Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.105 no.3-4 Pretoria Mar./Abr. 2009
REVIEW ARTICLES
A review of soybean rust from a South African perspective
J. Antony Jarvie
PANNAR (Pty) Ltd, P.O.Box 19, Greytown 3250, South Africa. E-mail: antony.jarvie@pannar.co.za
ABSTRACT
This review article describes the nature of the soybean rust pathogen, its interaction with the soybean host and documents some of the history of soybean rust in South Africa. Soybean rust has affected soybean cropping in parts of South Africa since 2001. The disease causes leaf lesions, which may progress to premature defoliation and ultimately result in grain yield loss in susceptible soybean genotypes. Chemical control measures have been successfully employed to limit commercial yield losses in South Africa; however, controlling the effects of this disease through host-resistance or tolerance mechanisms remains a long-term goal.
Key words: Phakopsora pachyrhizi, Glycine max, chemical control, tolerance
Introduction
Soybean rust, caused by the fungus Phakopsora pachyrhizi Sydow, was reported on soybeans (Glycine max L. Merr) in the Vryheid district of South Africa in February 2001,1 and later identified in several other parts of KwaZulu-Natal (KZN) and Eastern Highveld production regions. Epidemics of soybean rust have since occurred in these areas every season to date (2008) and chemical control has become a standard commercial practice in the affected growing regions. Shortly after rust was identified in neighbouring Zimbabwe in 1998, a soybean rust workshop2 was convened in Potchefstroom, South Africa, and a soybean rust task team was established to familiarize local researchers with the disease and develop a pre-emptive national soybean rust strategy. Through visits to Zimbabwe in the three-year period between the first outbreak in Zimbabwe and the first reported outbreak in South Africa, many local researchers gained valuable experience in identifying the disease and managing the epidemics.3 Consequently, commercial losses in the first two seasons were far less than they could have been, as chemicals and protocols used in Zimbabwe were adopted until local research could support the soybean cropping industry.
The pathogen
There are approximately 80 species of Phakopsora known worldwide,4 six of which occur on legumes. Soybean rust is caused by two species, P. pachyrhizi and, less commonly, P. meibomiae (Arthur) Arthur. The latter species (P. meibomiae), commonly known as the cause of Latin American rust or Legume rust, is found in the western hemisphere and is not known to cause severe yield losses.5 The nomenclature history of these two species of rust is complex and their correct assignment in early reports, especially from Africa, remains uncertain.4 The subject of this review is restricted exclusively to P. pachyrhizi, the cause of the disease known commonly as Asian soybean rust, or simply soybean rust hereafter.
Global distribution
Before 1992, soybean rust was known to cause significant losses in Asia and Australasia, inclusive of the following countries: Australia, India, Indonesia, Japan, Korea, Peoples Republic of China, Philippines, Taiwan, Thailand, Vietnam.6 Not much was documented about the distribution of soybean rust in Africa before 1996 (given the problems with nomenclature); however, the following sequence of first reports7 were confirmed: Uganda, Kenya and Rwanda, 1996; Zimbabwe and Zambia, 1998; Nigeria, 1999; Mozambique, 2000; South Africa, 2001. During 2001 P. pachyrhizi was detected in Paraguay8 and this was followed shortly by confirmation of its presence in Argentina in 20029 and Brazil and Bolivia in 2003.10 Uruguay, also a significant soybean producing country, recorded soybean rust for the first time in 2004.11 Soybean rust was detected in Hawaii in 199412 which stimulated the convening of a workshop to discuss the potential threat that this held for the soybean crop in the U.S.A. As correctly predicted by the delegates of this workshop,13 soybean rust had the potential to threaten crops on mainland U.S.A. In 2004, nine years later, Schneider et al.14 confirmed the presence of soybean rust in the U.S.A. From detection in Louisiana in 2004, it spread to nine states by 2005, and was detected in 15 states in 2006.15
Alternative hosts
The soybean rust pathogen is known to naturally infect 95 species from 42 genera of legumes, inclusive of important weed species like Kudzu vine (Pueraria lobata) and major crop species such as common bean (Phaseolus vulgaris).5 Such a broad host range is unusual amongst rust pathogens5 which normally have a narrow host range. The significance of the numerous alternative host possibilities for the soybean rust pathogen is that these may serve as an inoculum reservoir or a 'green bridge' from one soybean planting season to the next.
Epidemiology of soybean rust
The presence of a susceptible host, viable pathogen spores and suitable environmental conditions are requisites for the development of a soybean rust epidemic. The optimum temperature for urediniospore germination ranges between 12 and 27°C, depending on the source of the research.16–18 Urediniospore germination is greater in darkness, with light either inhibiting or delaying germination.18 A further requirement for urediniospore germination is a period of leaf wetness. This period is considered to be about 6 h when this occurs within the optimal temperature range.19 The optimum temperature for uredinia formation is reported by Kochman20 to be 17°C (night) or 27°C (day). Uredinia form on the leaves nine days post infection (DPI) under these conditions, with the urediniospores maturing two to three days later.21
Symptoms of soybean rust
First symptoms of soybean rust could be described as small water soaked lesions which develop into grey, tan to dark brown, or reddish brown lesions (uredinia) particularly on the abaxial leaf surface.22 The colour of the lesion is dependent on lesion age and interaction with the host genotype.6 Red-brown (RB) lesions with little sporulation indicates a semi-compatible reaction, whereas tan lesions with much sporulation (Fig. 1) indicates a fully compatible reaction. During the early stages of development, before sporulation, soybean rust may be confused with bacterial pustule disease [Xanthomonas campestris pv glycines (Nakano) Dye].22 Soybean rust symptoms generally occur first on the leaves at the base of the plant and progress up the canopy as the disease severity increases. Increased lesion density leads to leaf yellowing and ultimately premature leaf senescence, resulting in yield losses primarily through reduced grain size.23

Effect of soybean rust on yield
There is a dearth of published information on the effects of soybean rust on soybean yields in South Africa. Researchers that have published data relating to the effects of soybean rust on yield have recorded considerable variability over seasons and genotypes.24,25 McLaren25 evaluated all the commercial soybean genotypes over two seasons and concluded that there was no tolerance of economic value amongst them. He also observed that the yield loss sustained in shorter maturity genotypes was lower than the longer maturity genotypes. This observation confirmed the earlier work of Caldwell and McLaren24 who had come to a similar conclusion but had conducted their research on only one genotype per maturity class, leaving some doubt as to whether the effect was genotype specific or maturity-group related.
Initial indications from the research of Caldwell and McLaren24 showed that planting date did influence the yield loss, but their two seasons' data were not sufficient to substantiate a trend. Soybean rust symptoms were more severe in the 0.45 m than in 0.90 m row spacing, and this was attributed to poorer fungicide penetration into the canopy.24 McLaren25 found that disease severity, as measured by the area under the disease progress curve (AUDPC), was poorly correlated to yield loss percentage. Mean yield loss for 2003/04 season was 31.1% or 1.68 t ha–1 and in 2004/05 season it was a devastating 60.9% or 3.4 t ha–1. Genotype ranking for yield loss percentage between the two seasons was substantially different, highlighting the considerable variability of soybean rust epidemics over seasons and the difficulty in selecting for improved genotypic response.
Distribution and spread of soybean rust in South Africa
There has not yet been a formal attempt to survey the distribution of soybean rust in South Africa; however, the reports of positive identification of soybean rust sent in by members of the soybean rust task team have been collated for the period 2001–2008 (Table 1). The reports increased in frequency over the years surveyed, likely as a result of more scientists becoming involved in reporting rather than an increase in disease incidence. The distribution of locations with one or more soybean rust reports have been plotted on a rainfall map of South Africa (Fig. 2). The area with the highest incidence of soybean rust reports coincides with the high rainfall region east of the Drakensberg mountain range. Del Ponte et al.26 showed that cumulative rainfall in the period after initial rust detection was positively correlated to disease severity, which probably accounts for the similarity in the rainfall and soybean rust distribution patterns. During the 2006 season, reports of soybean rust were obtained atypically far west of the normal distribution, but mostly too late in the season (Table 1) to have a significant impact on yield.
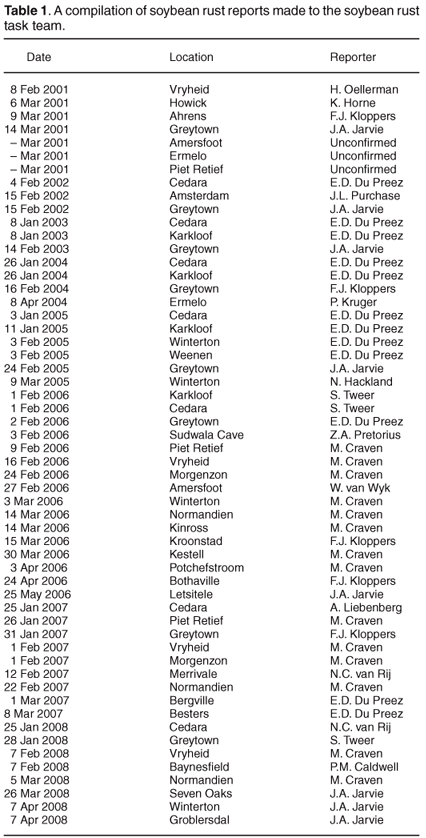
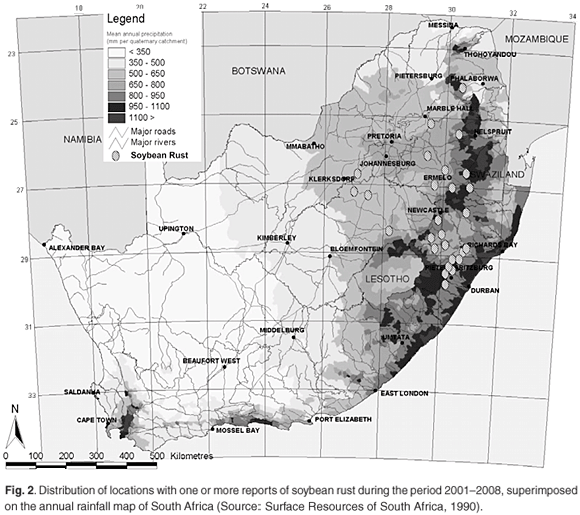
The collated reports are probably not ideally suited to making judgements on the progression of the disease, because the date of the report is not always a good indication of the start of the epidemic. However, in seasons that had sufficient reports to substantiate a trend (2006–2008), first reports for the season generally started in the east and progressed westward. While this may indicate a closer proximity to the inoculum source in the east of the production region, weather conditions favouring infection and development of symptoms may simply occur earlier in the season in the east compared to the west.
There is no literature on how the soybean rust pathogen survives from one season to the next in South Africa; however, Caldwell and McLaren24 established that it required a live host and did not survive on soybean stubble. Since most of the soybean production regions receive significant frosts in winter, the pathogen is presumed to overwinter in frost-free areas within the country. Soybean rust epidemics in the KZN midlands normally originate from a few clearly distinguishable foci within a field, which would infer that initial infections have been started by a low concentration of windborne urediniospores. Infections that have resulted from urediniospores generated from within these foci, are a lot more uniform, clearly a function of inoculum concentration around these foci.
Pivonia and Yang27 used a mathematical model to predict the likelihood of year-round survival of P. pachyrhizi across the world based only on historical temperature and moisture data. Host availability and presence of an inoculum source were not considered. They found that conditions for the survival of P. pachyrhizi were very favourable all along the east and southern coasts of South Africa. Since this area does not coincide with the soybean production area, it is likely then that the soybean rust pathogen survives the winter in this area on the many possible alternative hosts. Pretorius et al.28 established that Kudzu vine (Pueraria lobata) was one of the alternate hosts of P. pachyrhizi that provided a green bridge in South Africa for the survival of the pathogen through winter in the frost-free areas. It is speculated that this frost-free area then provides the initial inoculum source each season for the inland areas that have summer conditions favourable for the development of soybean rust. The consistency with which the epidemics have occurred since 2001 (Table 1) would tend to support the postulation that the source of urediniospores is, at the very least, regional and that local epidemics are not reliant on major weather phenomena for the deposition of urediniospores from the tropics of Africa.
Chemical control
Emergency registration of a number of chemicals made it possible for farmers to control epidemics during the first two seasons that soybean rust affected production in South Africa.29 Much debate in South African soybean workgroups revolved around the difference in rates used in Zimbabwe compared to the recommended chemical rates in South Africa. The fear existed that sub-optimal doses of chemical would promote the build up of pathogen resistance to the active ingredients that controlled soybean rust. With pathogen diversity and variability clearly demonstrated in host-pathogen relationships,5 this was a valid concern. Du Preez and Caldwell29 evaluated effective dosage rates, timing of application and frequency of applications. This research contributed towards a leaflet being published30 that made recommendations to soybean producers regarding control of soybean rust and included the registered chemicals. Du Preez and Caldwell29 established that effective chemical control varied in a time range from 10 d (triforine) to 19 d (flusilazole/carbendazim), which supported the generalization that spray intervals should be no longer than 21 d apart, and that between one and three sprays may be required. They also concluded that some chemicals (flusilazole/carbendazim) had limited curative action, whereas others (azoxystrobin) were only effective in preventative applications. This conclusion was very important to the national strategy used to control rust. If control was primarily preventative, then the timing of fungicide applications in the absence of symptoms would be crucial, a conclusion that was also reached by several other researchers.31 A reliable indicator of first spray was required, since spraying too early would mean unnecessary additional sprays, and spraying at first symptom would result in yield losses. As part of the national strategy to control soybean rust in South Africa, a series of 10 soybean indicator plots were planted throughout the production region, using early planting dates and genotypes which represented the extremes of maturity range for the country. These plots were not sprayed with fungicide and were monitored on a weekly basis from January through to April32 for the presence of rust, both in situ and via leaf samples in the laboratory. These plots were used as sentinel plots to give producers advance warning of the presence and severity of the disease in an area. Producers were notified of the first presence of soybean rust in their area via cell phone SMS or alerts on farm radio programmes.32 The system of sentinel crops is currently also one of the methods being applied in the U.S.A.33 for the advance warning of the presence of the disease. Systems that recommend spraying at predetermined soybean growth stages, for example at flower or at 60 days after planting (dap) as in Zimbabwe,34 do not take into consideration that the timing and severity of epidemics may have considerable seasonal variation. This could result in unnecessary spraying in some seasons. Hartman,15 however, reported that there were occasional yield benefits to spraying fungicides in the absence of rust which may make this system both cost effective and simple to apply.
In 2005, a report from Washington State University35 claimed that Roundup herbicide (glyphosate) had been found to have fungicidal action on P. pachyrhizi under laboratory conditions. Owing to the popularity of Roundup Ready (RR) soybean genotypes in South Africa, Kloppers and Jarvie (unpubl. data) performed a pilot study with sequential sprays of Roundup on an experimental RR genotype to establish whether there was a need to pursue this avenue of research further. The preliminary results showed that pre-flower applications of Roundup had no effect on soybean rust severity, but post-flower applications visibly reduced the premature defoliation due to rust. Since Roundup, when used as a herbicide, is primarily applied to soybeans at a pre-flower stage, it was felt that these findings would have little practical applicability and this line of research was not pursued further. The results of this pilot study were later confirmed by independent research conducted in the U.S.A. by Jurick and co-workers.36 In their study, control of soybean rust by applications of Roundup at the R2 and R4 stage significantly improved yield over the untreated control, but the yield benefit and control of the disease was inferior to that of conventional fungicide (azoxystrobin) applications.
Resistance
Screening for resistance
From the early 1960s through to the 1990s, much of the soybean rust research focused on resistance. Tschanz37 reported that he and his co-workers at the AVRDC (Asian Vegetable Research and Development Centre) had, over the years, screened more than 9000 accessions for resistance to soybean rust. Hartwig38 reported to have evaluated 1675 germplasm lines adapted to the southern U.S.A. for resistance to soybean rust in Taiwan. From this early screening work, it was clear that various levels of specific resistance, partial resistance and tolerance to soybean rust all occurred in soybean germplasm. One of the recent objectives of the USDA-ARS soybean rust research programme has been to evaluate the USDA germplasm collection for resistance. A set of 174 soybean genotypes, inclusive of the most important parental germplasm and the most promising sources of resistance, were screened against field populations of P. pachyrhizi in Brazil, China, Paraguay and Thailand.39 South Africa also participated in this evaluation, where soybean rust symptoms on this set of germplasm were recorded in the 2002/03 and 2003/04 seasons at Greytown, KZN. No lines were found to be resistant at all locations. With the threat of soybean rust looming in the U.S.A. at that time, the search for resistance intensified further, eventually involving the screening of 16 595 accessions in the Fort Detrick containment facility.15
Under field conditions, early maturing soybean genotypes will have a higher disease rating earlier in the season than the equivalent later maturing genotype. The rate of rust development in these genotypes is also higher than that of later maturing genotypes, and if a correction for host maturity is not made, erroneous conclusions from field data will result.40 To correct for maturity, relative life time (RLT) is calculated as the proportion of the life cycle completed relative to the complete life time (time from planting to harvest) of the genotype. Only rust severity ratings at comparable RLTs can be compared, which makes a single simple field severity rating meaningless unless all genotypes are of a similar maturity. McLaren25 showed that disease severity, as measured by the area under the disease progress curve (AUDPC), was poorly correlated with yield loss. For this reason, disease severity ratings are seldom used as a measure of resistance.
Specific resistance in soybean
McLean and Byth41 presented the first evidence of physiological races in P. pachyrhizi on soybean genotypes in Australia. Race 1 was virulent on Wills and avirulent on PI 200492. Race 2 was virulent on both varieties. Subsequent to this, considerable variation in isolate virulence (collected from the same field, as well as isolates from geographically-distant regions) has been shown to occur.5 Three infection types have been described: the Tan lesion is a fully susceptible reaction; the resistant RB reaction is a red-brown lesion with no or few sporulating uredinia; and the absence of any macroscopic symptoms is immunity.6 Eleven genotypes were used as a differential set to determine the physiological races of 42 purified P. pachyrhizi isolates by Wang and Hartman,6 and based on the infection type they were able to identify nine races. The data suggested that the pathogen races studied were complex and that they possessed multiple virulence genes for compatibility on many of the differential cultivars. Bromfield42 reported on a P. pachyrhizi race that had three virulence genes, more than were necessary to overcome host resistance. More recent research5 indicates that field pathogen populations are often mixtures of many races which may induce mixed infection types in the host. This is not uncommon in rust pathogens, as was shown to be the case with bean rust (Uromyces appendiculatus) where the more tropical locations (like South Africa) were found to induce greater race variability than more temperate climates.43 It is not known how many races are commonly found in South African soybean fields, but since mixed infection types on the same plant have been observed, at least two races must be present. Variability in race virulence is also known to occur. In inoculation studies conducted under controlled conditions, researchers reported that recent isolates collected from southern Africa and South America were significantly more virulent than Asian isolates collected in the 1970s.44 The most virulent isolate they reported was collected in Zimbabwe.
The specific resistance gene in PI 200492 was given the designation Rpp1,45 and since then three other independent dominant genes have been named: Rpp2;46 Rpp3;47 Rpp4.48 In Brazil, where the Rpp1 and Rpp3 genes are ineffective and Rpp2 and Rpp4 currently confer resistance, Neto49 reported that many 'new' (unnamed) gene sources of resistance have been discovered. These were tested for allelism to Rpp2 and Rpp4, and of the 26 sources reported, 23 were found to be at different loci to Rpp 2 and Rpp4. One of these sources of resistance was conditioned by a single recessive gene49 from the variety Abura, and this has been incorporated in a variety (BR01-18437) destined for release in Brazil during 2008. Neto49 also reported the preliminary findings that stacking Rpp2 and Rpp4 in a single genotype had no additive advantage in the expression of resistance.
The presence of multiple virulence genes in the pathogen population and the lack of multiple resistance genes in the host provides the soybean rust pathogen with a competitive advantage. The deployment of specific single genes for resistance is thus unlikely to be a successful strategy. As an example of gene failure, Hartman et al.5 quoted the examples cited by Bromfield, where the Rpp1, Rpp2 and Rpp3 lost their effectiveness in the field within 10 years of exposure. In Taiwan, Shanmugasudaram et al.50 quoted examples of Tainung 3, Tainung 4 and Kaohsiung 3 (all cultivars containing Rpp1) becoming susceptible within a few years of release. Genotypes PI 230970 and PI 230971 were identified as being resistant in Taiwan, and these were subsequently used as parents in crosses to generate a number of resistant lines (AGS 181, AGS 182, AGS 183, AGS 229, AGS 233, AGS 240, AGS 244, AGS 247). So too were the resistances of these lines short lived. Following that, new sources of resistance were identified in PI 459024, PI 459025 (Rpp4) and PI 339871 (G. soja) but have all since been defeated.5,50 In Brazil, Yorinori10 had a similar experience with germplasm that had shown resistance in 2002 being susceptible in 2003.
The use of gene pyramiding and gene rotation is also unlikely to be a stable solution because the pathogen retains unnecessary virulence genes at a high frequency in its population.51 In addition, resistance associated with the RB infection type is a semi-compatible host–pathogen reaction, which generally allows pathogen reproduction and has not been shown to significantly affect epidemic development.51
Partial resistance
Partial or rate-reducing resistance to soybean rust has been documented in soybean,51 but it has not been widely employed because of complexities in assessment. Plants or genotypes maturing at different times cannot be compared to each other in the field because of the different environmental conditions that they are exposed to at similar growth stages. Physiological differences can be partially corrected for by regressing relative life time (RLT) on the log transformation of rust severity. The slopes of these graphs can be compared to identify the 'slow rusting' genotypes. Collecting the data required to generate these graphs is laborious and cannot be conducted on a large number of genotypes, limiting its practical application. Hartman et al.5 suggested that measuring the latent period would help identify genotypes with a long latent period and hence a slower rate of rust development. The difficulties associated with identifying partial resistance and the ineffectiveness of specific resistance genes has led to the suggested use of tolerance as a breeding remedy for soybean rust.
Tolerance
Tolerance implies susceptibility, and can be defined as the relative ability of a genotype to yield under stress from rust.6 Tolerance is a characteristic that can only be evaluated in the target environment while under rust stress, as it implies a measure of genotypic adaptation to that environment. Tolerance is of little value unless the genotype is high yielding in that environment and it maintains yield stability despite rust infections. Selecting for yield stability in the presence of rust is not an easy task5 since over and above the normal genotype × environment interaction that breeders have to contend with for adaptation, seasonal variation in severity and timing of rust epidemics is superimposed. Whilst yield is normally the primary consideration, a consistent performance is also valuable to a producer, who may be willing to sacrifice some yield in order to achieve a stable yield over seasons.52 Tolerance is traditionally assessed by comparing yields of paired plots of fungicide protected versus unprotected plots. The percentage yield loss between fungicide protected and unprotected plots is not necessarily correlated to rust susceptibility ratings or to rust development rates5 and may be linked to other stress-tolerance mechanisms. Significant variation in tolerance levels exist in soybean, which could be exploited by breeders. From work conducted at the AVRDC in Taiwan, Hartman40 demonstrated yield losses of 12 genotypes ranging between 29 to 85%. Based on reduced pustule numbers, the two genotypes that had the smallest yield losses (29% and 31%) could conceivably have had some form of partial resistance. This, when compared to a possible 85%, appears to be significant but in reality is still far too high for practical benefit on a commercial scale. In more recent research conducted in Brazil,49 minor genes have contributed towards tolerance in the genotype EMGOPA 313, with yield losses in the order of magnitude where fungicide spraying would still be financially attractive.
Conclusion
High levels of tolerance or sustainable rust resistance in South African genotypes is not imminent, which means that for the foreseeable future control of soybean rust by a combination of chemical and cultural means will need to continue. An efficient warning system and effective fungicides have been instrumental in averting potentially large financial losses to producers. Whilst seasonal soybean rust epidemics will persist and control measures will continue to be required, the soybean rust crisis in South African soybean production is largely over as a result of the efforts of forward-thinking policy-makers and pro-active researchers.
I thank Paula Kruger, Maryke Craven, Rikus Kloppers for their contribution to the collection of data and gratefully acknowledge editorial assistance from Eve Dunlop and Paul Shanahan.
1. Pretorius Z.A., Kloppers F.J. and Frederick R.D. (2001). First report of soybean rust in South Africa. Plant Dis. 85, 1288. [ Links ]
2. Smit M.A. (1998). Proceedings of Workshop on Soybean Rust (Phakopsora pachyrhizi), October 1998. ARC Summer Grains Centre, Potchefstroom. [ Links ]
3. Smit M.A. (1999). Phakopsora pachyrhizi in Afrika: Verslag van Vergadering te Harare 9 Maart, 1999. ARC Summer Grains Centre, Potchefstroom. [ Links ]
4. Hennen J.F. (1996). The taxonomy of soybean rusts. In Proceedings of the Soybean Rust Workshop, 9–11 August 1995, eds J.B. Sinclair and G.L. Hartman, pp. 29–32. College of Agricultural, Consumer and Environmental Sciences, National Research Laboratory, Urbana, Illinois. [ Links ]
5. Hartman G.L., Miles M.R. and Frederick R.D. (2005). Historical viewpoint and soybean resistance to soybean rust. In Proceedings of the 2005 Illinois Crop Protection Conference, pp. 16–20. Available Online at: www.ipm.uiuc.edu/education/proceedings/index.html [ Links ]
6. Wang T.C. and Hartman G.L. (1992). Epidemiology of soybean rust and breeding for host resistance. Plant Prot. Bull. 34, 109–124. [ Links ]
7. Levy C. (2003). Measures to control soybean rust in southern Africa and initial investigation of the meteorological factors that favour its development. Phytopathology 93, 103. [ Links ]
8. Morel W., Scheid N., Amarilla V. and Cubilla L.E. (2004). Soybean rust in Paraguay, evolution in the last three years. In Proceedings of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, pp. 361–364. Embrapa, Londrina. [ Links ]
9. Rossi R.L. (2004). Current status of soybean production and utilization in Argentina. In Proceedings of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, pp. 38–49. Embrapa, Londrina. [ Links ]
10. Yorinori J.T. (2004). Country report and rust control strategies. In Proceedings of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, pp. 447–455. Embrapa, Londrina. [ Links ]
11. Stewart S., Guillin E.A and Díaz I. (2005). First report of soybean rust caused by Phakopsora pachyrhizi in Uruguay. Plant Dis. 89, 909. [ Links ]
12. Killgore E.M. and Heu R. (1994). First report of soybean rust in Hawaii. Plant Dis. 78, 1216. [ Links ]
13. Sinclair J.B. and Hartman G.L. (1996). Potential threat to U.S. continental soybeans. In Proceedings of the Soybean Rust Workshop, 9–11 August 1995, eds J.B. Sinclair and G.L. Hartman, p.6. College of Agricultural, Consumer and Environmental Sciences, National Research Laboratory, Urbana, Illinois. [ Links ]
14. Schneider R.W., Hollier C.A., Whitam H.K., Palm M.E., McKemy J.M., Hernández J.R., Levy L. and deVries-Paterson R. (2005). First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Dis. 89, 774. [ Links ]
15. Hartman G.L. (2007). Soybean rust: The first three years. Proceedings of the 2007 Illinois Crop Protection Conference, pp. 21–25. Online at: www.ipm.uiuc.edu/ education/proceedings/index.html [ Links ]
16. Keogh R.C. (1974). Studies on Phakopsora pachyrhizi Syd.: The causal agent of soybean rust. M.Sc. thesis, University of Sydney, Sydney, Australia. [ Links ]
17. Melching J.S. and Bromfield K.R. (1975). Factors influencing spore germination and infection by Phakopsora pachyrhizi and intensification and spread of soybean rust under controlled conditions. (Abstr) Proc. Am. Phytopathol. Soc. 2, 125. [ Links ]
18. Marchetti M.A., Melching J.S. and Bromfield K.R. (1976). Effects of temperature and due period on germination and infection by uredospores of Phakopsora pachyrhizi. Phytopathology 66, 461–463. [ Links ]
19. Tschanz A.T. and Shanmugasundaram S. (1984). Soybean rust. World Soybean Research Conference III 1984. Iowa State University, Iowa. [ Links ]
20. Kochman J.K. (1979). The effect of temperature on the development of soybean rust (Phakopsora pachyrhizi). Aust. J. Agric. Res. 30, 273–277. [ Links ]
21. Shanmugasundaram S. (1999). Epidemiology and control strategies for soybean rust – A knowledge base for Africa. In Proceedings of Workshop on Soybean Rust (Phakopsora pachyrhizi), ed. M.A. Smit, pp. 4–27. ARC Summer Grains Centre, Potchefstroom. [ Links ]
22. Sinclair J.B. and Hartman G.L. (1999). Soybean rust. In Compendium of Soybean Diseases, 4th edn., eds G.L. Hartman, J.B. Sinclair, and J.C. Rupe, pp. 25–26. American Phytopathological Society, St Paul, Minnesota. [ Links ]
23. Tichagwa J.S. (2004). Breeding for resistance to soybean rust in Zimbabwe. In Proceedings of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, pp. 349–353. Embrapa, Londrina. [ Links ]
24. Caldwell P.M. and McLaren N.W. (2004). Soybean rust research in South Africa. In Proceedings of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, pp. 354–360. Embrapa, Londrina. [ Links ]
25. McLaren N.W. (2008). Reaction of soybean cultivars to rust caused by Phakopsora pachyrhizi. S. Afr. J. Plant Soil 25, 49–54. [ Links ]
26. Del Ponte E.M., Godoy C.V., Li X. and Yang X.B. (2006). Predicting severity of Asian soybean rust epidemics with empirical rainfall data. Phytopathology 96, 797–803. [ Links ]
27. Pivonia S. and Yang X.B. (2004). Assessment of the potential year-round establishment of soybean rust throughout the world. Plant Dis. 88, 523–529. [ Links ]
28. Pretorius Z.A., Visser B. and du Preez P.J. (2007). First report of Asian soybean rust caused by Phakopsora pachyrhizi on Kudzu in South Africa. Plant Dis. 91, 1364. [ Links ]
29. Du Preez E.D. and Caldwell P.M. (2004). Chemical control of soybean rust (Phakopsora pachyrhizi Syd.) in South Africa. In Proceedings of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, pp. 431– 435. Embrapa, Londrina. [ Links ]
30. Pretorius Z.A. and McLaren N.W. (2006). Identification and control of soybean rust/Identifikasie en beheer van sojaboonroes. Pamphlet, ARC Summer Grains Centre, Potchefstroom. [ Links ]
31. Miles M.R., Hartman G.L., Levy C. and Morel W. (2003). Current status of soybean rust control by fungicides. Pesticide Outlook, October 2003, 197–200. [ Links ]
32. Craven M. (2008). Monitering help om sojaboonroes te bekamp. SA Graan 10, 46–47. [ Links ]
33. Schonyers L.E., Kemerait R.C., Brock J., Phillips D.V., Jost P.H., Sikora E.J., Gutierrez-Estrada A., Mueller J.D., Marois J.J., Wright D.L. and Harmon C.L. (2006). Asian soybean rust in 2005: a perspective of the southeastern United States. APSnet January 2006 feature, American Phytopathological Society, St Paul, Minnesota. Available Online from: www.apsnet.org/online/feature/sbr/ default.asp [ Links ]
34. Levy C. (2004). Zimbabwe – a country report on soybean rust control. In Proceedings of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, pp. 340–348. Embrapa, Londrina. [ Links ]
35. Feng P.C.C., Baley G.J., Clinton W.P., Bunkers G.J., Alibhai M.F., Paulitz T.C. and Kidwell K.K. (2005). Glyphosate inhibits rust diseases in glyphosate-resistant wheat and soybean. Proc. Natl. Acad. Sci. 102, 17290–17295. [ Links ]
36. Jurick W.M., Nequi N., Semer C.R, Mueller T., Marois J.J., Wright D.L., Harmon C.L., Gevens A.J. and Harmon P.F. (2007). The effect of Roundup on Asian soybean rust pathogen (Phakopsora pachyrhizi) in the field and in vitro. Proceedings of the 2007 National Soybean Rust Symposium, 12–14 December 2007, Louisville, Kentucky. [ Links ]
37. Tschanz A.T., Wang T.C. and Tsai B.Y. (1983). Recent advances in soybean rust research. International Symposium on Soybean in Tropical and Sub-tropical Cropping Systems, September 1983, Tsukuba, Japan. [ Links ]
38. Hartwig E.E. (1996). Resistance to soybean rust. In Proceedings of the Soybean Rust Workshop, 9–11 August 1995, eds J.B. Sinclair and G.L. Hartman, pp. 65–66. College of Agricultural, Consumer and Environmental Sciences, National Research Laboratory, Urbana, Illinois. [ Links ]
39. Miles M.R., Morel W., Yorinori J.T., Ma Z.H., Poonpogul S., Hartman G.L. and Frederick R.D. (2004). Preliminary report of Asian rust reaction on soybean accessions planted in Brazil, China, Paraguay and Thailand with seedling reactions from glasshouse screens in the United States. In Abstracts and Contributed Papers of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, poster P198. Embrapa, Londrina. [ Links ]
40. Hartman G.L. (1996). Highlights of soybean rust research at the Asian Vegetable Research and Development Centre. In Proceedings of the Soybean Rust Workshop, 9–11 August 1995, eds J.B. Sinclair and G.L. Hartman, pp. 19–28. College of Agricultural, Consumer and Environmental Sciences, National Research Laboratory, Urbana, Illinois. [ Links ]
41. McLean R.J. and Byth D.E. (1980). Inheritance of resistance to rust (Phakopsora pachyrhizi) in soybeans. Austr. J. Agric. Res. 31, 951–956. [ Links ]
42. Bromfield K.R. (1984). Soybean rust. American Phytopathological Society, St Paul, Minnesota. [ Links ]
43. Jochua C., Amane M.I.V., Steadman J.R., Xue X. and Eskridge K.M. (2008). Virulence diversity of the common bean rust pathogen within and among individual bean fields and development of sampling strategies. Plant Dis. 92, 401–408. [ Links ]
44. Hartman G.L., Bonde M.R., Miles M.R. and Frederick R.D. (2004). Variation of Phakopsora pachyrhizi isolates on soybean. In Proceedings of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, pp. 440–446. Embrapa, Londrina. [ Links ]
45. Bromfield K.R. and Hartwig E.E. (1980). Resistance to soybean rust and mode of inheritance. Crop Sci. 20, 254–255. [ Links ]
46. Bromfield K.R., Melching J.S. and Kingsolver C.H. (1980). Virulence and aggressiveness of Phakopsora pachyrhizi isolates causing soybean rust. Phytopathology 70, 17–21. [ Links ]
47. Hartwig E.E. and Bromfield K.R. (1983). Relationships among three genes conferring specific resistance to rust in soybeans. Crop Sci. 23, 237–239. [ Links ]
48. Hartwig E.E. (1986). Identification of a fourth major gene conferring resistance to soybean rust. Crop Sci. 26, 1135–1136. [ Links ]
49. Neto A. L. de F. (2007). Breeding for soybean rust resistance in Brazil. Proceedings of the 2007 National Soybean Rust Symposium, 12–14 December 2007, Louisville, Kentucky. [ Links ]
50. Shanmugasundaram S., Yan M.R. and Wang T.C. (2004). Soybean rust in Taiwan. In Proceedings of the VII World Soybean Research Conference, eds F. Moscardi, C.B Hoffmann-Campo, O.F. Saraiva, P.R. Galerani, F.C. Krzyzanowski and M.C. Carrão-Panizzi, pp. 365–368. Embrapa, Londrina. [ Links ]
51. Tschanz A.T. (1987). Soybean Rust Epidemiology – Final Report. Asian Vegetable Research and Development Center, Shanhua, Taiwan. [ Links ]
52. Kang M.S. (2002). Genotype-environment interaction: progress and prospects. In Quantitative Genetics, Genomics and Plant Breeding, ed. M.S. Kang, pp. 221–243. CABI publishing, Wallingford. [ Links ]
Received 18 September 2008. Accepted 4 February 2009.














