Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.104 no.11-12 Pretoria Nov./Dez. 2008
RESEARCH LETTERS
Enhanced stabilization of collagen-based dermal regeneration scaffolds through the combination of physical and chemical crosslinking
Q.B. Wessels; E. Pretorius
Department of Anatomy, Faculty of Health Sciences, University of Pretoria, P.O. Box 2034, Pretoria 0001, South Africa
ABSTRACT
The use of collagen in the biomedical device industry has led to major advances in soft tissue repair. This is attributed largely to the favourable biological and physiochemical properties of collagen. Regenerative medicine and tissue engineering favoured the use of this biomaterial and various commercial products have become available in the past few decades. This study aims to develop a collagen and chondroitin-6-sulphate dermal regeneration scaffold with enhanced resistance against enzymatic degradation. Frozen slurries (0.5% collagen) were dried under vacuum, coated with silicone, crosslinked and then thoroughly rinsed. The scaffolds were subjected to a range of quantitative and qualitative tests that included: scanning electron microscopy analysis, collagenase enzymatic degradation, and cytotoxicity assessment. Scaffold resistance to enzymatic degradation was manipulated after dehydrothermal treatment by employing combinations of crosslinking agents, such as glutaraldehyde and/or carbodiimide, with or without the presence of L-lysine. Results indicate that highly porous (mean pore diameter of 87.3 µm), bioactive, non-cytotoxic tissue engineering matrices were obtained. Enhanced stability of these scaffolds was achieved through extensive crosslinking and suggests the potential to prevent in vivo wound contraction sufficiently.
Introduction
Reparative medicine employs current technology and various materials in its aim to replace, repair or enhance tissue and organs. The clinical need to address injuries, birth defects and degeneration beyond the scope of a single scientific discipline has become the driving force behind reparative medicine. Several strategies are employed in reparative medicine and include: substitution of one body part with another; the use of synthetic materials and devices; the application of allografts or xenografts; the use of an external device to augment or replace a non-functioning organ; and the allocation of living cells. The last strategy is known as tissue engineering and serves to repair, preserve, or improve the function of tissues and organs through the synergy of molecular biology (including biopolymer chemistry) and engineering.1
The need for advanced wound care products is motivated by the pathophysiology of severe dermal injuries. Adult dermal regeneration does not occur spontaneously.2–5 Unassisted wound closure of deep dermal injuries results in excessive scarring and wound contraction.5,6 This in return impairs or inhibits the mobility of the affected area. The representative epidermal and dermal components of engineered dermal regeneration matrices, such as Integra®, are known to enhance wound healing and closure of deep dermal injuries.7 The stable extracellular matrix is placed on the excised wound bed and is overgrafted after a few weeks. A very thin skin graft is used from an appropriate donor site such as the posterior aspect of the thigh. This method has been shown to resist the recurrence of scarring, compared to the use of traditional skin grafts.8 In so doing, skin substitutes, in general, not only save lives, as in the case of full-thickness burn wounds, but also improve the quality of life of those affected.
The efficiency of tissue engineering scaffolds rests upon a few essential features. They include biocompatibility, controlled biodegradability, low or non-antigenicity, and a suitable microstructure or architecture. The scaffold architecture in turn relies on three variables, namely, the pore size, pore structure, and the surface area of the scaffold. These variables must be manipulated in order to yield a suitable homogeneous three-dimensional lattice that will allow cellular phenotype, growth, migration and adhesion.9 This has been confirmed, based on studies conducted on endothelial cells, fibroblasts, osteoblasts, vascular smooth muscle cells, rat marrow cells, chondrocytes, preadipocytes, and adipocytes.10–17
The in vivo and in vitro rate of degradation of collagen porous scaffolds has become the most critical design consideration in research and for clinical applications.18 Research has shown that scaffolds with enhanced resistance towards enzymatic degradation prevent wound contraction adequately and promote tissue regeneration.19,20 This in turn will ensure minimal impairment or inhibition of the mobility of the affected area. The rate of scaffold degradation depends largely on the method of crosslinking used. Crosslinking of collagen-derived medical devices is achieved through two established methods, namely, chemical crosslinking and physical crosslinking.21 Chemical crosslinking is the most popular22 and most thoroughly studied of these methods.
The study reported here aims to develop a dermal regeneration scaffold composed of a silicone-based artificial epidermis and a collagen-GAG (glycosaminoglycan) dermis for cutaneous tissue engineering applications. We also aim to enhance the stabilization of collagen-based scaffolds through the unique combination of physical crosslinking and various chemical crosslinkers.
Materials and methods
Collagen-GAG scaffold preparation
Bovine Achilles tendon atelocollagen, obtained from Southern Cryoscience (Pty) Ltd, was used to prepare the scaffolds. Atelocollagen is collagen extracted by using proteolytic enzymes, such as pepsin, which retains the intact tertiary super-helical structure of collagen, free from telopeptides. The non-helical terminal telopeptides are regarded to be the predominant antigenic determinant of this biomaterial.23,24
Bovine type I atelocollagen suspensions (0.56%) were prepared in 0.05 M aqueous acetic acid according to the methods of Yannas and colleagues.9,25–27 Chondroitin-6-sulphate in 0.05 M aqueous acetic acid was added to each of the prepared collagen suspensions to give a final collagen: GAG mass ratio of 92:8. The final solutions, with constituent collagen concentrations of 0.5%, were blended for an additional 90 min to form a homogeneous co-precipitate. The co-precipitates were subjected to either controlled or uncontrolled (also known as conventional quenching) freezing.9,28 The frozen slurries were dried under vacuum for 17 hours.
The silicone epidermal portion, aimed at temporary restoration of barrier function of each of the scaffolds, was prepared as follows. Biomedical grade silicone rubber (Q7-4840 Silastic®, Dow Corning) was used according to the manufacturer's specifications to form a film with an approximate thickness of 0.5 mm on an acetate-based backing. The surface of the collagen sponge that was in contact with the atmosphere was placed onto the wet, unpolymerized silicone. The selected silicone thickness not only ensured adequate bonding with the collagen but also pliability. The acetate backing ensured easy removal after the silicone had polymerized. Polymerization was induced at 105°C for 30 min as directed by the manufacturer. The dried scaffolds were crosslinked and examined as described below.
Scanning electron microscopy and pore analysis
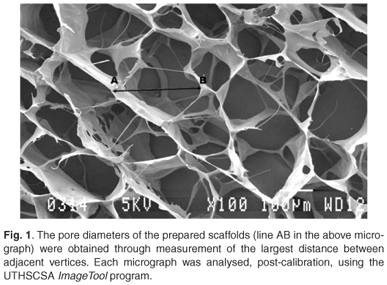
The uncrosslinked dry scaffolds were prepared by fixation with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 90 min. Post-fixation was done in a 0.5% aqueous osmium tetroxide solution for 60 min. Critical point CO2 freezing followed, in order to dry the samples, after which they were coated with gold. The samples were viewed and photographed using a JEOL (JSM-840) scanning microscope.28,29 Each sample was examined for the presence of the following: collagen fibre aggregates or strands, collagen sheets, and polygonal shapes of open pores.28 The pore diameters were determined through measurement of the largest distance between adjacent vertices (Fig. 1). These diameters were measured after calibration, and analysed using the UTHSCSA ImageTool program (developed at the University of Texas Health Science Center at San Antonio, Texas, and available from the Internet by anonymous ftp from maxrad6uthscsa.edu)
Crosslinking
Initial crosslinking of the scaffolds was achieved through dehydrothermal treatment (DHT) at 105°C and c. 0.2 mbar for 24 h.30,31 The formation of crosslinking bridges was induced by the addition of 2.5 µM L-lysine in solution at a pH of 5.5.31 Scaffolds were then immersed in EDAC/NHS (1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and N-hydroxysuccinimide) at room temperature for 24 h.32 The final crosslinking was achieved through the use of 0.5% glutaraldehyde as described by Olde Damink et al. 33 The control group of collagen-GAG scaffolds were only glutaraldehyde (GA) crosslinked post-DHT.
The known cytotoxicity of residual GA in these medical devices is well documented. The procedure that was followed to remove any unbound GA was based on the work of Speer et al.34 The samples were washed in three changes of 500 ml MilliQ water which had been through a 0.2-µm filter. The water is available from Glycar (Pty) Ltd, and a Stuart orbital shaker was used to ensure efficient rinsing. Each wash cycle lasted 10 min. An additional wash was carried out in Dulbecco's phosphate buffered saline (DPBS), available from Whitehead Scientific (Pty) Ltd, to ensure that the scaffold equilibrated to a pH of about 7. The pH was verified with a CRISON pH meter. The final wash entailed the use of Fibroblast Basal Medium (FBM) supplemented with 10% fetal calf serum for 10 min. The efficacy of the wash procedure was verified through exposure of sample extracts to normal foreskin dermal fibroblasts.
Enzymatic degradation assay
The assay that was performed was based on earlier work done by Moore and Stein in 194835 as well as that of Mandl et al.36 in 1948 and more recent (2004) work by Pek et al.37 The principle of the test rests on the fact that collagenase I cleaves two of the three helical chains of collagen. Concentrations of released peptides and amino acids are measured by ninhydrin-colourimetric absorbance at 570 nm.35,36 Freshly prepared collagenase (0.1 mg/ml PBS, Sigma, St Louis, MO) was used. Scaffold samples were cut into 10 mm × 10 mm pieces, thoroughly washed and placed in 15 ml centrifuge tubes. Buffer (5 ml of a 50 mM TES buffer) with calcium chloride with a final pH of 7.4 was added to the sample material. This was done in triplicate and the average of the three representative results of each sample was used for the data analysis. Collagenase was added to the sample material and incubated at 37°C for 12 and 24 h. The light absorbance of each ninhydrin-treated sample was read at 570 nm using a Bio-Tek, model ELx800 plate reader. A standard curve was prepared using 4.0 mM L-leucine in 10 mM hydrochloric acid and used to determine the quantity of L-leucine equivalents liberated through the enzymatic action of collagenase for each sample.
Cytotoxicity assay
Normal human foreskin dermal fibroblasts were used to determine the efficacy of the free aldehyde removal after GA crosslinking. Second passage cells were counted and cell viability was assessed using the trypan blue dye exclusion assay. These cells were then seeded into 24-well plates at a density of 5 × 104 cells per well. Incubation for 48 h (at 37°C and 5% CO2) in supplemented FBM followed. Extracts were prepared by placing 1 mm × 1 mm sample pieces in test tubes containing 8 ml supplemented FBM. The samples were incubated for 24 h at 37 °C in order to obtain extracts. The culture medium of the incubated cells was replaced after the 48-h incubation period with sample extract. Further incubation for 48 h followed, after which the MTT assay was performed. The assay relies on the mitochondrial reduction of yellow MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to purple formazan by viable cells. This assay thus provides an indication of the number of viable cells remaining after exposure to the extracts. Light absorbance at 450 nm was read using a Bio-Tek, model ELx800 plate reader.38,39 The data were graphically depicted to represent the cell viability versus sample type. The standard deviation of each data set was included and a P-value of less than 0.05 was accepted as being statistically significant.
Statistical methods
Quantifiable data were statistically analysed and any comparison made included the calculation of means, standard deviations and P-values (confidence interval of 95%).
Results and discussion
Scaffold architecture
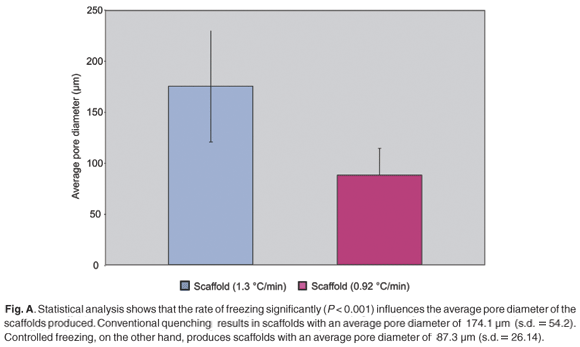
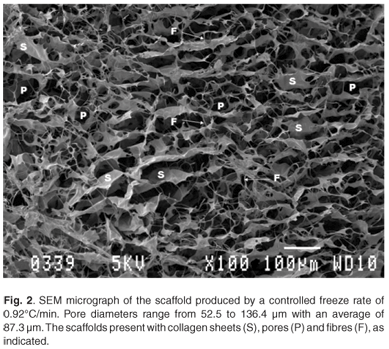
Results indicate that highly porous bioactive tissue engineering matrices have been obtained by either controlled freezing or uncontrolled freezing. The average pore diameter of the most homogeneous scaffolds (Fig. 2) ranged between 52.5 and 136.4 µm, with a mean of 87.3 µm. These templates were formed by using a 0.5% collagen concentration and a controlled freeze rate of 0.92°C/min. The scaffolds show large amounts of sheets, fibres and polygonal pores.
Uncontrolled freezing (1.3°C/min) of a 0.5% collagen concentration resulted in the formation of an irregular scaffold (Fig. 3) with a pore diameter ranging between 93.4 and 278.1 µm (average = 174.1 ± 54.2 µm).
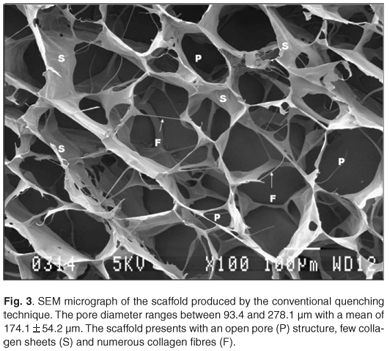
Scaffolds presented with an open structure, with few collagen sheets and numerous collagen fibres.
Statistical analysis (Fig. A in supplementary material online) of the two data sets showed that the rate of freezing significantly influenced the resulting average pore diameter of the scaffolds (P < 0.001).
Optimal porosity and homogeneity, as previously described,23,24 has been achieved through the use of a controlled freeze rate, based on previous work done by O'Brien et al. in 2004.9 The scaffolds presented with average pore diameters of 87.3 ± 26.1 µm (Fig. A). This falls within the critical range of 20 and 120 µm as required for dermal regeneration.23,24
Enzymatic degradation assay
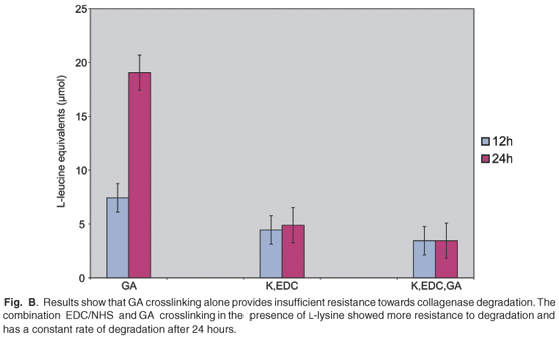
Results from the collagenase-induced scaffold degradation assay show that the GA crosslinked scaffolds (Fig. B online) liberate the largest amount of L-leucine equivalents after 12 h (7.4 ± 1.1 µmol) and 24 h (19.1 ± 2.0 µmol) of incubation. Thus, GA crosslinking alone provided insufficient resistance to enzymatic degradation, with a high rate of degradation over 24 h. The highest resistance against enzymatic degradation was achieved through a combination of L-lysine (K) bridging, and EDC/NHS and GA crosslinking. Only 3.4 ± 1.0 µmol L-leucine equivalent was liberated after 12 h and 3.4 ± 1.8 µmol after 24 h. The rate of degradation after 24 h was more constant than the scaffolds crosslinked with EDC/NHS in the presence of K, which released 4.4 ± 1.5 µmol and 4.9 ± 1.2 µmol L-leucine equivalents after 12 and 24 h of enzyme exposure, respectively.
Work done by others30,35 has found that EDC/NHS treatment provides greater resistance to collagenase degradation compared with non-crosslinked or only DHT-treated matrices. The pre-treatment of scaffolds with L-lysine is known to enhance EDC/NHS crosslinking.29
Cytotoxicity assay
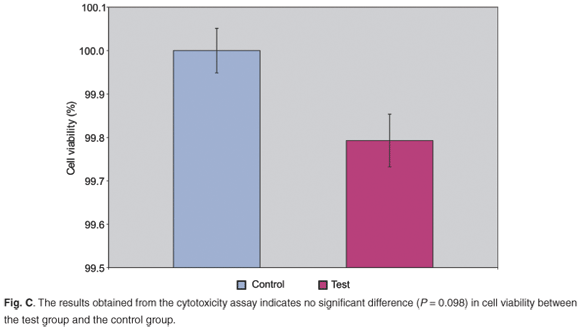
The results of the cytotoxicity assay (Fig. C online) confirmed effective removal of unbound aldehydes. There was no significant difference in the cell viability between the control group and the test group (P = 0.098). The data obtained match the study by Speer et al.32
Concluding remarks
The use of collagen in the biomedical device industry has led to major advances in soft tissue repair. This is largely attributed to the favourable biological and physiochemical properties of collagen. Regenerative medicine and tissue engineering has favoured the use of this biomaterial, and various commercial products have become available in the past few decades.37
The implications for the design and development of an artificial skin or tissue engineering scaffold are vast. The key elements of biocompatibility, pore volume and diameter, effective crosslinking, and the optimal rate of degradation are influenced by a multitude of factors. The rate of scaffold degradation has become the most critical design consideration for both research and clinical applications.18 Research has shown that scaffolds with enhanced resistance towards enzymatic degradation sufficiently prevent wound contraction.38,39
We report on homogeneous, highly porous, non-cytotoxic, collagen-GAG tissue engineering scaffolds that are a first for South Africa. This was achieved through the use of a constant freeze rate of 0.92°C/min, with well-established crosslinking methods. Initial crosslinking was achieved through dehydrothermal treatment and was followed by the employment of combinations of crosslinking agents, such as glutaraldehyde and/or carbodiimide, with or without the presence of L-lysine. Results show that enhanced resistance against enzymatic degradation was obtained after DHT, treatment with L-lysine, followed by crosslinking with EDC/NHS and GA, respectively. This particular scaffold would thus have a longer life of structural integrity in vivo. The combination of physical and chemical crosslinking, as has been described, will thus have the potential to reduce wound contraction in vivo.
1. Sipe J.D. (2002). Tissue engineering and reparative medicine. Ann. N.Y. Acad. Sci. 961, 1–9. [ Links ]
2. Bansky R. (2005). The use of dermal template INTEGRA in scalpation injury of the hand. Bratisl. Lek. Listy. 106(6-7), 221–225. [ Links ]
3. Billingharm E. and Medawar P.B. (1951). The technique of free skin grafting in mammals. J. Exp. Biol. 28, 385–394. [ Links ]
4. Billingharm E. and Medawar P.B. (1955). Contracture and intussusceptive growth in the healing of extensive wounds in mammalian skin. J. Anat. 89, 114–123. [ Links ]
5. Yannas I.V. (2005). Similarities and differences between induced organ regeneration in adults and early foetal regeneration. J. R. Soc. Interface 2(5), 403–417. [ Links ]
6. Williams W. (2002). Pathophysiology of the burn wound. In Total Burn Care, 2nd edn, pp. 783–797. Saunders, London. [ Links ]
7. Vacanti C.A. (2006). History of tissue engineering and a glimpse into its future. Tissue Eng. 12(5), 1137–1142. [ Links ]
8. Heimbach D., Luterman A., Burke J.F. et al. (1988). Artificial dermis for major burns: a multi-center randomized clinical trial. Ann. Surg. 208(3), 313–320. [ Links ]
9. O'Brien F.J., Harley B.A., Yannas I.V. and Gibson L. (2004). Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 25, 1077–1086. [ Links ]
10. Zeltinger J., Sherwood J.K., Graham D.A., Mueller R. and Griffith L.G. (2001). Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 7(5), 557–572. [ Links ]
11. Wake M.C., Patrick C.W. and Mikos A.G. (1994). Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell. Transplant. 3(4), 339–343. [ Links ]
12. Salem A.K., Stevens R., Peason R.G., Davies M.C., Tendler S.J., Roberts C.J., Willams P.M. and Shakesheff K.M. (2002). Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. J. Biomed. Mater. Res. 61(2), 212–217. [ Links ]
13. Nehrer S., Breinan H.A., Ramappa A., Young G. et al. (1997). Matrix collagen type and pore size influence behavior of seeded canine chondrocytes. Biomaterials 18(11), 769–776. [ Links ]
14. LiVecchi A.B., Tombes R.M. and LaBerge M. (1994). In vitro chondrocyte collagen deposition within porous HDPE: substrate microstructure and wettability effects. J. Biomed. Mater. Res. 28(8), 839–850. [ Links ]
15. Kuberka M., von H.D., Schoof H., Heschel I. and Rau G. (2002). Magnification of the pore size in biodegradable collagen sponges. Int. J. Artif. Organs 1, 67–73. [ Links ]
16. Claase M.B., Grijpma D.W., Mendes S.C., De Bruijn J.D. and Feijen J. (2003). Porous PEOT/PBT scaffolds for bone tissue engineering: preparation, characterization, and in vitro bone marrow cell culturing. J. Biomed. Mater. Res. 64, 291–300. [ Links ]
17. Borden M., El-Amin S.F., Attawia M. and Laurencin C.T. (2003). Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials 24, 597–609. [ Links ]
18. Ma L., Gao C., Mao Z., Zhou J. and Shen J. (2004). Enhanced biological stability of collagen porous scaffolds by using amino acids as novel cross-linking bridges. Biomaterials 25, 2997–3004. [ Links ]
19. Yannas I.V., Burke J.F., Orgill D.P. and Skrabut E.M. (1982). Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 215, 174–176. [ Links ]
20. Yannas I.V. (1998). Studies on the biological activity of the dermal regeneration template. Wound Rep. Regen. 6(6), 518–524. [ Links ]
21. Orban J.M., Wilson L.B., Kofroth J.A., El-Kurdi M.S., Maul T.M. and Vorp D.A. (2004). Crosslinking of collagen gells by transglutaminase. J. Biomed. Mater. Res. 68A, 756–762. [ Links ]
22. Hara M. (2006). Various crosslinking methods for collagens: merit and demerit of methods by radiation. J. Oral Tissue. Eng. 3(3),118–124. [ Links ]
23. Steffen C., Timpl R. and Wolff I. (1968). Immunogenicity and specificity of collagen V. Demonstration of three different antigenic determinants on calf collagen. Immunology 15(1), 135–144. [ Links ]
24. Piez K.A. (1967). In Treatise on Collagen, vol. 1, pp. 207–252. Academic Press, New York. [ Links ]
25. Yannas I.V. (2002). Models of organ regeneration process induced by templates. Ann. N.Y. Acad. Sci. 961, 280–293. [ Links ]
26. Yannas I.V., Lee E., Orgill D.P., Skrabut E.M. and Murphy G.F. (1989). Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl Acad. Sci. USA. 86(3), 933–937. [ Links ]
27. Burke J.F., Yannas I.V., Quinby W.C., Bondoc C.C. and Jung W.K. (1981). Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 194(4), 413–428. [ Links ]
28. Doillon C.J., Whyne C.F., Brandwein S. and Silver F.H. (1986). Collagen-based wound dressings: control of the pore structure and morphology. J. Biomed. Mater. Res. 20, 1219–1228. [ Links ]
29. Paggiaro A.O., Kamamoto F., Rodas A.C.D., Mathor M.B., Herson M.R. and Ferreira M.C. (2003). Scanning electron microscopy as a tool for the evaluation of collagen lattices. Microsc. Acta 21(1), 205–208. [ Links ]
30. Ma L., Gao C., Mao Z., Shen J., Hu X. and Han C. (2003). Thermal dehydration treatment and glutaraldehyde crosslinking to increase the biostability of collagen–chitosan porous scaffolds used as dermal equivalent. J. Biomater. Sci.– Polym. E. 14(8), 861–874. [ Links ]
31. Ma L., Gao C., Mao Z., Zhou J. and Shen J. (2004). Enhanced biological stability of collagen porous scaffolds by using amino acids as novel crosslinking bridges. Biomaterials 25, 2997–3004. [ Links ]
32. Olde Damink L.H.H., Dijkstra P.J., Feijen J., van Luyn M.J.A., van Wachem P.B. and Nieuwenhuis P. (1996). Crosslinking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 17, 765–773. [ Links ]
33. Olde Damink L.H.H, Dijkstra P.J., Van Luyn M.J.A., Van Wachem P.B., Niewenhuis P. and Feijen J. (1995). Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci.–Mater. M. 6, 460–472. [ Links ]
34. Speer D.P., Chvapil M., Eskelson C.D. and Ulreich J. (1980). Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 14, 753–764. [ Links ]
35. Moore S. and Stein W.H. (1948). Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 176(1), 367–388. [ Links ]
36. Mandl I., MacLennan J.D., Howes E.L., DeBellis R.H. and Sohler A. (1953). Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J. Clin. Invest. 32(12), 1323–1329. [ Links ]
37. Pek Y.S., Spector M., Yannas I.V. and Gibson L.J. (2004). Degradation of a collagen–chondroitin-6-sulphate matrix by collagenase and by chondroitinase. Biomaterials 25, 473–482. [ Links ]
38. Paddle-Ledinek J.E., Nasa Z. and Cleland H.J. (2006). Effect of different wound dressings on cell viability and proliferation. Plast. Reconstr. Surg. 117(7S), 110–118. [ Links ]
39. Pachence J.M. (1996). Collagen-based devices for soft tissue repair. J. Biomed. Mater. Res–A. 33, 35–40. [ Links ]
Received 22 April. Accepted 14 October 2008.
This article is accompanied by graphical results online at www.sajs.co.za
Author for correspondence and present address: Southern Biotech (Pty) Ltd, P.O. Box 17198, Lyttelton 0140, South Africa. E-mail: quenton.w@southmed.co.za
Supplementary material to:
Wessels Q.B. and Pretorius E. (2008). Enhanced stabilization of collagen-based dermal regeneration scaffolds through the combination of physical and chemical crosslinking. S. Afr. J. Sci. 104, 513–516.