Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.104 no.7-8 Pretoria Set./Ago. 2008
RESEARCH LETTERS
Skeletochronology of the endangered side-neck turtle, Podocnemis expansa
Anusuya ChinsamyI, *; Nicole ValenzuelaII
IZoology Department, University of Cape Town, Private Bag, Rondebosch 7701, South Africa
IIDepartment of Ecology, Evolution and Organismal Biology, Iowa State University, Ames, Iowa 50011, U.S.A
ABSTRACT
Previous preliminary mark–recapture studies, and assessment of carapace length and annuli of the endangered giant Amazonian river turtle, Podocnemis expansa, have provided some insight into various aspects of their population structure and overall biology. Many questions still remain, however, particularly pertaining to the attainment of sexual maturity, nesting age of females, and longevity of individuals. The current study examines the feasibility of using skeletochronology on the bones of Podocnemis expansa, to obtain data pertaining to these questions, as well as to acquire individual ontogenetic age data. Material for the analysis was opportunistically obtained from 'kitchen remains' and leftovers of natural predators. Our results showed that even after being subjected to such harsh treatment, all the bones in our sample preserved histological detail. By the application of skeletochronology, we estimate the individual ages of all specimens and these compared favourably with age data obtained previously. In spite of our limited sample size, we found a positive relationship between the number of growth rings and carapace length, a slower increase in body size for the larger individuals, and we tentatively suggest that sexual maturity may have occurred at about 5–6 years of age. On the basis of the findings of this pilot study, we suggest that skeletochronology can be effectively used on this endangered taxon. Furthermore, as skeletochronology can also reliably permit deductions about the age profile of individuals that fall prey to predators, it also has the potential of assisting in the development of effective conservation strategies.
Introduction
Management of endangered species requires knowledge of the age structure of populations.1–3 Often this is ascertained by mark–recapture studies. However, such studies usually require an extensive time span for long-lived organisms, whereas the endangered status of many species calls for faster data collection that can be used in risk assessment and, ultimately, decision making. The study reported here examines the potential of using skeletochronology as an alternative approach to mark–recapture studies of the endangered giant Amazonian river turtle, Podocnemis expansa, by examining the microscopic structure of the bones of these turtles recovered from kitchen remains and natural predation events.
This method involves the determination of the age of the animal from growth marks evident in the skeleton.4–9 In many poikilotherms, skeletal growth is cyclical, that is, in a single year an animal has alternating periods of fast and slowed growth.5,10 Bone fluorescent labelling experiments have demonstrated that a period of fast growth, represented by a so-called zone, which is deposited during the favourable growing season, which usually corresponds to spring and summer months or periods of rainfall.5,11,12 The period of slowed growth is termed an annulus, and it is often associated with a line of arrested growth (LAG). In some cases, when periodic interruption is abrupt, only a LAG is formed. Thus, assuming that the growth rings (that is, annuli and/or LAGs) are annual, counting the number of these rings present in the skeleton provides a reasonable estimate of the age of the individual.5,8,9,12 Skeletochronology has been, and remains largely, used to determine the age structure of amphibian populations,13,14 including a threatened species,15 as well as some reptilian populations.4,7,16 More specifically, the validity and annual growth marks in marine turtles has been validated in Caretta caretta17 and in Lepidochelys kempii.18
Methods
Since Podocnemis expansa is an endangered taxon, large-scale collection of specimens for skeletochronology is not feasible. However, often these turtles end up as a food source for humans.19,20 We therefore decided to collect remains of Podocnemis opportunistically from kitchen leftovers, as well as from the remains of individuals attacked by felids (Felis concolor or Panthera onca) while nesting. Specimens were collected along the middle Caquetá river in Colombia, from the localities of Tres Islas, Puerto Solarte, Puerto Remanso, and Quinché. Individuals were all females except one whose sex is indeterminate. Straight-line carapace length was available for four out of the six individuals.
At the time of collection, bones were preserved in 4% formalin. Later, bones were submerged in commercial bleach overnight to prevent rotting of the soft tissue still attached. The bones were then placed in a detergent solution for two days and then scrubbed to complete the cleaning process. Finally, the bones were left at room temperature until they dried. Undecalcified fragments of femora were embedded in resin and thin-sectioned according to the method outlined in refs 12 and 21. Femora were selected, as it is well recognized that long bones (femora, humeri, and tibiae), sectioned along the neutral axis of the bone (that is, the midshaft region), tend to preserve the history of bone growth best.12 Undecalcified, thin sections were prepared of the mid-shaft regions of the six femora collected, and were studied by polarized and ordinary light microscopy. The bone microstructure was documented, and a count of the growth rings present in the cortical bone was made to deduce the age of the individual. The latter was done 'double-blind' and the results were compared. In most cases there was a high agreement between the results obtained separately; where there were differences, the slides were viewed again to reach a consensus count. In some cases where uncertainties arose because of closely spaced LAGs or discontinuities, a 'bracketed count' is provided.
Results
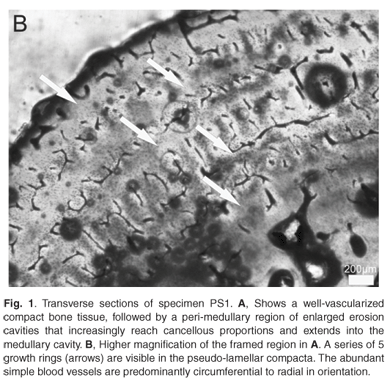
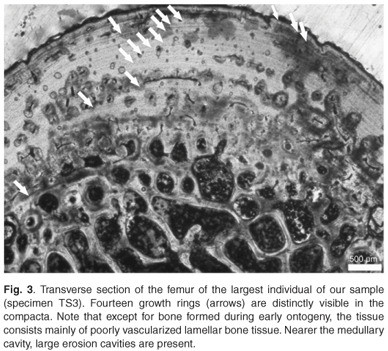
Considering the fairly harsh treatment of the bones prior to analyses, it is noteworthy that the microstructure of the bone (histology) was still preserved, and that it was fairly similar in all the mid-shaft sections examined. Cross sections of the femora revealed a distinct compacted region of cortical bone, which surrounds a medullary cavity that contains a large amount of cancellous bone tissue (Figs 1–3). In general, the cortical bone is well vascularized by numerous simple blood vessels, which are embedded in a pseudo-lamellar or lamellar type of bone tissue. Primary osteons occur infrequently in the bone tissue formed during early stages of growth. A large amount of secondary reconstruction is evident in the peri-medullary region, and a number of secondary osteons are present in this region. Distinct cycles of growth were observed in all individuals studied (Table 1). With an increase in the number of growth rings, the texture of the bone changes from a pseudo-lamellar type to a more poorly vascularized lamellar type of bone tissue (Figs 2 and 3). The intensive secondary reconstruction in the peri-medullary region may have resulted in the obliteration of bone (and growth rings) formed early during ontogeny (Figs 2 and 3).

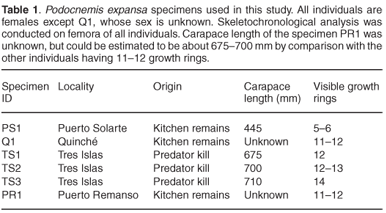
The youngest individual in the sample was specimen PS1, with a carapace length of 445 mm, and five growth rings are evident in the cortical bone (Fig. 1B). Resorption of bone around vascular canals is already under way, and a large number of enlarged erosion cavities are evident, with several reaching cancellous dimensions (Fig. 1A). Given the level of secondary reconstruction evident in the peri-medullary region (Fig. 1A), it is possible that one or more growth rings may have been removed but we do not have younger individuals with which to compare.
Studies by Alho et al.22 and Hildebrand et al.23 have suggested that the size of P. expansa nesting females ranges from 450–820 mm carapace length. Thus, PS1, with a carapace length of 445 mm, is of a similar size to the smallest nesting female reported thus far.
After the attainment of sexual maturity, a narrowing of consecutive zones occurs, so it is possible that deductions can be made regarding the onset of sexual maturity in Podocnemis expansa.5,12 However, it is noteworthy that the spacing of annuli/LAGs is quite complex as it varies in a cross section because of the overall morphology of the bone, and because of local growth conditions.5 Bearing this is mind, it is nevertheless evident that in the youngest individual studied, specimen PS1, the zonal regions following the 3rd annulus visible from the medullary cavity are substantially narrow. The two earlier zones closest to the medullary cavity measure (maximally) about 578 and 455 µm, respectively, and there is about 2164 µm of resorptive bone right up to the middle of the medullary cavity, so that it is quite likely that 2–3 growth rings may have been resorbed (assuming a conservative average zonal size of about 516 µm). Unfortunately, we do not have younger individuals with which to measure the amount of bone formed in the zonal regions during early stages of ontogeny, and especially that formed during the first year of growth. Because of this lack of juveniles, we were unable also to measure the diameter of the bone of a hatchling in the first year of growth, which could have provided a good estimate of how much bone is resorbed at different stages of ontogeny.9 Given that hatchlings tend to deposit bone rapidly and have fast growth rates, however, it can be assumed that the initial zones were probably much wider. If it were assumed that two or three growth rings were removed during the growth of this individual, then sexual maturity occurred around 5–6 years of ontogeny (as represented by the substantial narrowing of the zones after the third visible annulus). Previous studies using mark and recapture data have suggested that maturity in P. expansa is attained in 4–5 years24 (although the author cautions that there may have been some uncertainty in the marking year of some specimens). Interestingly, Ojasti25 reported that a Venezuelan fisherman stated that it takes seven years for female P. expansa to reach maturity, but these data are unconfirmed. Pritchard and Trebbau26 suggested that sexual maturity in Podocnemis may be attained closer to 15 years of age, based on carapace annuli data from Venezuelan specimens, whereas captive animals kept at the Goeldi museum in Belém, Brazil, reached maturity in eight years.19 Finally, captive animals kept by FUDECI at the Estación Experimental Amazonas in Puerto Ayacucho, Venezuela, did not attain maturity after five years even though they exhibited accelerated growth induced by intensive feeding (O. Hernandez, pers. comm.). Thus, it appears that our skeletochronological data most closely agree with Ramirez,24 and we suggest that skeletochronology on younger individuals will shed further light on this aspect of their reproductive biology. This may require targeted collection of smaller/younger individuals rather than the opportunistic sampling used in this analysis. Alternatively, a sampling strategy using digits of individuals of all age/size classes could be effectively employed.
The largest individual in our sample, specimen TS3, has 14 growth rings visible in the cortical bone (Fig. 3). However, because substantial secondary reconstruction has occurred around the medullary cavity (Fig. 3), earlier growth rings may have been removed. This individual is therefore likely to be relatively old. Longevity of the species has been estimated at between 40–50 years and the largest reported size for females is 820 mm.23 The largest individual in our sample has a carapace length of 710 mm, and has 14 growth rings present in its cortical bone. Even if there were substantial reconstruction in the peri-medullary region, resulting in the removal of earlier growth rings, it is unlikely that it was as old as Hildebrand's23 largest individual. It should be noted in particular that the earliest growth ring preserved in this specimen is fairly wide (Fig. 3), which suggests that it was formed relatively early in ontogeny. The fact that subsequent zones are all much narrower, suggests that this zone is equivalent to the third visible zone in the youngest individual, PS1, and could possibly represent sexual maturity. If this were the case, this widest zone represents the fifth or sixth year of growth, thus giving an estimate of at least 20 years for this individual. It should be noted, however, that longevity estimated by skeletochronology is always a minimum because osteogenesis can stop whereas ageing continues.4
Conclusions
The results from this preliminary survey are very encouraging for several reasons. First, it is evident that the alternating periods of growth reflected in the bone microstructure of Podocnemis expansa can be used for skeletochronology. Second, it is noteworthy that some samples for this study were collected from kitchen remains. Thus, even though the bones had been cooked, their microstructure was unaltered. Histological analysis permitted the application of skeletochronology, which allowed the deduction of life-history information about the endangered turtle. The results of our pilot study suggest that more complete sampling schemes can be designed using specimens that are normally harvested by local people (or from natural predation events), without posing any additional pressure on the populations of this endangered turtle species. We suggest, however, that future studies make every effort to sample multiple bones from each individual to assess growth mark count variation within individuals.
Although our sample was fairly limited, our data showed a positive relationship between the number of growth rings and carapace length, and a slower increase in body size for the larger individuals (Table 1), as would be expected for species with indeterminate growth as is the case of P. expansa.25 Our results further emphasize the great potential of using skeletochronology for future surveys of P. expansa and other endangered poikilothermic species, as it directly addresses important questions such as longevity, sexual maturity, and population age structure. Answering these questions appropriately will require much larger sample sizes incorporating males and females from a wider range of body sizes to assess the growth patterns of the taxon. Given that P. expansa is endangered, however, and that human and predator exploitation may be confined to a particular size/age class of individuals, there is obviously a difficulty in obtaining samples of other segments of the population using our collection method. It is possible that wider sampling opportunities could come from the marketplace in some localities where this species is found, but this would most likely also be restricted to particular age/size classes. Thus, it seems that it may be necessary to undertake a targeted collection of digits of different age/size classes from the natural population.
From a conservation standpoint, it is important to compare the subset of exploited females with the general distribution of females in natural populations (perhaps by sampling a digit from the latter) to confirm if local people are collecting older females preferentially because they are larger in size. In this case, to standardize the skeletochronological assessments, digits from exploited females should also be sampled. Age-biased exploitation would affect the age structure of the population, while size-biased exploitation may impact population sex ratios because larger P. expansa females build deeper nests, which experience colder temperatures and tend to produce more male-biased sex ratios.27,28 Thus, it appears that carefully constrained studies making use of skeletochronology have the potential to provide information to enable the development of effective conservation strategies for P. expansa.
We are grateful to D. Muñoz and E. Moreno for providing us with the specimens used in this study, and to K. von Willig and K. Mvumvu-Sidinile for technical assistance. Jacques Castanet and an anonymous reviewer are acknowledged for having commented on this manuscript. This study was supported in part by the NRF (South Africa), Colciencias COD 6218-13-143-95 RC-288-96 (Colombia), the National Science Foundation IBN-9800679 (U.S.A.), and the Ford Foundation grant 960–0929 (U.S.A).
1. Dennis B., Munholland P.L. and Scott J.M. (1991). Estimation of growth and extinction parameters for endangered species. Ecol. Monogr. 61, 115–144. [ Links ]
2. Durant S.M. and Harwood J. (1992). Assessment of monitoring and management strategies for local populations of the Mediterranean monk seal Monachus monachus. Biol. Conserv. 61, 81–92. [ Links ]
3. Williams L.R, Echelle A.A., Toepfer C.S., Williams-Marsha M.G. and Fisher F.L. (1999). Simulation modeling of population viability for the leopard darter (Percidae: Percina pantherina). Southw. Nat. 44, 470–477. [ Links ]
4. Castanet J. Newman D.G. and Saint Girons H. (1988). Skeletochronological data on the growth, age, and population structure of the tuatara, Sphenodon punctatus, on Stephens and Lady Alice Islands, New Zealand. Herpetologica 44, 25–37. [ Links ]
5. Castanet J., Vieillot H.F., Meunier F.J and Ricqles De A. (1993). Bone and individual aging. Bone (Bone growth). Bone 7, 245–283. [ Links ]
6. Chinsamy A. (1993). Bone histology and growth trajectory of the prosauropod dinosaur Massopondylus carinatus Owen. Mod. Geol. 18, 319–329. [ Links ]
7. Chinsamy A., Hanrahan S.A., Neto R.M. and Seely M. (1995). Skeletochronological assessment of age in Angolosaurus skoogi, a cordylid lizard living in an aseasonal environment. J.Herpetol. 29, 457–460. [ Links ]
8. Zug G.R., Wynn A.H. and Ruckdeschel C. (1986). Age determination of loggerhead sea turtles Caretta caretta by incremental growth marks in the skeleton. Smithson. Contrib. Zool. 427, 1–34. [ Links ]
9. Zug G.R., Chaloupka M. and Balaz G.H. (2006). Age and growth in olive ridley seaturtles 9. (Lepidochelys olivacea) from the north-central Pacific: a skeletochronological analysis. Mar. Ecol. 27, 263–270. [ Links ]
10. Chinsamy A. and Dodson P. (1995). Inside a dinosaur bone. Am. Sci. 83, 174–180. [ Links ]
11. Hutton J.M. (1986). Age determination of living Nile crocodiles from the cortical stratification of bone. Copeia 1986, 332–341. [ Links ]
12. Chinsamy-Turan A. (2005). The Microstructure of Dinosaur Bone – Deciphering Biology with Fine Scale Techniques. Johns Hopkins University Press, Baltimore. [ Links ]
13. Sagor E.S., Ouellet M., Barten E. and Green D.M. (1998). Skeletochronology and geographic variation in age structure in the wood frog, Rana sylvatica. J. Herpetol. 32, 469–474. [ Links ]
14. Guarino F.M. and Erismis U.M. (2008). Age determination and growth by skeletochronology of Rana holtzi, an endemic frog from Turkey. Ital. J. Zool. 75, 237–242. [ Links ]
15. Driscoll D.A. (1999). Skeletochronological assessment of age structure and population stability for two threatened frog species. Aust. J. Ecol. 24, 182–189. [ Links ]
16. Castanet J. and Smirina E. (1990). Introduction to the skeletochronological method in amphibians and reptiles. Ann. Scien. Nat., Zool., Paris 13e Sér. 11, 191–196. [ Links ]
17. Coles W.C., Musick J.A. and Williamson L.A. (2001). Skeletochronology validation from an adult loggerhead (Caretta caretta). Copeia 1, 240–242. [ Links ]
18. Snover M.L. and Hohn A.A. (2004). Validation and interpretation of annual skeletal marks in loggerhead (Caretta caretta) and Kemp's ridley (Lepidochelys kempii) sea turtles. Fishery Bull. 100, 876–880. [ Links ]
19. Alho C.J.R. (1985). Conservation and management strategies for commonly exploited Amazonian turtles. Biol. Conserv. 32, 291–298. [ Links ]
20. Klemens M.W. and Thorbjarnarson J.B. (1995). Reptiles as food source. Biodiversity and Conservation 4, 281–298. [ Links ]
21. Chinsamy A. and Raath M.A. (1992). Preparation of bone for histological study. Palaeont. afr. 29, 39–44. [ Links ]
22. Alho C.J.R., Carlvalho A.G. and Pádua L.F.M. (1979). Ecologia da tartaruga da Amazônia e avaliaçâo de seu manejo na reserva biológica do Trombetas. Brasil Forestal 9, 29–47. [ Links ]
23. Hildebrand V.P., Bermúdez N. and Peñuela M.C. (1997). La tortuga charapa (Podocnemis expansa) en el río Caquetá, Amazonas, Colombia. Aspectos de su biología reproductiva y técnicas para su manejo. Disloque Editores, Santafé de Bogotá, Colombia. [ Links ]
24. Ramírez M.V. (1956). La tortuga, estudio biológico de la tortuga 'arrau' del Orinoco, Venezuela. El Agricultor Venzolano 21, 44–63. [ Links ]
25. Ojasti J. (1971). La tortuga Arrau del Orinoco. Defensa de la Naturaleza 1, 3–9. [ Links ]
26. Pritchard P.C.H. and Trebbau P. (1984). The Turtles of Venezuela. Society for the Study of Amphibians and Reptiles, Athens, Ohio. [ Links ]
27. Valenzuela N. (2001). Constant, shift and natural temperature effects on sex determination in Podocnemis expansa turtles. Ecology 82, 1108–1122. [ Links ]
28. Valenzuela N. (2001). Maternal effects on life history traits in the Amazonian giant river turtle Podocnemis expansa. J. Herpetol. 35, 368–378. [ Links ]
Received 12 December 2007. Accepted 18 May 2008.
* Author for correspondence. E-mail: anusuya.chinsamy-turan@uct.ac.za