within the laboratory to prevent contamination. Shop attendants did not give any advice on the hygienic preparation and storage of the medicines. However, all medicines were kept at room temperature at the different shops.

Standard curves
The presence of sterols and sterolins was determined by TLC on silica gel plates, using a solvent phase consisting of chloroform:ethylacetate:formic acid (5:4:1). The plate was subjected to a spray reagent consisting of anisaldehyde:sulphuric acid: ethanol (1:1:18), and then left to dry in an oven at 70°C for 10 min16. A sterol standard curve (Fig. 1) was prepared by using a β-sitosterol standard (Sigma). Four hundred and ninety-five microlitres of chloroform were added to 5 µl β-sitosterol (20 mg ml–1 in choloroform), vortexed for 5 min and centrifuged at 8 600 g for 5 min before loading. Duplicate samples were loaded on a silica gel plate to yield 1–6 µg sterols, prior to chromatography. The Rf value for β-sitosterol was 0.55. In a previous study, other sterols (stigmasterol, cholesterol, campesterol, desmosterol, ergosterol, fucosterol), as well as the stenol stigmastenol, were tested and all gave the same Rf value on TLC plates17. The spots detected at this Rf value would therefore represent the total of the most common and abundant phytosterols.

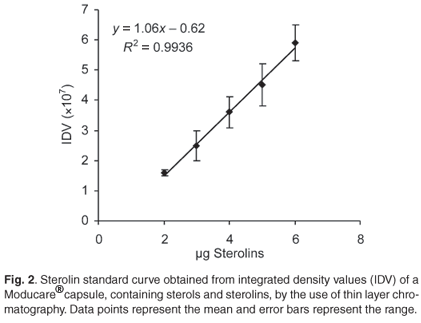
There is no commercially-available sterolin standard. Therefore, a Moducare® capsule was used as a sterolin standard. A Moducare® capsule containing 20 mg β-sitosterol and 0.2 mg β-sitosterol glucoside per capsule was used for the sterolin standard curve (Fig. 2). The contents of the capsule were added to 1 ml of chloroform, vortexed for 5 min, left at room temperature for 15 min, and then centrifuged for 5 min at 8 600 g. Duplicate samples of the supernatant (chloroform layer) were loaded onto a TLC plate to yield 1–6 µg of the β-sitosterol glucoside prior to chromatography. The Rf value for this sterolin was 0.75. The integrated density value (IDV) was determined using the AlphaInnotech Alpha-imager computer software program.
Detection of sterols and sterolins from African potato, rooiwortel and ready-made mixtures
African potato and rooiwortel were grated and 1 ml sterile water or chloroform added to 0.5 g of grated tuber. Tubes containing chloroform were vortexed for 5 min and left at room temperature for 30 min; tubes containing water were boiled for 5 min. Fifty microlitres of each sample were used for TLC. Fifty-microlitre samples of ready-made mixtures were used for TLC. Concentrations were determined from the slopes of the standard curves of sterols (R2 = 0.9893) and sterolins (R2 = 0.9934).
Effect of isolated microorganisms on sterols and sterolins in rooiwortel
The rooiwortel was washed and soaked in 5% hypochlorite solution for 15 min and an extract was prepared by grating a small piece of rooiwortel (about 3 cm long and 4 cm in diameter) on a sterile surface with a grater prewashed in 70% ethanol. The grated product was added to 750 ml of sterile water in a Schott bottle. The extract was boiled for 5 min and 1 ml aliquots were then transferred to Eppendorf tubes and inoculated (2 × duplicates) with 1 ml of the contaminating microorganism. Previous detection of the following microbial contaminants15 and preliminary screening suggested that Bacillus spp., Pseudomonas putida, Aspergillus spp., Penicillium spp. and Mucor spp. showed potential biodegradative ability, hence only these isolates were used for biodegradation experiments. For Bacillus spp. and Pseudomonas putida, 1 × 105 CFU ml–1, 5 × 104 CFU ml–1, and 2.5 × 104 CFU ml–1 were incubated with rooiwortel extract (in duplicate). For Aspergillus spp., Penicillium spp. and Mucor spp., a disk of agar (5 mm diameter, 0.07 g) was added to 1 ml of sterile water, and the suspension was inoculated into 1 ml of rooiwortel extract (in duplicate). These duplicates were incubated at 28°C for 11 days and at 4°C for 25 days, respectively. Four hundred microlitres of rooiwortel + microbe preparation were transferred to a new Eppendorf tube, and 400 µl of chloroform added to each aliquot, vortexed for 5 min and centrifuged at 8 600 g for 5 min. Fifty microlitres of the chloroform layer were used for TLC and the IDV of the compounds determined.
Results
No sterols were detected in water extracts of the African potato. Sterols were, however, detected in the chloroform extract. By contrast, sterols were detected in both water and chloroform extracts of rooiwortel.
Sterol and sterolin concentrations of bacteria (Bacillus spp. and Pseudomonas putida) and rooiwortel mixtures and fungi (Aspergillus spp., Penicillium spp. and Mucor spp.) and rooiwortel mixtures decreased during incubation time. The amounts of sterols and sterolins remaining over time were expressed as a percentage of the concentrations obtained at day zero. P. putida and Mucor spp. showed the highest rates of biodegradation at both 28°C (Fig. 3) and 4°C (Fig. 4). The rate of biodegradation was slower at 4°C than at 28°C. The minimum concentrations of total sterols and sterolins detected were 9.15 × 10–3 µg µl–1 and 1.09 × 10–2 µg µl–1, respectively.


Thin layer chromatography of the five ready-made mixtures indicated the presence of sterols and sterolins in only one sample – the ready-made mixture 1B, which was known to contain rooiwortel. If these sterols and sterolins were detected at their minimum detectable levels, as mentioned above, a person would ingest approximately 2.3 mg sterols and 2.7 mg sterolins per day (if the recommended dosage is one cup per day). The recommended daily dosage of Moducare® contains 60 mg β-sitosterol and 0.6 mg of the corresponding sterolin. Therefore, if the active compounds were present, but not detected due to low concentrations, a person would ingest insufficient amounts of sterols, but enough sterolins on a daily basis.
Discussion
Sterols from the African potato were only soluble in chloroform. This implies that African potatoes prepared according to the herbalist's instructions do not contain sterols. Only one of the five ready-made mixtures contained sterols due to the presence of rooiwortel.
It was shown that the microorganisms isolated contributed to the degradation of sterols and sterolins within the medicines. However, the stability of sterols and sterolins in the absence of microorganisms could not be determined, due to degradation and binding during heat sterilisation and filtration, respectively. Although information on the stability of sterols and sterolins in the absence of contaminating organisms would be useful, it should be noted that the extracts were prepared in such a way that the level of contamination was relatively low compared to the amounts of organisms added. The fact that there was increased degradation with increased inoculum levels clearly illustrates the role of the contaminating organism in accelerating the rate of degradation.
Biodegradation of sterols and sterolins was slower at 4°C than at 28°C, since most of the fungi and bacteria tested were mesophiles. Sterols and sterolins could not be detected in certain rooiwortel + fungi or rooiwortel + bacteria mixtures after 25 days of incubation. This could be due to biodegradation by these microorganisms inoculated into the extract. It is common practice for herbalists to prepare medicines and store them in a refrigerator. However this study indicates that after 25 days of storage there may be little or no active compounds present due to spontaneous biodegradation by naturally-occurring microbes. Patients who do not have a refrigerator would store the medicines at room temperature and after eleven days, the sterols and sterolins (active compounds) may be totally degraded.
Herbal shops usually supply prepared medicine in 750 ml bottles. If the recommended dose is one cup per day, this volume will be sufficient for three days. Hence, patients who buy medicines for one month or longer are at risk of buying medicines without any active compounds. There was no expiry date on the medicines; hence they could have been stored for months before getting to the consumer, compromising the stability of the active ingredients.
As the bacterial inoculum increased there was an increase in the rate of biodegradation. However, some values were variable, especially towards the fourth day at 28°C and the fifteenth day at 4°C, due to the depletion of growth medium (rooiwortel) and the poor visibility of sterols and sterolins on the TLC plate. Since the same amounts of the three fungal species were incubated with the rooiwortel extract, the rate of biodegradation may be reflective of the abilities of these fungi to degrade sterols and sterolins rather than the amount of inoculum. The implication is that microbial contaminants such as Bacillus spp., Pseudomonas putida, Aspergillus spp., Penicillium spp. and Mucor spp. may degrade sterols and sterolins or other active compounds in medicines.
Patients are therefore made to believe that they are buying medicine that will be good for them; however, this medicine may not contain the active compounds, as illustrated in this study on the biodegradation of sterols and sterolins.
Conclusion
To impact on community health, the introduction of guidelines for the microbial quality of traditional herbal medicines is of paramount importance, as microbial contamination may have adverse effects on the stability of sterols and sterolins and possibly of the stability of other active ingredients also.
The authors thank the National Research Foundation for financial support (GUN 2069228). Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regard thereof.
1. Erasto P., Adebola P.O., Grierson D.S. and Afolayan A.J. (2005). An ethnobotanical study of plants used for the treatment of diabetes in the Eastern Cape province, South Africa. Afr. J. Biotechnol. 4(12), 1458–1460. [ Links ]
2. Bodeker G., Kabatesi D., King R. and Homsy J. (2000). A regional task force on traditional medicine and AIDS. Lancet 355, 1284. [ Links ]
3. Vermani K. and Garg S. (2002). Herbal medicines for sexually transmitted diseases and AIDS. J. Ethnopharmacol. 80, 49–66. [ Links ]
4. Bouic P.J.D., Etsebeth S., Liebenberg R.W., Albrecht C.F., Pegel K. and van Jaarsveld P.P. (1996). Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 18, 693–700. [ Links ]
5. Bouic P.J.D. and Lamprecht J.H. (1999). Plant sterols and sterolins: a review of their immune modulating properties. Altern. Med. Rev. 4, 170–177. [ Links ]
6. Bouic P.J.D. (2002). Sterols and sterolins: new drugs for the immune system? Drug Discov. Today 7, 775–778. [ Links ]
7. Breytenbach U., Clark A., Lamprecht J. and Bouic P.J.D. (2001). Flow cytometry analysis of the Th1-Th2 balance in healthy individuals and patients infected with the human immunodeficiency virus (HIV) receiving a plant sterol/sterolin mixture. Cell Biol. Int. 25, 43–49. [ Links ]
8. Quilez J., Garcia-Lorda P. and Salas-Salvado J. (2003). Potential uses and benefits of phytosterols in diet: present situation and future directions. Clin. Nutr. 22, 343–351. [ Links ]
9. Louw C.A.M., Regnier T.J.C. and Korsten L. (2002). Medicinal bulbous plants of South Africa and their traditional relevance in the control of infectious diseases. J. Ethnopharmacol. 82, 147–154. [ Links ]
10. Bent S. and Ko R. (2004). Commonly used herbal medicines in the United States: a review. Am. J. Med. 116, 478–485. [ Links ]
11. Tona L., Kambu K., Ngimbi N., Cimanga K. and Vlietinck A.J. (1998). Antiamoebic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharmacol. 61, 57–65. [ Links ]
12. Adewunmi C.O., Agbedahunsi J.M., Adebajo A.C., Aladesanmi A.J., Murphy N. and Wando J. (2001). Ethno-veterinary medicine: screening of Nigerian medicinal plants for trypanocidal properties. J. Ethnopharmacol. 77, 19–24. [ Links ]
13. Alshawsh M.A., Mothana R.A., Al-shamahy H.A., Alsllami S.F. and Lindequist U. (2007). Assessment of antimalarial activity against Plasmodium falciparum and phytochemical screening of some Yemeni medicinal plants. Evid. Based Complement. Alternat. Med., online at http://ecam.oxfordjournals.org/cgi/ content/abstract/nem148doi:10.1093/ecam/nem148 [ Links ]
14. Liang Y., Xie P. and Chan K. (2004). Quality control of herbal medicines. J. Chromatogr. B 812, 53–70. [ Links ]
15. Govender S., du Plessis-Stoman D., Downing T.G. and van de Venter M. (2006). Traditional herbal medicines: microbial contamination, consumer safety and the need for standards. S. Afr. J. Sci. 102, 253–255. [ Links ]
16. Retief A.C. (2001). Analysis of sterols and sterolins in Hypoxis hemerocallidea and related herbal medicine. M.Sc. thesis, University of Pretoria, South Africa. [ Links ]
17. Boukes G.J, van de Venter M. and Oosthuizen V. (2008). Quantitative and qualitative analysis of sterols/sterolins and hypoxoside contents of three Hypoxis (African potato) spp. Afr. J. Biotechnol. 7(11), 1624–1629. [ Links ]
Received 29 February. Accepted 12 December 2008.
* Author for correspondence E-mail: sharlene.govender@nmmu.ac.za














