Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.104 no.11-12 Pretoria nov./dic. 2008
RESEARCH IN ACTION
Surface polarity determination of wood fibres after different pre- treatments and bisulphite pulping
M. Meincken; N.C. Matyumza
Department of Forest and Wood Science, University of Stellenbosch, Private Bag X1, Matieland 7602, South Africa
ABSTRACT
The surgace polarity of pulp fibres originating from four different wood species commonly used for pulping in South Africa has been determined after various pre-treatments, and after magnesium bisulphite pulping. The presence as well as the distribution of polar groups on the fibre surface strongly affects inter-fibre bonding in paper. Bonding consists mostly of hydrogen bonds between free hydroxyl groups on the fibre surface. Surface polarity was examined by atomic force microscopy in pulsed-force mode. This technique allows the imaging of the polarity of a surface with a nanometre-scale molecular resolution. It is thus sensitive to individual functional groups, mostly hydrophilic hydroxyl groups. Polarity differences between the various wood species have been observed. We compare these observations with the varying pulp quality that arises from the pulp composition. Improvement in pulp quality may be possible if fibre surface properties are used as guidance criteria for the choice of a specific pre-treatment method.
Introduction
Paper properties, such as mechanical strength and water absorption, are influenced significantly by the surface properties of its constituent pulp fibres. The amount and distribution of polar groups on the fibre surface is one important element, as this influences the inter-fibre bonding as well as the retention of filler particles or other additives to the paper composition.
Hydrophobic components, including lignin, are reported to impair paper strength.1,2 The presence of anionic structures on the fibre surface, on the other hand, is stated to increase paper strength.3 Polar (hydrophilic) groups on the fibre surface are claimed to improve the interaction with filler or binder particles and other additives that attach to the fibre via hydrostatic forces.4 The main contributors of free hydroxyl groups at the fibre surface are cellulose and polyoses.
We have compared the surface polarity and morphology (roughness) of fibres from four different wood species and have determined the changes in characteristic values after various forms of pre-treatment and subsequent sulphite pulping.
The species investigated—Acacia mearnsii, Eucalyptus dunnii, E. grandis and E. macarthurii—are commonly used in the South African paper milling industry for pulping. Differences in pulp quality, mainly in the yield and kappa number, have been reported.5 A. mearnsii and E. grandis are commonly blended to attain a requisite pulp quality. Although E. dunnii and E. macarthurii are found to produce a different pulp quality, they are often used together with the former two wood species to augment material available for paper manufacture.
We propose that a difference in relevant surface properties may be linked to differing pulp qualities and could provide a better understanding of the surface characteristics. This would allow for optimization of pre-treatment and pulping conditions for different pulpwood fibres. The aim is to maximize both the pulp yield and its quality.
The surface roughness and the surface polarity of fibres from the four investigated wood species were determined with atomic force microscopy (AFM). This technique has been employed to study the topography and morphology of fibre surfaces by several research groups6–11 at a molecular scale.
An image of the surface polarity can be simultaneously determined with a topographic image in the digital pulsed force mode (DPFM). This additional facility allows the determination of the adhesion between the sample and a probe at each scan point,12,13 generating a surface 'map' where different adhesive force values are depicted as different colours. If a silicon tip is used, the adhesive force is mainly due to the interaction of polar groups, and can therefore be regarded as an indication for the polarity of the sample. It is possible from this image to distinguish polar and non-polar parts of the surface, and thus determine the distribution as well as the average polarity.14,15
We have determined the surface roughness from the topographic images by measuring the mean deviation from the average height.
Experimental
See Appendix.
Results and discussion
Figure 1(a) gives a view of the fibres through an optical microscope (×50 magnification) and two typical scan areas, in which 2 × 2 µm2 AFM topography (b) and adhesive force (c) images were acquired.

The surface roughness determined from acquired topography images of untreated fibres, of fibres after hot water extraction (HWE), hot water extraction and biopulping (HWE & BP) and subsequent sulphite pulping of all three pre-treated fibre samples is displayed in Fig. 2(a)–(d). All four species show an average surface roughness of about 20 nm, with broad error bars, irrespective of the type of pre-treatment. Sulphite pulping increases the average surface roughness to about 30 nm with an even larger distribution of values.
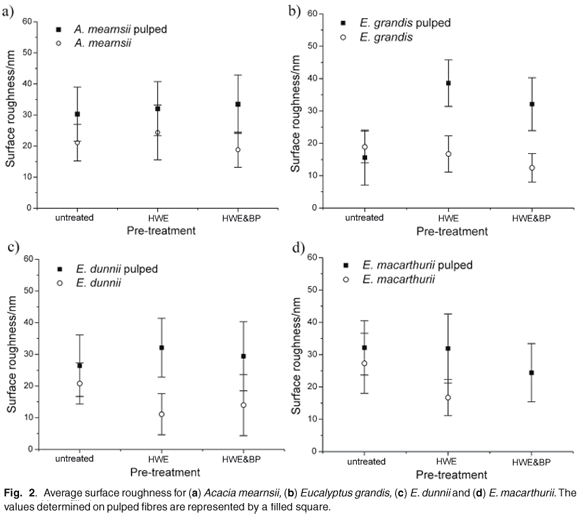
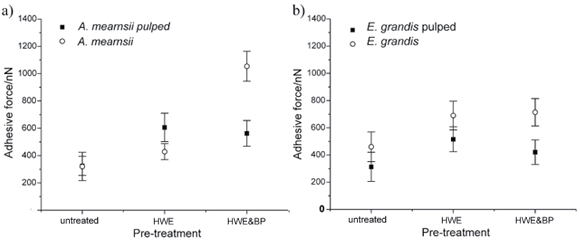
Figure 3(a)–(d) shows, for the four observed species, the surface polarity of untreated fibres, of fibres after HWE, HWE & BP and subsequent sulphite pulping of all three pre-treated fibre samples. For A. mearnsii [Fig. 3(a)] and E. grandis [Fig. 3(b)], the surface polarity is increased by HWE and further increased by HWE & BP. For A. mearnsii the average polarity increases by about 50% after HWE and by about 250% after HWE & BP in relation to the untreated sample. For E. grandis the average polarity increases by about 45% after HWE and by about 55% after HWE & BP.
Subsequent sulphite pulping further changes the surface polarity. In the case of E. grandis the average polarity of the pulped fibres lies about 30% below the unpulped fibres (33% lower for the untreated fibres, 24% lower for the HWE fibres and 36% lower for the HWE & BP fibres). In the case of A. mearnsii the average polarity of the untreated fibres remains the same after pulping; it increases after HWE by about 45% and decreases by about 55% after HWE & BP. The surface polarity is increased by the pre-treatments in both cases. Subsequent sulphite pulping increased the surface polarity, compared with untreated samples, but the measured values were lower compared with the pre-treated samples. This similar trend can explain why these two species pulp well together and produce a pulp of comparable yield. The results also indicate that HWE, as a pre-treatment, is sufficient, and that a combination of HWE & BP has no advantages for subsequent sulphite pulping. The surface polarity of E. dunnii [Fig. 3(c)] and E. macarthurii [Fig. 3(d)], on the other hand, differs visibly from that of A. mearnsii and E. grandis. The polarity on untreated fibres from E. dunnii is about three times higher than on fibres from A. mearnsii and E. grandis. The polarity on fibres from E. macarthurii is about 50% higher.
Unlike A. mearnsii and E. grandis, the surface polarity decreases for both species after pre-treatment. The polarity of E. dunnii fibres decreases by about 10% after HWE and by about 55% after HWE & BP. The polarity of E. macarthurii fibres decreases by about 60% after HWE and only by about 25% after HWE & BP.
Compared to A. mearnsii and E. grandis, the polarity of E. dunnii fibres is much higher after HWE and the polarity of E. macarthurii fibres is much lower. After HWE & BP the polarity of fibres from E. dunnii and E. macarthurii is comparable, but lower than on fibres from A. mearnsii and E. grandis.
The surface polarity of untreated fibres from E. dunnii and E. macarthurii is reduced by sulphite pulping but remains higher than for A. mearnsii and E. grandis. Pulping after HWE decreases the polarity on E. dunnii fibres by about 30% and raises it on E. macarthurii by about 60%, which makes their surface polarity values comparable to A. mearnsii and E. grandis. Pulping after HWE & BP does not change the surface polarity significantly for fibres from E. dunnii and E. macarthurii, resulting in a similar surface polarity as obtained on fibres from A. mearnsii and E. grandis with the same treatment.
The AFM measurements show a clearly visible change in surface polarity after pre-treatment of the fibres. Pulping changed the surface polarity further. A clear difference between the four wood species is evident and the same treatment leads to different surface polarities on fibres from different species.
Conclusions
Topographical images show that the surface morphology—that is, the surface roughness—of the fibres does not vary greatly with wood species or different pre-treatments. Magnesium bisulphite pulping increases the surface roughness on fibres from all four species similarly, independent of the pre-treatment. The surface morphology of the fibres does not therefore account for different pulp qualities.
The surface polarity of the pulp fibres, which influences pulp quality greatly, shows significant differences between the four wood species, which can explain differences in pulp quality. Whereas A. mearnsii and E. grandis show similar trends and comparable values, E. dunnii and E. macarthurii deviate to higher and lower values, respectively. Various pre-treatments do not have the same effect on the different wood species. We have not yet determined whether a higher or lower surface polarity results in a better pulp quality, but it can be assumed that similar surface properties of the fibres would be preferable in a pulpwood mix. If a blend of pulpwood is used, each species should therefore be pre-treated in a different way, to obtain fibres with similar surface properties for further processing, such as pulping, thereby enhancing the pulp quality.
We thank R. Sanderson from the Department of Chemistry and Polymer Science for his contribution and the use of the Veeco Multimode SPM, which he has on loan from the Centre for Macromolecular Chemistry and Technology in Tripoli, Libya. Wood samples were provided by TWK Agricultural Ltd. Funding of this project has been by the National Research Foundation, grant number ICD2006060600004.
1. Lindstroem T., Soeremark C. and Westman L. (1977). The colloidal behaviour of kraft lignin. Colloid Polym. Sci. 258, 168–173. [ Links ]
2. Zhang X., Beatson R.P., Cai Y.J. and Saddler, J.N. (1999). Accumulation of specific dissolved colloidal substances during white water recycling affecting paper properties. J. Pulp Pap. Sci. 25, 206–210. [ Links ]
3. Fardim P. and Holmbom B. (2005). Origin and surface distribution of anionic groups in different papermaking fibres. Colloids and Surfaces A 252, 237–242. [ Links ]
4. Hannuksela T., Holmbom B., Mortha G. and Lachenal D. (2003). Effect of sorbed galactoglucomannans on the strength properties of pulp and paper handsheets. Proc. 5th International Paper and Coating Chemistry Symposium, Montreal, June 16–19, 229–232. [ Links ]
5. Clarke C.R.E. (1995). Variation in growth, wood, pulp and paper properties of nine eucalypt species with commercial potential in South Africa. Ph.D. thesis, University College of North Wales, Bangor. [ Links ]
6. Futura T. and Gray D.G. (1998). Direct force–distance measurements on wood pulp fibres in aqueous media. J. Pulp Pap. Sci. 24, 320–324. [ Links ]
7. Fardim P., Gustafsson J., von Schoultz S., Peltonen J. and Holbom B. (2005). Extractives on fiber surfaces investigated by XPS, ToF-SIMS and AFM. Colloids and Surfaces A 255, 91–103. [ Links ]
8. Gustafsson J., Ciovica L. and Peltonen J. (2003) The ultrastructure of spruce kraft pulps studied by AFM and XPS. Polymer 44, 661–670. [ Links ]
9. Gustafsson J., Letho J.H., Tienvieri T., Ciovica L. and Peltonen J. (2003). Surface characteristics of thermomechanical pulps. Colloids and Surfaces A 225, 95–104. [ Links ]
10. Kangas H. and Kleen M. (2004). Surface chemical and morphological properties of mechanical pulp fines. Nordic Pulp Pap. Res. J. 19, 191–199. [ Links ]
11. Koljonen K. (2004). Effect of surface properties of fibres on some paper properties of mechanical and chemical pulp. Ph.D. thesis, Helsinki University of Technology, Finland. [ Links ]
12. Marti O., Stifter T., Waschipky H., Quintus M. and Hild S. (1999). Scanning probe microscopy of heterogeneous polymers. Colloids and Surfaces 154, 65–73. [ Links ]
13. Krotil H., Stifter T., Waschipky H., Weishaupt K., Hild S. and Marti O. (1999). Pulsed force mode: a new method for the investigation of surface properties. Surf. Interf. Anal. 27, 336–340. [ Links ]
14. Frisbie C.D., Rozsnyai L.F., Noy A., Wrighton M.S. and Lieber C.M. (1994). Functional group imaging by chemical force microscopy. Science 265, 2071–2074. [ Links ]
15. Akari S., Horn D. and Keller H. (1995). Chemical imaging by scanning force microscopy. Adv. Mater. 7, 549–551. [ Links ]
16. Han J.S., Mianowski T. and Lin Y. (1999). Validity of plant fiber length measurement. Forest Products Laboratory, Ag. and Bio. Eng. chap. 14, 149–167. [ Links ]
17. Meincken M., Roux S.P. and Jacobs E.P. (2005). Determination of the hydrophilic character of membranes by pulsed force mode atomic force microscopy. Appl. Surf. Sci. 252, 1772–1779. [ Links ]
18. Meincken M. (2007). Atomic force microscopy used to determine the surface roughness and surface polarity of different cell types of hardwoods commonly used for pulping. S. Afr. J. Sci. 103, 4–6. [ Links ]
Author for correspondence. E-mail: mmein@sun.ac.za
Experimental
Wood from four species (A. mearnsii , E. grandis, E. dunnii and E. macarthurii) was chipped using a Wigger pilot size machine, with four blades adjusted to produce approximately 20-mm-long woodchips. Those with a thickness range of 6–8 mm were used for the experiments and received the following pre-treat- ments: no treatment (control material); hot water extraction for one hour at 140°C; hot water extraction for one hour at 140°C with subsequent biopulping. These three different batches were then pulped with magnesium sulphite in a laboratory-scale digester, as described below. The method was intended to emulate the pulping process conditions used by the industries that provided the samples for this project.
Biopulping
Biopulping using co-cultures of Pycnoporus sanguineus and Aspergillus flavipes was carried out, to loosen the wood structure and facilitate lignin removal in the subsequent chemical pulping process. P. sanguineus is a lignin-digesting fungus, whereas A. flavipes is known to use readily-available sugars, and does not alter the wood structure extensively. Cultures of A. flavipes and P. sanguineus were inoculated on one kilogram of wood chips, which were placed on a grid, 5 cm from the bottom of a closed, cylindrical, plastic bio-reactor. The bio-reactor was incubated for 14 days at 30°C and aerated from below the grid with 10 l/min sterile, moist air, blown through a water trap. The chips were then harvested.
Magnesium bisulphite pulping
The chips were impregnated at 100°C for 2 hours prior to pulping and subsequently pulped in micro-bombs accommodating 80 g of oven-dry (dried at 103°C for 24 hours) chips for 12 hours at 140°C with magnesium bisulphite (bisulphite pulping) at a sulphite concentration of 5%. The filled micro-bombs were then placed in a 15-litre digester and covered with water. The temperature of the water was kept constant by a thermostat.
Fibre preparation
Samples from untreated, extracted and biopulped wood were separated into single fibres by mild maceration in Jeffery's solu- tion.16 Fibres were kept in distilled water at 4°C prior to AFM analysis.
Atomic force microscopy
Single fibres were spread from a water suspension onto a glass slide and left to dry for 12 hours. The adhesion due to capillary forces between the two substrates was sufficient to keep the fibres in position for AFM analysis. Images were acquired with the fast scan axis parallel to the longitudinal fibre axis, in order to minimize shear forces.
Images (2 × 2 µm2) were acquired with a multimode AFM combined with a Witec DPFM controller. This allows simultaneous acquisition of a surface (topographical) image and an image displaying the adhesive force acting between the tip and the sample. A silicon tip (k = 2.8 N/m, from Nanosensors) was used for these experiments. The adhesive force acting between sample and tip is thus a measure of the polarity of the sample. A higher adhesive force translates directly to a higher surface polarity, as has been detailed elsewhere.17,18
The average polarity of a single fibre was determined from one adhesion image, which consists of 65 536 (256 × 256) single measurements in the observed area. This was done on five different fibres for each species, resulting in one average value of the polarity, with a standard deviation. An average surface roughness value was determined from the topography images by measuring the deviation from the average recorded height for five fibres of each species and treatment.