Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.104 no.7-8 Pretoria Set./Ago. 2008
RESEARCH LETTERS
Sterols and sterolins in Hypoxis hemerocallidea (African potato)
Division of Pharmaceutics, Faculty of Pharmacy, Rhodes University, Grahamstown 6140, South Africa
ABSTRACT
Commercially available health supplements and herbal remedies containing sterols and sterolins, either from African potato (Hypoxis hemerocallidea) alone, or whether enriched with sterols and sterolins, are claimed to be efficacious in the treatment of a variety of ailments. Sterols and sterolins in African potato are purported to be the relevant constituents that are required for the therapeutic claims of such products. A patent describing the extraction of sterolins from African potato plant material has claimed that approximately 9 mg sterolins can be isolated from 100 g of an enriched aqueous African potato extract. Our analysis of African potato plant material and its sterol and sterolin content, when similarly prepared, shows that the measureable content of sterols and sterolins in African potato is far less than the amounts of these compounds that have been claimed to be necessary for therapeutic benefit. We conclude that therapeutic claims relating to sterol and sterolin content in African potato are unsubstantiated, in view of the extremely low content of such compounds that we have isolated from our plant material, and in products containing African potato, or extracts thereof.
Introduction
African potato (AP), Hypoxis hemerocallidea, apart from its perceived nutritional value, is of active medical interest, and is purported by many South Africans as possibly being the best-known medicinal plant. Although African potato is the common name, its underground growth is not a tuber, but a corm. Furthermore, the term African potato is also, and confusingly, attributed to another plant, Plectrantus esculentus. The name Hypoxis will therefore be used throughout this text when referring to the AP. Extracts of the corms have been ingested by man as a dietary supplement and for a diversity of ailments.1–6 In recent years, the increasing interest in functional foods and the use of phytosterols, their glycosides (sterolins) and steryl esters (>2 g/day) for reducing serum cholesterol and increasing immunity has resulted in these compounds regaining considerable attention.1 There are many herbal formulations containing sterols for this reason, most of which have been fortified with additional amounts of free sterols, stanols and sterolins. Commercially available health supplements and herbal remedies containing sterols and sterolins as well as Hypoxis, alone or enriched with sterols and sterolins, are claimed to be efficacious for a variety of ailments. In particular, purported sterol and sterolin content in products containing Hypoxis is identified to be the important feature for the therapeutic claims of these products.
Sterols and stanols are claimed to play an important role in the realm of health supplements, with extensive scientific argument for their prophylactic and therapeutic use for various medical conditions and ailments, such as atherosclerosis,2,3 benign prostatic cancer4 and colon cancer.5, 6 However, many reports on the medicinal properties of sterols are largely based on in vitro analyses or unrealistic high in vivo dosages, making the therapeutic claims for these compounds disputable.
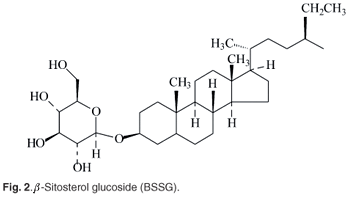
The average daily human dietary consumption of phytosterols is approximately 250 mg, mostly derived from vegetable oils, cereals, fruits and vegetables.7 Dietary phytosterols have been estimated to be almost twice this intake in the case of vegetarians. Phytostanols are much less abundant in nature than phytosterols and are consequently consumed in relatively lower amounts (c. 25 mg/day) in human diets.1 Phytosterols have also been used since the mid-1990s in strategies for lowering cholesterol, and for reducing the risk of cardiovascular diseases (CVD). The advent of the 'statin' compounds has resulted in a rapidly diminished use of phytosterol products. Lipophilic extracts of Hypoxis corms have been used in Europe for the treatment of prostate problems.8 These extracts are claimed to have antiinflammatory activity, and to relieve the symptoms of prostate adenoma. The components claimed to be responsible for this effect are mixtures of phytosterols, and it is claimed that β-sitosterol (Fig. 1) is the main active compound. A commercial product, Harzol®, was launched in 1974, and consists of a combination of b-sitosterol and its glucoside, BSSG (Fig. 2) and has gained wide acceptance in Germany. The glycosides (including BSSG) were purported to be obtained from Hypoxis, and their action in the treatment of benign prostate hypertrophy was attributed to inhibition of 5α-reductase, or to diminished binding of dihydrotestosterone within the prostate gland.9 Various other commercial preparations were subsequently launched, and were claimed to contain Hypoxis extracts, or other plant material, specifically for their sterol/sterolin content. One such formulation, initially purported to contain Hypoxis, was stated to contain sterols-to-sterolins in the mass ratio of 100:1. This specific ratio has been claimed to enhance the in vitro proliferative response of human T cells, more so than individual sterols at the same concentration, and this claim was used to market and promote sales of this particular product.5–10 The Hypoxis component, however, was subsequently removed from this product, and the included phytosterols have been subsequently claimed to be sourced from elsewhere.

With the absence of convincing ethnomedical information relating to the efficacy of the 100:1 ratio of sterols-to-sterolin, coupled with the non-availability of data from other sterol and sterolin ratios, the situation raises doubt regarding the validity of the efficacy and therapeutic claims for such products. Claims of phytosterol content in Hypoxis nevertheless continue to be made, such as a recent advertisement for Santjie Marx Products, which includes the statement: 'The plant sterols and sterolins that are essential to proper immune system function, are highly concentrated in the Africa Potato plant' (http://www.smarxprodukte.com/).
Early claims relating to the medicinal properties of β-sitosterol and its glycoside in Hypoxis have been reported in the literature.11 Although not substantiated by definitive pharmacological data, several patents have been filed on the usage and medicinal claims of sterolin-enriched extracts of Hypoxis for various therapeutic purposes.12–17 In particular, a patent filed by Pegel and Liebenberg in 197318 claimed that the potency of an extract from Hypoxis species in the treatment of prostate hypertrophy was correlated with chemically non-defined phytosterol glycosides (sterolins such as BSSG).
It should be noted that due to poor aqueous solubility and limited bioavailability of free phytosterols, their purported serum cholesterol-lowering effects were not always found to be consistent, and very high oral doses (up to 25–50 g/day) appear to be required for efficacy. The problems of solubility and bioavailability have led to many contradictory results in early clinical studies.1
The highest yield recorded in the US patent entitled 'Extraction of sterolins from plant material', was about 9 mg sterolin from 100 g of an enriched aqueous extract of Hypoxis.17–20 The extractive value of the above aqueous isolate, defined as the content of extractable matter in milligrams per gram of plant material, was not given in the patent. Repeated experiments with the extraction of Hypoxis, conducted in our laboratories, has consistently yielded about 30% w/w dried aqueous extract from fresh corms of Hypoxis. From our data, the patent's claimed 9 mg of sterolin (BSSG) in 100 g of aqueous extract of Hypoxis can be estimated to have originated from about 300 g of fresh Hypoxis plant material.
Sterolins are deglycosylated to their corresponding sterols in the gastrointestinal tract (GIT) of humans, following ingestion, and the resulting sterol is claimed to be responsible for the purported therapeutic effect.7 Comparing the molecular weight ratio of BSSG (molecular weight, 552.89) with BSS (molecular weight, 414.65), 9 mg will stoichiometrically convert to about 6.8 mg β-sitosterol, which is far lower than the reported therapeutic dose required for efficacy.7 To date, except for the claims made in the aforementioned patent, to our knowledge there have been no research publications describing the isolation and analysis of sterols or sterolins from Hypoxis. Nor are there any scientific data to substantiate the amount of these phytoconstituents that is claimed to be required for therapeutic efficacy. Their presence in adequate therapeutic concentration in any species of the genus Hypoxis deserves scrutiny. The issue has been raised by Nicoletti et al.24 and Hostetmann et al.8
Based on our argument, we propose that any nutritional or therapeutic value of Hypoxis is unlikely to be due to the presence of sterolins in Hypoxis or extracts therefrom, as final extractive recovery of these compounds is <0.01% w/w of enriched extract.
Content of sterols and sterolins in Hypoxis
A Hypoxis extract was prepared from Hypoxis plant material, in accordance with the example procedure recorded in the patent.16 We measured the content of sterols (β-sitosterol and stigmasterol) and BSSG recovered. Extractive procedure is similar to the Hypoxis decoctions prepared according to the common recipe used by traditional healers (sangomas)inthe Eastern Cape province of South Africa. Analysis involved a validated HPLC procedure, coupled with evaporative light-scattering detection for the qualitative and quantitative determination of sterols and BSSG.25 The aqueous extract did not show any detectable quantities of BSSG or BSS and this was confirmed by spiking the sample solutions with known concentrations of BSS and BSSG standards.
The content of sterols (stigmasterol, β-sitosterol and stigmastanol) and sterolins in the preparation outlined above is less than our analytical detection limit of 0.002%w/w. This equates to an amount of less than about 6 mg BSSG/100 g of aqueous extract, in contrast to the content of 9.01 mg/100 g aqueous extract, as given in the patent information. Notwithstanding the patent claims, we confirm a lower sterolin content of Hypoxis, and we show that this so-called enriched extract of Hypoxis contains less than 0.002% sterolins.
We conclude that because the daily consumption by consumers of Hypoxis is about 200–500 ml of such an aqueous decoction (typically containing about 37 mg fresh Hypoxis per ml, or c.18.5 g of Hypoxis plant material),26 the daily intake of BSS and BSSG, if present in the decoction, will be negligible (about a milligram) and will be inadequate for any therapeutic efficacy (Table 1).

Health-product preparations of Hypoxis hemerocallidea are readily available from retail outlets, and more recently via the internet, and the suppliers and manufacturers of these products continue to make a variety of claims regarding the efficacy of such products. It is the purpose of this communication to present a scientific appraisal of these claims, and to afford evidence that they are suspect.
We thank C. Albrecht for the supply of BSSG and the Medical Research Council of South Africa for financial support.
1. Moreau R.A., Bruce D.W. and Kevin B.H. (2002). Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 41, 457–500. [ Links ]
2. Hendriks H. F. J., Westerate J. A., van Vliet T. and Meijer G.W. (1999). Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 53, 319–327. [ Links ]
3. Miettinen T. A., Puska P., Gylling H. and Vartiainen E. (1995). Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N. Engl. J. Med., 333, 1308–1312. [ Links ]
4. Awad A. B. and Fink C.S. (2000). Phytosterols as anticancer dietary components: evidence and mechanism of action. J. Nutr. 130, 2127–2130. [ Links ]
5. Rao A. V. and Janezic S.A. (1992). The role of dietary phytosterols in colon carcinogenesis. Nutrition and Cancer 18, 43–52. [ Links ]
6. Rao A.V. and Koratkar R. (1997). Antinutrients and Phytochemicals in Food, ed. F. Shadhidi, pp. 313–324. ACS Symposium Series, American Chemical Society, Washington, DC. [ Links ]
7. Moreau R.A., Singh V. and Hicks K.B. (2001). Comparison of oil and phytosterol levels in germplasm accessions of corn, teosinte, and Job's tears. J. Agric. Food Chem. 49, 3793–3795. [ Links ]
8. Hostetmann K., Marston A., Ndjoko K. and Wolfender J.L. (2000). The potential of African plants as a source of drugs. Curr. Org. Chem. 4, 973–1010. [ Links ]
9. Bruneton J. (1995). Pharmacognosy, Phytochemistry, Medicinal Plants. Intercept, Hampshire, U.K. [ Links ]
10. Bouic P.J., Etsebeth S. and Liebenberg R.W. (1996). Beta-Sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 18, 693–700. [ Links ]
11. Bouic P.J., Clark A. and Lamprecht J. (1999). The effects of β-sitosterol (BSS) and β-sitosterol glucoside (BSSG) mixture on selected immune parameters of marathon runners: inhibition of post marathon immune suppression and inflammation. Int. J. Sports Med. 20, 258–262. [ Links ]
12. Bouic P.J. (2001). The role of phytosterols in immune modulation: a review of the past 10 years. Curr. Opin. Clin. Nutr. Metab. Care 4, 471–475. [ Links ]
13. Bouic P.J., Clark A. and Brittle W. (2001). Plant sterol/sterolin supplement use in a cohort of South African HIV-infected patients–effects on immunological and virological surrogate markers. S. Afr. Med. J. 91, 848–850. [ Links ]
14. Bouic P.J. (2002). Sterols and sterolins: new drugs for the immune system? Drug Discovery Today 7, 775–778. [ Links ]
15. Drewes S.E. and Khan F. (2004). The African potato (Hypoxis hemerocallidea): a chemical–historical perspective. S. Afr. J. Sci. 100, 425–430. [ Links ]
16. Pegel K. H. (1973). Extraction of sterolins from plant material. [ Links ]South African Patent, 72 01, 855.
17. Pegel K.H. (1973). Extraction of sterolins from plant material. [ Links ]US Patent Application, 338057.
18. Pegel K.H. (1977). Active plant extracts of hypoxidaceae and their use. [ Links ]US Patent Application, 856507.
19. Pegel K.H. (1977). Sterolins and their use. [ Links ]US Patent Application, 843496.
20. Pegel K.H. (1979). Active plant extracts of hypoxidaceae and their use. [ Links ]US Patent Application, 016387.
21. Pegel K.H. and Colin B.R. (1979). Sterolin products. [ Links ]US Patent Application, 053735.
22. Pegel K.H. and Walker H. (1979). Sterol glycoside with activity as prostaglandin synthetase inhibitor. [ Links ]US Patent Application, 000599.
23. Pegel K.H. and Liebenberg R.W. (1973). Extraction of phytosterol glycosides from Hypoxis tubers. [ Links ]German Offenlegungsschrift Patent, 2312285.
24. Nicoletti M., Galeffi C., Messana I. and Marini Bettolo G.B. (1992). Hypoxidaceae. Medicinal uses and the nor-lignan constituents. J. Ethnopharmacol. 36, 95–101. [ Links ]
25. Nair V.D. P., Kanfer I. and HoogMartens J. (2006). Determination of stigmasterol, β-sitosterol and stigmastanol in oral dosage forms using high performance liquid chromatography with evaporative light scattering detection. J. Pharmaceut. Biomed. Anal. 41(3), 731–737. [ Links ]
26. Steenkamp V., Gouws M.C., Gulumian M., Elgorshi E.E. and Vanstaden J. (2006). Studies on antibacterial, antiinflammatory and antioxidant activity of herbal remedies used in the treatment of benign prostatic hyperplasia and prostatitis. J. Ethnopharmacol. 103, 71–75. [ Links ]
Received 23 December 2007. Accepted 5 June 2008.
* Author for correspondence. E-mail: i.kanfer@ru.ac.za
a Present address: College of Pharmacy, University of Kentucky, Lexington, KY 40536, U.S.A.














