Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.105 no.3-4 Pretoria mar./abr. 2009
RESEARCH ARTICLES
A spatial assessment of Brassica napus gene flow potential to wild and weedy relatives in the Fynbos Biome
M.A. McGeochI, II, *; J.M. KalwijI, III; J.I. RhodesI, IV
IDST-NRF Centre for Invasion Biology
IICape Research Centre, South African National Parks, P.O. Box 216, Steenberg 7947, South Africa
IIIDepartment of Conservation Ecology and Entomology, University of Stellenbosch, Private Bag X1, Matieland 7602, South Africa. Currently: Department of Botany and Zoology, University of Stellenbosch
IVBiosafety South Africa, 105 Wentworth, Somerset Links Office Park, De Beers Avenue, Somerset West 7130, South Africa
ABSTRACT
Gene flow between related plant species, and between transgenic and non-transgenic crop varieties, may be considered a form of biological invasion. Brassica napus (oilseed rape or canola) and its relatives are well known for intra- and inter-specific gene flow, hybridisation and weediness. Gene flow associated with B. napus poses a potential ecological risk in the Fynbos Biome of South Africa, because of the existence of both naturalised (alien, weedy) and native relatives in this region. This risk is particularly pertinent given the proposed use of B. napus for biofuel and the potential future introduction of herbicide-tolerant transgenic B. napus. Here we quantify the presence and co-occurrence of B. napus and its wild and weedy relatives in the Fynbos Biome, as a first step in the ecological risk assessment for this crop. Several alien and at least one native relative of B. napus were found to be prevalent in the region, and to be spatially congruent with B. napus fields. The first requirement for potential gene flow to occur has thus been met. In addition, a number of these species have elsewhere been found to be reproductively compatible with B. napus. Further assessment of the potential ecological risks associated with B. napus in South Africa is constrained by uncertainties in the phylogeny of the Brassicaceae, difficulties with morphology-based identification, and poor knowledge of the biology of several of the species involved, particularly under South African conditions.
Key words: ecological risk assessment, herbicide resistance, Brassicaceae, hybridisation, species distribution range, biotechnology, transgenic
Introduction
Gene flow from crops to wild relatives has been associated with the evolution of weediness in seven of the world's 13 most important crops.1 In addition, of the environmental risks posed by transgenic crops, those associated with the transfer of transgenes are considered to be most important.2,3 Gene flow between crops and wild relatives is well documented,1,4 and the possible movement of transgenes from crop plants to wild relatives must thus be considered in (i) the ecological risk assessment for transgenics, (ii) the introduction and development of novel crops, and (iii) agricultural expansion for biofuel production.2,5,6
Gene flow from transgenic crops to wild relatives may have a number of negative effects.3 For instance, transgenic technology has the potential to exacerbate the invasiveness of plant species.7 Hybridisation with transgenic varieties could increase the fitness of a weed species (by for example conferring traits such as drought tolerance), or compound the effects of existing invasive species.8–10 The possible long-term ecological effects of such invasion may be considerable.11–13 Furthermore, gene flow from transgenic plants is difficult to contain.2 This has been clearly demonstrated by transgene movement in maize,14,15 rice,16 creeping bentgrass17,18 and oilseed rape.19,20 Indeed, Snow and Morán-Palma2 suggest that 'if gene flow is possible then it is probable'. Nonetheless, for hybridisation to occur between a crop plant and a wild relative, a number of barriers to gene flow must be overcome.21 For example, the respective taxa must be geographically proximate, must overlap at least partially in flowering time, must share a pollination mechanism, must show reproductive compatibility, and hybrids must be viable and at least partially fertile.22
One of the species of concern is Brassica napus L. (Brassicaceae), i.e. canola or oilseed rape.23–25 Brassica napus has a number of characteristics favouring gene flow and a potential increase in weediness. These include the ability of B. napus to form volunteer populations, as well as its propensity to become weedy in other cultivated crops.26 Gulden et al.19 reported B. napus seed losses during harvest as 20 times the normal seeding rate. These seeds can remain in the soil seedbank for several years after harvest.26 Brassica napus seed and pollen also have high mobility. For instance, for a number of seed lots containing certified transgenic and non-transgenic B. napus seed, 97% had adventitious contamination.27 Oilseed rape has large pollen dispersal potential and it can outcross and hybridise with wild relatives, such as Brassica rapa and Raphanus ra phanistrum.20 Indeed, hybridisation between several representatives of the genus Brassica and sexually compatible (non-transgenic) wild relatives has been regularly reported.28
Brassica napus is becoming one of the most important sources of oil and protein in the world.29 It is currently the fourth most important oilseed and global production continues to increase rapidly.30 In addition, transgenic, insect-resistant and herbicide-tolerant B. napus varieties have been developed and tested in field experiments.31,32 Brassica napus (non-transgenic) was introduced to South Africa fairly recently, with 5 000 ha planted in 1994, and 40 200 ha planted to the crop in 2005/06.33 It has also been identified as a possible crop for the production of biofuel in South Africa,34 and the area planted to B. napus may thus increase significantly in the future. With a larger area planted to B. napus there may well also be more interest in the use of transgenic B. napus. Transgenic B. napus with glufosinate ammonium tolerance was approved for trial release in South Africa in 2000,35 although to date it has not received market approval. There has been little attempt to identify the possibility of gene flow from transgenic crops to wild or weedy relatives in the country, despite a constant increase in the number of field trials of a range of transgenic crops that have been approved.35,36 Although elsewhere there have been several studies of gene flow in Brassica species and their wild relatives,23,37 the likelihood and consequences of gene flow in this system in South Africa have to date not been considered, nor have the consequences of increasing area planted to the crop.38
Here we: (i) quantify the diversity of wild and weedy B. napus relatives, and (ii) assess the spatial congruence of their distributions as a basis for understanding the potential for gene flow from commercially-produced B. napus to wild and weedy relatives in the Fynbos Biome of the Cape Floristic Region. This is a globally significant centre of biodiversity and endemism that is highly susceptible to plant invasions.39–41 The Fynbos Biome also encompasses the majority of the area planted to B. napus in South Africa. The results reported here will inform ecological risk assessment and regulatory decisions for envisaged biofuel, and potential transgenic, B. napus cultivation,42,43 and will narrow the range of potential taxa for further experimental assessment of reproductive compatibility.
Methods
The study area was the Fynbos Biome of the Western Cape Province, South Africa (Fig. 1). In this area B. napus L. is commercially produced in six municipalities and is locally referred to as canola (and less commonly in South Africa as oilseed rape).

A literature study was conducted to determine which plant taxa may have the potential to hybridise with B. napus. All species present in the tribe Brassiceae (containing B. napus) and the closely-related tribe Sisymbrieae were considered.44 We subsequently compared this list of taxa with plant specimens from the Compton Herbarium (South African National Biodiversity Institute (SANBI), Cape Town) and with the Pretoria Computerised Information System (PRECIS) database of the National Herbarium (SANBI, Pretoria) to finalise the target species list. Nomenclature followed Germishuizen and Meyer.45
A rapid assessment field survey was conducted to estimate the spatial overlap in ranges between B. napus and its wild and weedy relatives in the Fynbos Biome. The rapid assessment approach was adopted based on the assumption that the greatest potential risk for gene flow is with prevalent, wide-ranging species that frequently co-occur with B. napus. The study area was divided into quarter-degree square (QDS) grid cells of approximately 23 × 28 km. Of these grids 98 consecutive QDS were selected for the field sampling, encompassing the Fynbos Biome. Field sampling targeted mainly disturbed road verges with agricultural activities in the hinterland. These are common habitats for weedy relatives of B. napus, and also represent localities where putative gene flow is most likely to occur (road verges abut B. napus fields). Road verges were visited between September and October 2008, as close as possible to the centre of each QDS. Based on the herbarium specimens of the Compton and PRECIS collections, the survey period coincided with the main flowering seasons of the target species. At each site we carefully examined the area for approximately ten minutes and recorded the presence of target species. When present, target species were always found within the first two minutes of examination. Specimens were collected for all observations to verify species identification and for future genetic analysis. Additional specimens of target species were collected ad hoc while travelling between the QDS centres. A total of 425 records for South Africa and 69 for the Western Cape Province were obtained from the Compton and PRECIS Herbarium databases, and 222 records (different QDS by species combinations) were obtained from the field survey.
The qualitative spatial risk assessment was based on the following factors: (i) Indigenous relatives were considered to present a greater risk than alien relatives, because gene flow with indigenous species potentially represents the greater risk to biodiversity. (ii) Relative prevalence. The higher the relative prevalence of the species in the Western Cape Province and in the field survey, the greater the potential gene flow risk. Here the frequency of occurrence of herbarium records of the species, and/or its sampled prevalence in the field survey, was used to calculate relative prevalence. Relative prevalence was scored based on the species representing <5% (score 1), 5–10% (score 2), 11–25% (score 3) and >25% (score 4) of the records in the Western Cape Province or in the field survey. (iii) Spatial overlap. Relatives found to overlap spatially with B. napus were considered to present a higher risk for gene flow compared with those with distributions that did not overlap spatially. (iv) Reproductive compatibility. Where available, literature evidence on reproductive compatibility and the formation of hybrids was used to assess hybridisation risk.46 Within indigenous and alien relative categories, relatives considered to present the greatest risk for gene flow were thus those that had high relative prevalence, overlapped spatially with B. napus, and for which there was significant literature evidence of reproductive compatibility. Species were then ranked (1 = highest risk) based on their performance according to these risk factors.
Results
Based on literature information and the field survey conducted as part of this study, 27 relatives of B. napus were identified as occurring in South Africa. These relatives include both alien (mostly naturalised) and indigenous species (Table 1). Because the field survey may be considered a rapid assessment (restricted to road-verge sampling), the list is not necessarily complete. Nonetheless, it is likely to be representative of the most common wild relatives. Further, because some specimens were difficult to identify, a few taxa in the list are not identified to species level. The group is well known for its phenotypic plasticity, and hybrids may share morphological features of parent taxa,44,47,48 making morphological identification difficult in some cases.

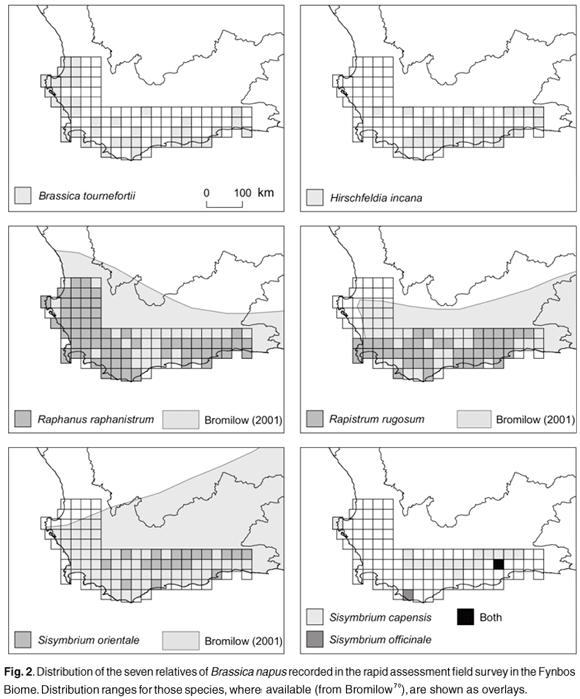
Of the 27 relatives of B. napus in South Africa, 13 occur in the Western Cape Province (including the Fynbos Biome), and of these at least seven were sampled in the field survey (including six alien and at least one indigenous species). Raphanus raphanistrum, Brassica tournefortii and Sisymbrium capense were most prevalent in herbaria collections for the Western Cape Province, and R. raphanistrum, Rapistrum rugosum, B. tournefortii and Hirschfeldia incana were most prevalent in the field survey (Table 1). Sisymbrium orientale and S. capense were also moderately prevalent in the field survey (Table 1).
There is significant overlap between B. napus fields and the distribution of several wild and weedy relatives of B. napus in the Fynbos Biome (Figs 1 and 2). The maximum number of species per QDS recorded was seven, in the vicinity of Sandkraal (QDS 3321CD) (Fig. 1). Thirty-seven of the sampled QDSs included three or more relatives, and 12 of these high wild relative-species-rich cells overlapped with the presence of B. napus (Fig. 1).
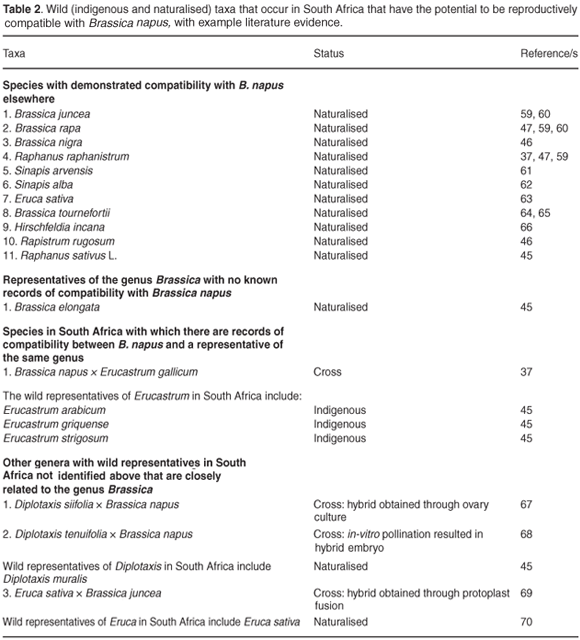
There is comparatively little information in the literature on the reproductive compatibility of B. napus and wild and weedy relatives found in South Africa (Table 2). However, at least nine naturalised relatives of B. napus in South Africa have elsewhere been demonstrated to have some degree of reproductive compatibility with B. napus (Table 2). It is important to emphasise that the absence of records of compatibility represents a lack of knowledge and not necessarily the absence of compatibility. No information is available on the potential for reproductive compatibility between B. napus and indigenous relatives in South Africa.
The qualitative spatial risk assessment for gene flow potential between B. napus and relatives in the Fynbos Biome was based primarily on documented overlap in their spatial distributions, and relative prevalence in herbaria and field survey samples (Fig. 2, Table 3). Sisymbrium capense was ranked as the highest priority indigenous species for further examination (Table 3), on the basis that it was most prevalent and spatially congruent with B. napus.
Indigenous species in the genus Erucastrum should also be considered a priority for further assessment, because elsewhere in the world species in the genus have been shown to be reproductively compatible with B. napus (Table 3).
Amongst the naturalised (and weedy) species, R. rugosum, B. tournefortii, R. raphanistrum and H. incana are considered to be of the highest priority for further gene flow and hybridisation risk assessment (Table 3). All four species are prevalent in the Fynbos Biome, are spatially congruent with B. napus and have elsewhere been shown to have some degree of reproductive compatibility with B. napus (Table 3).
Discussion
A general model for assessing the risks associated with gene flow includes three components: (i) factors that affect the likelihood of gene flow, (ii) factors that will affect the likelihood of transgene establishment and proliferation, and (iii) the potential negative consequences of gene flow.49 One of the first questions included in risk assessment guidelines for transgenic organisms is what factors affect the likelihood of intra- and interspecific gene flow.50,51 This includes determining if related taxa are present in the region, and if so, their frequencies and distributions.50 Here we have shown that several taxa, that are closely related to B. napus, co-occur with B. napus fields in the Fynbos Biome. We have also shown that related taxa are widespread across the Fynbos Biome, and that a number of these are also prevalent (occurring in a high proportion of sites visited). Therefore, based on the co-occurrence and prevalence of relatives of B. napus in the Fynbos Biome, at least one hurdle to potential gene flow (i.e. spatial co-occurrence) has been overcome for several taxa.
The spatial overlap in the distribution of relatives is of course in itself insufficient for gene flow and hybridisation to occur. In addition, the majority of attempts to form hybrids reported in the literature have been unsuccessful. One review46 showed that 47% of reported attempts at hybridisation with B. napus were unsuccessful, but that at least one instance of successful hybridisation was found in the remaining species (n = 23). The review, however, also found high variability in the success of hybridisation attempts across studies.46 This suggests that single studies demonstrating unsuccessful hybridisation are insufficient for reaching conclusions about hybridisation potential in this group of taxa.
Cross pollination between populations and reproductive compatibility, including the fertility, fitness and persistence of hybrids, are also necessary for gene flow to pose a potential risk to biodiversity.24,52 Although information on the phenology, especially flowering times, of B. napus and its relatives in South Africa is patchy, most of these taxa flower in spring and summer, and disjunct flowering seasons are thus unlikely to form a barrier to potential gene flow between them.53 Again, little is known about the reproductive mechanisms, compatibility and possible fertility of hybrids in these species, particularly under South African conditions. Although in some cases hybrids are known to generally be self-incompatible (e.g. B. napus × B. rapa) or to have low fitness,54 the absence of such information on several of these taxa, and under local conditions, is cause for concern. Within South Africa there are five naturalised species in the genus Brassica, as well as a number of native and naturalised representatives from closely-related genera where there may be the potential for gene flow. For example, of the naturalised species in the genus Brassica in South Africa, B. rapa is one of the diploid parents resulting in tetraploid oilseed rape.20 Cross-compatibility, without the use of in vitro ovule and embryo rescue techniques, has been demonstrated between these two species.20 Representatives of genera that are closely related to, and form hybrids with members of Brassica, include the native genus Erucastrum. For instance, E. gallicum (non-native) has elsewhere been shown to hybridise with B. napus.37
Finally, if hybrids or transgenes were to spread and persist as feral populations, potential risks to agricultural production and biodiversity would include, for example, the development of, or an increase in weediness, development of herbicide resistance, invasion of natural habitat, movement of populations into new ecological niches and possible extinction of native species.11–13,55,56 One example of this concerns R. raphanistrum—one of the four naturalised alien species considered to present the greatest gene flow risk based on the findings of this study. Herbicide resistance has already been demonstrated in this species in the Fynbos Biome.57 Similarly in Australia, herbicide resistance has evolved in E. austroafricanum (a close relative of B. napus) (http://www. weedscience.org/Case/). Such potential unintended and negative consequences of gene flow between B. napus and its relatives in South Africa have previously not been investigated nor, to our knowledge, considered.
The results we show here thus provide a first step in the risk assessment for gene flow between B. napus and its relatives in the Fynbos Biome. These results clearly demonstrate that further attention must be given to ecological risk assessment for B. napus in this biodiversity-rich region of South Africa, and we have narrowed the list and identified priority species for further attention. Ecological risk assessment will be particularly critical if transgenic B. napus is to be considered for release, or if B. napus is to be used for biofuel production (with a likely concomitant increase in area planted38 in the country). However, significant hurdles to ecological risk assessment for B. napus currently include: (i) the remaining uncertainties in the phylogeny and relatedness of taxa in the Brassicaceae,44 (ii) difficulties with morphology-based identification of these species generally and in South Africa,58 (iii) the lack of taxonomic expertise, and (iv) poor knowledge of the distribution, phenology, pollination syndromes and reproductive mechanisms, particularly, but not only, of indigenous taxa.
We thank Sarah Davies for initiating the funding opportunity for this research, and for her facilitation thereof and comments on the output. We thank C.A.P.E. and the Centre for Invasion Biology for financial support, A.Y. van Zonneveld for field assistance, and the Compton Herbarium in Cape Town for taxonomic assistance and access to data records.
1. Ellstrand N.C., Prentice H.C. and Hancock J.F. (1999). Gene flow and introgression from domesticated plants into their wild relatives. Annu. Rev. Ecol. Syst. 30, 539–563. [ Links ]
2. Snow A.A. and Morán-Palma P. (1997). Commercialization of transgenic plants: potential ecological risks. Bioscience 47, 86–97. [ Links ]
3. Andow D.A. and Zwahlen C. (2006). Assessing environmental risks of transgenic plants. Ecol. Lett. 9, 196–214. [ Links ]
4. Daehler C.C. (1998). The taxonomic distribution of invasive plants: ecological insights and comparison to agricultural weeds. Biol. Conserv. 84, 167–180. [ Links ]
5. Ellstrand N.C. (2001). When transgenes wander, should we worry? Plant Physiol. 125, 1543–1545. [ Links ]
6. Koh L.P. and Ghazoul J. (2008). Biofuels, biodiversity, and people: understanding the conflicts and funding opportunities. Biol. Conserv. 141, 2450–2460. [ Links ]
7. Halfhill M.D., Millwood R.J., Raymer P.L. and Stewart C.N. (2002). Bt-transgenic oilseed rape hybridization with its weedy relative, Brassica rapa. Environ. Biosafety Res. 1, 19–28. [ Links ]
8. Abbott R.J. (1992). Plant invasions, interspecific hybridization and the evolution of new plant taxa. Trends Ecol. Evol. 7, 401–405. [ Links ]
9. Raybould A.F. and Gray A.J. (1994). Will hybrids of genetically modified crops invade natural communities? Trends Ecol. Evol. 9, 85–89. [ Links ]
10. Snow A.A., Andow D.A., Gepts P., Hallerman E.M., Power A., Tiedje J.M. and Wolfenbarger L.L. (2005). Genetically engineered organisms and the environment: current status and recommendations. Ecol. Appl. 15, 377–404. [ Links ]
11. Rhymer J.M. and Simberloff D. (1996). Extinction by hybridization and introgression. Annu. Rev. Ecol. Syst. 27, 83. [ Links ]
12. Huxel G.R. (1999). Rapid displacement of native species by invasive species: effects of hybridization. Biol. Conserv. 89, 143–152. [ Links ]
13. Petit R.J. (2004). Biological invasions at the gene level. Divers. Distrib. 10, 159–165. [ Links ]
14. Quist D. and Chapela I.H. (2001). Transgenic DNA introgressed into traditional maize landraces in Oaxaca, Mexico. Nature 414, 541–543. [ Links ]
15. Smyth S., Khachatourians G. and Phillips P.W.B. (2002). Liabilities and economics of transgenic crops. Nature Biotech. 20, 537. [ Links ]
16. Ledford H. (2007). Out of bounds. Nature 445, 132–133. [ Links ]
17. Watrud L.S., Lee E.H., Fairbrother A., Burdick C., Reichman J.R., Bollman M., Storm M., King G. and Van de Water P.K. (2004). Evidence for landscape-level, pollen-mediated gene flow from genetically modified creeping bentgrass with CP4 EPSPS as a marker. Proc. Natl. Acad. Sci. U.S.A. 101, 14533–14538. [ Links ]
18. Reichman J.R., Watrud L.S., Lee E.H., Burdick C.A., Bollman M.A., Storm M.J., King G.A. and Mallory-Smith C. (2006). Establishment of transgenic herbicide-resistant creeping bentgrass (Agrostis stolonifera L.) in nonagronomic habitats. Mol. Ecol. 15, 4243–4255. [ Links ]
19. Gulden R.H., Shirtliffe S.J. and Thomas A.G. (2003). Harvest losses of canola (Brassica napus) cause large seedbank inputs. Weed Sci. 51, 83–86. [ Links ]
20. Warwick S.I., Simard M.J., Légère A., Beckie H.J., Braun L., Zhu B., Mason P., Séguin-Swartz G. and Stewart C.N. (2003). Hybridization between transgenic Brassica napus L. and its wild relatives: Brassica rapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) O.E. Schulz. Theor. Appl. Genet. 107, 528–539. [ Links ]
21. Richards A.J. (2005). Hybridisation – reproductive barriers to gene flow. In Gene Flow from GM Plants, eds G.M. Poppy and M.J. Wilkinson, pp. 78–112. Blackwell Publishing, Oxford. [ Links ]
22. Chapman M.A. and Burke J.M. (2006). Letting the gene out of the bottle: the population genetics of genetically modified crops. New Phytol. 170, 429–443. [ Links ]
23. Baranger A., Chèvre A.M., Eber F. and Renard M. (1995). Effect of oilseed rape genotype on the spontaneous hybridisation rate with a weedy species: an assessment of transgene dispersal. Theor. Appl. Genetics 91, 956–963. [ Links ]
24. Wilkinson M.J., Davenport I.J., Charters Y.M., Jones A.E., Allainguillaume J., Butler H.T., Mason D.C. and Raybould A.F. (2000). A direct regional scale estimate of transgene movement from genetically modified oilseed rape to its wild progenitors. Mol. Ecol. 9, 983–991. [ Links ]
25. Pilson D. and Prendeville H.R. (2004). Ecological effects of transgenic crops and the escape of transgenes into wild populations. Annu. Rev. Ecol. Evol. Syst. 35, 149–174. [ Links ]
26. Tolstrup K., Andersen S.B., Boelt B., Buus M., Gylling M., Holm P.B., Kjellson G., Pedersen S., Østergaard H. and Mikkelsen S. (2003). Report from the Danish Working Group on the co-existence of genetically modified crops with conventional and organic crops. DIAS Report 94, p. 275, Danish Institute of Agricultural Sciences, Denmark. [ Links ]
27. Friesen L.F., Nelson A.G. and Van Acker R.C. (2003). Evidence of contamination of pedigreed canola (Brassica napus) seedlots in Western Canada with genetically contaminated engineered herbicide resistance traits. Agron. J. 95, 1342–1347. [ Links ]
28. Ellstrand N.C. (2003). Dangerous liaisons? When cultivated plants mate with their wild relatives. In Syntheses in Ecology and Evolution, ed. S.M. Scheiner, pp. 137–204. The John Hopkins University Press, Baltimore. [ Links ]
29. Lamb R.J. (1989). Entomology of oilseed Brassica crops. Annu. Rev. Entomol. 34, 211–229. [ Links ]
30. Rashid U. and Anwar F. (2008). Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 87, 265–273. [ Links ]
31. Stewart Jr C.N., Adang M.J., All J.N., Raymer P.L., Ramachandran S. and Parrott W.A. (1996). Insect control and dosage effects in transgenic canola containing a synthetic Bacillus thuringiensis cryIAc gene. Plant Physiol. 112, 115–120. [ Links ]
32. Stewart C.N., All J.N., Raymer P.L. and Ramachandran S. (1997). Increased fitness of transgenic insecticidal rapeseed under insect selection pressure. Mol. Ecol. 6, 773–779. [ Links ]
33. DOA (2006). South African variety list for fruit crops as maintained by the registrar of plant improvement: January. Department of Agriculture, Pretoria. [ Links ]
34. DME (2007). Draft Biofuels Industrial Strategy of the Republic of South Africa. Department of Minerals and Energy, Pretoria. [ Links ]
35. DOA (2005). Genetically Modified Organisms Act, 1997. Annual Report 2004/2005, Directorate: Genetic Resources Management, Department of Agriculture, Pretoria. [ Links ]
36. McGeoch M.A. and Pringle K.L. (2005). Science and advocacy: the GM debate in South Africa. S. Afr. J. Sci. 101, 7–9. [ Links ]
37. Lefol E., Séguin-Swartz G. and Downey R. (1997). Sexual hybridisation in crosses of cultivated Brassica species with the crucifers Erucastrum gallicum and Raphanus raphanistrum: potential for gene introgression. Euphytica. 95, 127–139. [ Links ]
38. Ceddia M.G., Bartlett M. and Perrings C. (2009). Quantifying the effect of buffer zones, crop areas and spatial aggregation on the externalities of genetically modified crops at landscape scale. Agric. Ecosyst. Environ. 129, 65–72. [ Links ]
39. Breytenbach G.J. (1986). Impacts of alien organisms on terrestrial communities with emphasis on communities of the south-western Cape. In Ecology and Management of Biological Invasions in Southern Africa, eds I.A.W. Macdonald, F.J. Kruger and A.A. Ferrar, pp. 229–238. Oxford University Press, London. [ Links ]
40. Kareiva P., Parker I.M. and Pascual M. (1996). Can we use experiments and models in predicting the invasiveness of genetically engineered organisms? Ecology 77, 1670–1675. [ Links ]
41. Mucina L. and Rutherford M.C. (2006). The Vegetation of South Africa, Lesotho and Swaziland, Strelitzia 19. South African National Biodiversity Institute, Pretoria. [ Links ]
42. Hill R.A. (2005). Conceptualizing risk assessment methodology for genetically modified organisms. Environ. Biosafety Res. 4, 67–70. [ Links ]
43. McGeoch M.A. and Rhodes J.I. (2006). Ecological risk assessment of genetically modified organisms in South Africa: an assessment of the current policy framework. C.I.B Occasional Paper, No. 2. Online at: www.sun.ac.za/cib/occasion.asp. [ Links ]
44. Bailey C.D., Koch M.A., Mayer M., Mummenhoff K., O'Kane S.L., Warwick S.I., Windham M.D. and Al-Shehbaz I.A. (2006). Toward a global phylogeny of the Brassicaceae. Mol. Biol. Evol. 23, 2142–2160. [ Links ]
45. Germishuizen G. and Meyer N.L. (2003). In Plants of Southern Africa: an Annotated Checklist, Strelitzia 14. National Botanical Institute, Pretoria. [ Links ]
46. Fitzjohn R.G., Armstrong T.T., Newstrom-lloyd L.E., Wilton A.D. and Cochrane M. (2007). Hybridisation within Brassica and allied genera: evaluation of potential for transgene escape. Euphytica 158, 209–230. [ Links ]
47. Warwick S.I. and Sauder C.A. (2005). Phylogeny of tribe Brassiceae (Brassicaceae) based on chloroplast restriction site polymorphisms and nuclear ribosomal internal transcribed spacer and chloroplast trnL intron sequences. Can. J. Bot. 83, 467–483. [ Links ]
48. Iqbal M., Akhtar N., Zafar S. and Ali I. (2008). Genotypic responses for yield and seed oil quality of two Brassica species under semi-arid environmental conditions. S. Afr. J. Bot. 74, 567–571. [ Links ]
49. Andow D.A. and Hilbeck A. (2004). Science-based risk assessment for non-target effects of transgenic crops. Bioscience 54, 637–649. [ Links ]
50. Andow D.A. (2005). Characterizing ecological risks of introductions and invasions. In Invasive Alien Species: a New Synthesis, ed. H.A. Mooney, pp. 84– 103. Island Press, Washington. [ Links ]
51. Jorgensen R.B. and Wilkinson M.J. (2005). Rare hybrids and methods for their detection. In Gene Flow, GM Plants, eds G.M. Poppy and M.J. Wilkinson, pp. 113–142. Blackwell Publishing, Oxford. [ Links ]
52. Guéritaine G., Bonavent J.F. and Darmency H. (2003). Variation of prezygotic barriers in the interspecific hybridization between oilseed rape and wild radish. Euphytica 130, 349–353. [ Links ]
53. Pallett D.W., Huang L., Cooper J.I. and Wang H. (2006). Within-population variation in hybridisation and transgene transfer between wild Brassica rapa and Brassica napus in the UK. Ann. Appl. Biol. 148, 147–155. [ Links ]
54. Toxopeus H. and Baas J. (2004). Brassica rapa L. [Internet] record from Protabase. PROTA (Plant Resources of Tropical Africa/Ressources végétales de l'Afrique tropicale), eds G.J.H. Grubben and O.A. Denton. Wageningen, Netherlands. Online at: http://database.prota.org/search.htm. [ Links ]
55. Ellstrand N.C. and Schierenbeck K.A. (2000). Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl Acad. Sci. U.S.A. 97, 7043–7050. [ Links ]
56. Hall L., Topinka K., Huffman J., Davis L. and Good. A. (2000). Pollen flow between herbicide-resistant Brassica napus is the cause of multiple-resistant B. napus volunteers. Weed Sci. 48, 688–694. [ Links ]
57. Smit J.J. and Cairns A.L.P. (2001). Resistance of Raphanus raphanistrum to chlorsulfuron in the Republic of South Africa. Weed Res. 41, 41–47. [ Links ]
58. Ball P.W., Heywood V.H. and Akeroyd J.R. (1993). Cruciferae. In Flora Europaea, 2nd edn, vol. 1, eds T.G. Tutin, N.A. Burges, A.O. Chater, J.R. Edmondson, V.H. Heywood, D.H. Moore, D.H. Valentine, S.M. Walters and D.A.Webb, pp. 313– 417. Cambridge University Press, Cambridge. [ Links ]
59. Scheffler J.A. and Dale P.J. (1994). Opportunities for gene transfer from transgenic oilseed rape (Brassica napus) to related species. Transgenic Res. 3, 263–278. [ Links ]
60. Bing D.J., Downey R.K. and Rakow G.F.M. (1996). Hybridizations among Brassica napus, B. rapa and B. juncea and their weedy relatives B. nigra and Sinapis arvensis under open pollination conditions in the fields. Plant Breed. 115, 470– 473. [ Links ]
61. Moyes C.L., Lilley J.M., Casais C.A., Cole S.G., Haeger P.D. and Dale P.J. (2002). Barriers to gene flow from oilseed rape (Brassica napus) into populations of Sinapis arvensis. Mol. Ecol. 11, 103–112. [ Links ]
62. Lelivelt C.L.C., Leunissen E.H.M., Frederiks H.J., Helsper J.P.F.G. and Krens F.A. (1993). Transfer of resistance to the beet cyst nematode (Heterodera Schachtii Schm.) from Sinapis alba L. (white mustard) to the Brassica napus L. gene pool by means of sexual and somatic hybridization. Theor. Appl. Genet. 85, 688–696. [ Links ]
63. Fahleson J., Lagercrantz U., Mouras A. and Glimelius K. (1997). Characterization of somatic hybrids between Brassica napus and Eruca sativa using species-specific repetitive sequences and genomic in situ hybridization. Plant Sci. 123, 133–142. [ Links ]
64. Janeja H.S., Banga S.S. and Lakshmikumaran M. (2003). Identification of AFLP markers linked to fertility restorer genes for tournefortii cytoplasmic male-sterility system in Brassica napus. Theor. Appl. Genet. 107, 148–154. [ Links ]
65. Liu Clarke J.H., Chèvre A.M., Landgren M. and Glimelius K. (1999). Characterization of sexual progenies of male-sterile somatic cybrids between Brassica napus and Brassica tournefortii. Theor. Appl. Genet. 99, 605–610. [ Links ]
66. Darmency H. and Fleury A. (2000). Mating system in Hirschfeldia incana and hybridization to oilseed rape. Weed Res. 40, 231–238. [ Links ]
67. Batra V., Prakash S. and Shivanna K.R. (1990). Intergeneric hybridization between Diplotaxis siifolia, a wild species and crop brassicas. Theor. Appl. Genet. 80, 537–541. [ Links ]
68. Zenkteler M. (1990). In-vitro fertilization of ovules of some species of Brassicaceae. Plant Breed. 105, 221–228. [ Links ]
69. Sikdar S.R., Chatterjee G., Das S. and Sen S.K. (1990). 'Erussica', the intergeneric fertile somatic hybrid developed through protoplast fusion between Eruca sativa Lam. and Brassica juncea (L.) Czern. Theor. Appl. Genet. 79, 561–567. [ Links ]
70. Bromilow C. (2001). In Problem Plants of South Africa: a Guide to the Identification and Control of More Than 300 Invasive Plants and Other Weeds. Briza Publications, Pretoria. [ Links ]
Received 9 February. Accepted 4 May 2009.
* Author for correspondence E-mail: melodiem@sanparks.org














