Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.104 n.3-4 Pretoria Mar./Apr. 2008
RESEARCH LETTERS
Characterization of a succession of small insect viruses in a wild South African population of Nudaurelia cytherea capensis (Lepidoptera: Saturniidae)
Cheryl T. WalterI; Michele TomasicchioI; Valerie HodgsonI; Donald A. HendryI; Martin P. HillII; Rosemary A. DorringtonI, *
IDepartment of Biochemistry, Microbiology and Biotechnology, Rhodes University, P.O. Box 94, Grahamstown 6140, South Africa
IIDepartment of Zoology and Entomology, Rhodes University
ABSTRACT
The Tetraviridae are a family of small insect RNA viruses first discovered in South Africa some 40 years ago. They consist of one or two single-stranded (+) RNAs encapsidated in an icosahedral capsid of approximately 40 nm in diameter, with T = 4 symmetry. The type members of the two genera within this family, Nudaurelia β virus (NβV) and Nudaurelia ω virus (NωV), infect Nudaurelia cytherea capensis (pine emperor moth) larvae. The absence of N. capensis laboratory colonies and tissue culture cell lines susceptible to virus infection have limited research on the biology of NβV and NωV because the availability of infectious virus is dependent upon sporadic outbreaks in the wild N. capensis populations. In September 2002, dead and dying N. capensis larvae exhibiting symptoms similar to those reported previously in other tetravirus infections were observed in a wild population in a pine forest in the Western Cape province of South Africa. We report here the isolation of three small insect viruses from this population over a period of three years. Transmission electron microscopy and serological characterization indicate that all three are tetra-like virus isolates. One isolate was shown by cDNA sequence analysis to be NβV, which was thought to have been extinct since 1985. The two other isolates are likely new tetraviruses, designated Nudaurelia ψ virus (NψV) and Nudaurelia ζ virus (NζV), which are morphologically and serologically related to NωV and NβV, respectively.
Introduction
The Tetraviridae are a family of small insect RNA viruses consisting of one or two single-stranded, positive sense genomic RNAs encapsidated in an icosahedral capsid of approximately 40 nm in diameter, with T = 4 symmetry.1 Members of this family are classified into two genera: the monopartite betatetraviruses and bipartite omegatetraviruses, of which the type strains are Nudaurelia capensis β virus (NβV) and Nuda urelia capensis ω virus (NωV), respectively. The tetraviruses are the only viruses that exclusively infect insects, with a remarkably narrow host range limited to closely related species of the order Lepidoptera.2
The single genomic RNA of betatetraviruses, of approximately 6500 nucleotides (nts) in length, encodes the viral replicase and an overlapping capsid precursor protein, which is translated from a sub-genomic RNA.3–5 In the omegatetraviruses, RNA1 (approximately 5300 nts in length) encodes the replicase and three predicted small open reading frames.6,7 RNA2, of about 2500 nts, encodes the capsid precursor protein as well as an additional open reading frame designated p17,7–9 which does not appear to be essential for capsid assembly.10,11 The structure and assembly of tetravirus capsids, in particular NωV, have been the subject of intense interest. Tetravirus particles assemble as procapsids composed of 240 identical subunits that undergo maturation via assembly-dependent autoproteolytic cleavage of the capsid precursor protein at its carboxy terminus, leading to the production of the beta and gamma subunits.12 The release of the gamma peptide results in the stabilization of the mature virus particle, which is proposed to be important for infectivity.13,14 In some betatetraviruses, the capsid protein precursor undergoes additional cleavage at the amino terminus prior to assembly into procapsids.4,5,15
NβV was one of five small viruses (termed the Nudaurelia α, β, γ, δ and ε viruses) isolated more than thirty-five years ago from moribund larvae of Nudaurelia capensis (now classified as Imbrasia cytherea cytherea) and commonly known as the pine emperor moth.16,17 Structural characterization of NβV led to its classification as the first member of a new family of viruses, the Tetraviridae.18 A decline in the prevalence of NβV in the population coincided with the isolation of a second tetravirus, NωV, in 1985,19 the capsid of which has since been the focus of extensive structural studies.12,13 20–22 In contrast, little is known about the biology and life cycle of Nudaurelia viruses due to the absence of a laboratory colony of N. capensis and tissue culture cell lines susceptible to infection. Research on NβV and NωV has therefore been dependent upon the sporadic outbreaks within the wild N. capensis populations in the pine plantations of the Western Cape province in South Africa.
In September 2002, dead and dying N. capensis larvae, exhibitingsymptoms similar to those reported previously,16,17 were observed in a wild population in a pine forest in the Western Cape. We report here the characterization of a succession of small insect viruses from this population over a period of three years. Transmission electron microscopy and serological characterization indicate that all three are tetra-like virus isolates. One isolate was shown by cDNA sequence analysis to be NβV, which was thought to have been extinct since 1985. The two other isolates are likely new tetraviruses, which are morphologically and serologically related to NωV and NβV.
Materials and methods
See Appendix.
Results
In September 2002, dead and dying N. capensis larvae (VP2002) exhibiting symptoms similar to those reported previously16,17 were collected from a wild population in the Western Cape and subjected to a tetravirus purification protocol. Symptoms included lethargy, flaccidity of the larvae, cessation of feeding, vomiting and diarrhoea. Moribund larvae were subsequently also collected from the same population in 2003 and 2004 (VP2003 and VP2004, respectively) for further virus preparations. VP2002 were observed to exhibit more vomiting episodes whereas VP2003 and VP2004 larvae exhibited more discolouration among the other typical symptoms.
Isolation and characterization of VP2002, VP2003 and VP2004
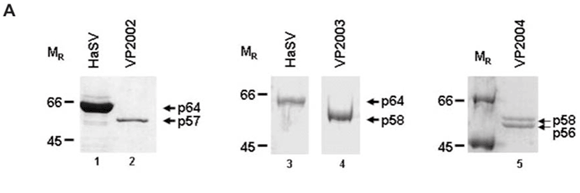
SDS–PAGE analysis of virus samples VP2002 revealed a major protein migrating at approximately 57 kDa (Fig. 1A, lane 2). This was significantly smaller than the mature capsid protein of Helicoverpa armigera stunt virus (HaSV), which is 64 kDa in size (Fig. 1A, lane 1). A protein migrating at approximately 58 kDa was detected in VP2003 (Fig. 1A, lane 4), which was also significantly smaller than the major capsid protein of HaSV (Fig. 1A, lane 3). This protein was also present in VP2004, which contained a second protein migrating at approximately 56 kDa (Fig. 1A, lane 5). We detected a small protein of between 6 and 7 kDa in size in VP2002 and VP2003, but not in VP2004 owing to the small amount of virus at our disposal (data not shown). As tetravirus capsids contain a major and a minor capsid protein between the sizes of 58–64 kDa and 6–8 kDa, respectively,1 the data suggested the presence of tetra-like viruses in the virus samples.
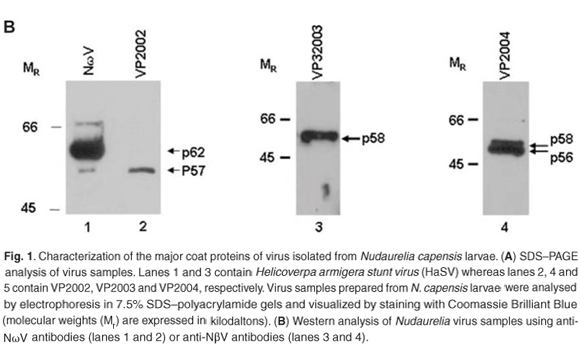
Western analysis confirmed that all three virus samples were serologically related to tetraviruses, with VP2002 cross-reacting with the NωV-specific antibodies whereas VP2003 and VP2004 were both detected by NβV-specific antibodies (Fig. 1B). However, the major band in VP2002, p57, was significantly smaller than that of the NωV control, which is 62 kDa in size (Fig. 1B, lane 2 vs lane 1), leading us to conclude that while VP2002 was serologically related, it was distinct from NωV with respect to the size of the major capsid protein. The 58 kDa protein in VP2003 and VP2004 was detected by the NβV-specific antibodies, which also cross-reacted with the 56 kDa protein in VP2004 (Fig. 1B, lanes 3 and 4). Since the predicted molecular weight (MR) for NβV is 58 kDa,3 we concluded that NβV was most likely present in both samples, and that p56 might represent a serologically related, but distinct NβV-like virus present in VP2004.
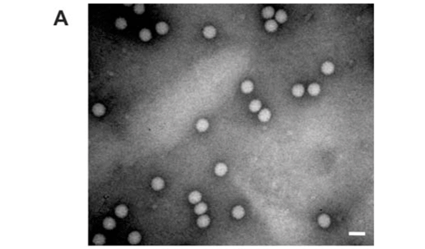
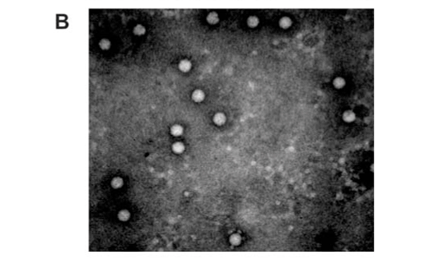
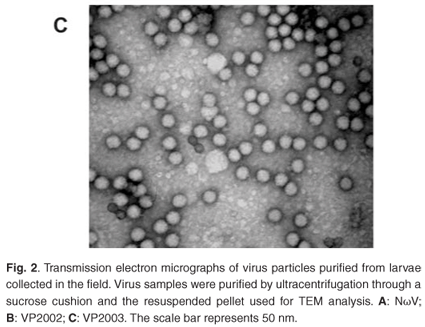
Analysis of the samples by transmission electron microscopy (TEM) confirmed the presence of tetravirus-like particles in VP2002 and VP2003 (Fig. 2B and C). As expected, particles in the NωV control sample were on average 40 nm in diameter with a surface morphology consistent with that observed by Hendry et al.19 and Canady et al.21 (Fig. 2A). Particles from VP2002 and VP2003 had an average diameter of 37.0 nm and 38.5 nm, respectively, with surface morphologies distinct from those of NωV (Fig. 2, compare panels B and C with A). The particles of VP2003 exhibited the typical pitted surface of NβV.18 The diameter and surface structure, together with the detection of a 58 kDa protein that cross-reacted with NβV-specific antibodies, led to the conclusion that the VP2003 was most likely NβV. However, although VP2002 cross-reacted with anti-NωV antibodies (data not shown), it appeared to be distinct from NωV with respect to the size of the major capsid protein (57 kDa vs 62 kDa for NωV), particle diameter (37 nm versus 40 nm for NωV) and surface morphology (Fig. 2, compare panels A and B). This suggested that VP2002 contained an NωV-like virus that was distinct from NωV.
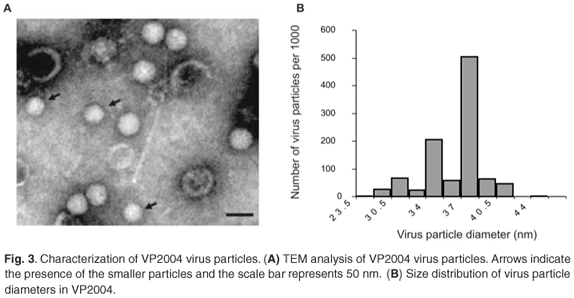
TEM analysis of VP2004 showed the presence of virus particles with the distinctive pitted surface of NβV (Fig. 3A). However, there appeared to be two types of particles with diameters of approximately 37 nm and 34 nm (Fig. 3A), representing 50% and 20% of the population, respectively (Fig. 3B). The remaining 30% of the diameters were found to be evenly distributed among the remainder of the diameter increments. Because p56 and the 34-nm particles are absent in VP2003, it is possible that p56 might be derived from the 34-nm particles in VP2004, which might therefore represent a tetravirus related to but distinct from NβV. Our attempts to separate the particles by density gradient ultracentrifugation were unsuccessful.
Molecular characterization of Nudaurelia virus isolates
We were unable to obtain cDNA from VP2002 or VP2004. In the case of VP2002, all attempts at RT–PCR using NωV capsid-specific primers were unsuccessful. As we had used the same primer set to characterize a confirmed NωV isolate,9 it was likely there might be significant divergence between the capsid coding sequence of VP2002 and NωV. The small sample available to us precluded further molecular analysis of VP2004.
RNA extracted from VP2003 particles appeared to be quite heterogeneous with several species ranging from 1.0 to 4.0 kb in size. None corresponded to the 6.6 kb (full-length) transcript generated from NβV cDNA (Fig. 4B). Since Northern analysis indicated the presence of NβV RNA (data not shown), we used RT–PCR to amplify three overlapping cDNA fragments covering a region of 1637 nucleotides corresponding to the 5' end of the replicase ORF and the entire NβV capsid gene (Fig. 4A), which confirmed the presence of NβV RNA in VP2003 (Fig. 4C). The DNA sequence of the two largest fragments (CapF × CapR and Rep2 × Cap2), covering all 2207 nt of the 6625 nt genome of NβV, was 99.5% identical to the published NβV sequence,3 confirming that VP2003 contained NβV. In all, there were 12 nucleotide substitutions, of which five would result in amino-acid substitutions in either the replicase and/or the capsid gene. Whether or not these polymorphisms are representative of the wild-type virus sequence is unknown because the cDNA sequence was derived from only two recombinant clones of each cDNA.
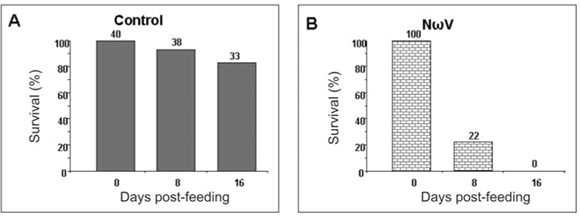
Virus-feed bioassays
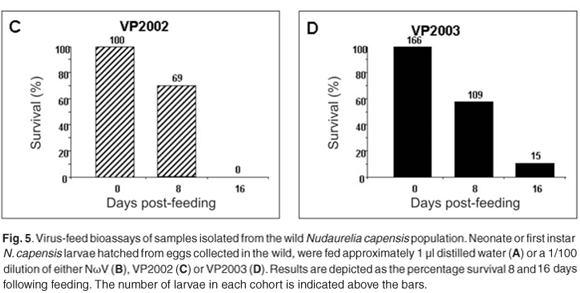
We have thus far been unable to establish a laboratory colony of N. capensis. Consequently, we were reliant upon eggs collected seasonally from the wild population to develop a virus-feed bioassay in the laboratory. There was a relatively low mortality rate among the control larvae (Fig. 5A), whereas animals fed with NωV (isolated in 1996 from a different location)9 showed symptoms of regurgitation and diarrhoea and all had died after 16 days post-feeding (Fig. 5B). Similarly, there was 100% mortality among larvae infected with VP2002, which exhibited similar symptoms to those fed with NωV (Fig. 5C). Animals fed with VP2003 were discoloured, lethargic and flaccid, with most having died 16 days post-feeding (Fig. 5D). These symptoms are similar to those observed by Jukes16 and Hendry et al.19 in larvae infected with NβV or NωV. We were unable to bioassay VP2004 because we were unable to collect sufficient numbers of viable N. capensis eggs during the 2006 season.
Virus samples extracted from the larvae fed with NωV, VP2002 and VP2003 as well as the control group were subjected to SDS–PAGE and Western analysis with NωV- and NβV-specific antibodies. A 62 kDa protein corresponding to the expected NωV mature capsid protein was detected in larvae infected with NωV, whereas those fed VP2002 showed the presence of a protein migrating at 57 kDa, which also cross-reacted with the NωV antibodies (Fig. 6, lanes 2 and 3). There was no evidence of either protein in the control sample (Fig. 6, lane 1). The presence of faint bands with a relative MR of 70 kDa and 63 kDa in the NωV and VP2002 lanes, respectively, probably indicates the co-purification of immature virus particles represented by the capsid precursor protein. Based on this and SDS–PAGE analysis, we estimated the relative MR of the VP2002 minor capsid protein to be approximately 6 kDa, which is smaller than the 8 kDa γ peptide of NωV. This needs to be confirmed by mass spectroscopy.
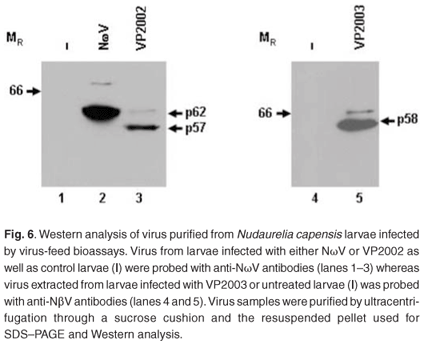
A 58 kDa protein cross-reacting with the NβV-specific antibodies was detected in larvae fed with VP2003, but not in the control sample (Fig. 6, lane 5 vs lane 4). Small amounts of this protein were subsequently detected in undiluted control samples (data not shown), indicating a low background level of NβV infection in the insects, which may have been present in the wild population from which the eggs were collected. The presence of the capsid protein precursor migrating at approximately 66 kDa, which is consistent with the predicted MR for NβV,3 was also detected.
Discussion
The data presented here indicate the presence of three distinct tetra-like viruses in a wild population of N. capensis, one of which is NβV, which has not been detected since 1985.19 There are two other viruses present in VP2002 and VP2004, which are serologically related to NωV and NβV, respectively. In the case of VP2002, there are differences in the size of the major capsid protein and average particle diameter as compared with those of NωV. Our inability to produce cDNA using primers specific to the NωV capsid coding sequence suggests the potential for significant sequence variation between the two viruses. We therefore hold that VP2002 may represent a new, NωV-like virus, which we have designated Nudaurelia ψ virus (NψV). The similar symptoms exhibited by NωV- and NψV-infected larvae might reflect a related pathology. With respect to VP2004, the presence of p56 together with a population of virus particles with a smaller diameter than that of NβV suggests the presence of potentially another new Nudaurelia virus, which we propose to name Nudaurelia ζ virus (NζV). Their size, morphology, serological relatedness and the characteristics of the capsid proteins suggest that NψV and NζV may be tetraviruses. However, this can only be confirmed by obtaining the cDNA sequence of these viruses. Although the lack of a laboratory colony of N. capensis and limited availability of eggs collected from the field have made future studies on this virus difficult, we intend to pursue this further should we be able to collect viable eggs in future seasons.
Replication of RNA viruses is generally known to have a high error rate28 with the presence of virus quasi-species proposed to be important for the long-term persistence in the host.29 It has been reported that RNA genomes can mutate at a rate of between 10–3 and 10–5 substitutions per nucleotide copied.28 The high degree of sequence conservation between VP2003 and the NβV genome sequence, which was derived from a 1985 NβV isolate found in a geographically distinct N. capensis population,3 is therefore unexpected. The same sequence conservation has also been observed between geographically and temporally distinct NωV isolates.9 It would be interesting to determine whether the apparent lack of sequence diversion among the NβV and NωV virus populations is due to selection pressure or an unusually low mutation rate.
Since 1965, the emergence and in some cases disappearance of eight viruses (including those identified in this study) have been recorded in the wild populations of N. capensis in South Africa (refs 19, 30, 31 and this study]. Of these, NωV and NβV appear to have been the most dominant, but our data indicate that two other potential tetraviruses may also exist in the wild N. capensis population. The Tetraviridae family currently comprises thirteen confirmed members along with several as yet unclassified isolates. This study has characterized two potentially new Nudaurelia tetraviruses, which together with NωV and NβV bring the total of tetraviruses that infect N. capensis to four. It is reasonable to expect that the same could be true for other lepidopteran species, which implies that tetraviruses may be relatively ubiquitous in the lepidopteran insects and that the majority may as yet be undiscovered.
This research was supported by a grant from the Rhodes University Joint Research committee. Cheryl Walter and Michele Tomasicchio were holders of National Research Foundation scholarships. The authors acknowledge the technical assistance of Bintou Ahmadou Ahidjo and wish to thank Terry Hanzlik of CSIRO, Division of Entomology, Canberra, Australia, for kindly providing wild-type HaSV, and Mark Wilmot of MTO in Stellenbosch for his help in collecting N. capensis eggs and larvae in the field.
1. Ball L.A., Hendry D.A., Johnson J.E., Rueckert R.R. and Scotti P.D. (2005). Family Tetraviridae. In Virus Taxonomy, eds C.M. Fauqet, M.A. Mayo, J. Maniloff, U. Desselberger and L.A. Ball, Eighth Report of the International Committee on Taxonomy of Viruses, pp. 873–883. Elsevier Academic Press, San Diego and London. [ Links ]
2. Hanzlik T.N. and Gordon K.H.J. (1997). The Tetraviridae. Adv. Virus Res. 48, 101–168. [ Links ]
3. Gordon K.H.J., Williams M.R., Hendry D.A. and Hanzlik T.N. (1999). Sequence of the genomic RNA of Nudaurelia β virus (Tetraviridae) defines a novel virus genome organization. Virology 258, 42–53. [ Links ]
4. Gorbalenya A.E., Pringle F.M., Zeddam Z-L., Luck B.T., Cameron C.E., Kalmakoff J., Hanzlik T.N., Gordon K.H. and Ward V.K. (2002). The palm subdomain-based active site is internally permuted in viral RNA-dependent RNA polymerases of an ancient lineage. J. Mol. Biol. 324, 47–62. [ Links ]
5. Pringle F.M., Johnson K.N., Goodman C.L., McIntosh A.H. and Ball L.A. (2003). Providence virus: a new member of the Tetraviridae that infects cultured insect cells. Virology 306, 359–370. [ Links ]
6. Gordon K.H.J., Johnson K.N. and Hanzlik T.N. (1995). The larger genomic RNA of Helicoverpa armigera stunt virus encodes the viral RNA polymerase and has novel 3'-terminal tRNA-like structure. J. Virol. 208, 84–98. [ Links ]
7. Yi F., Zhang J., Yu H., Liu C., Wang J. and Hu Y. (2005). Isolation and identification of a new tetravirus from Dendrolimus punctatus larvae collected from Yunnan Province, China. J. Gen. Virol. 86, 789–796. [ Links ]
8. Hanzlik T.N., Dorrain J., Gordon K.H.J. and Christian P.D. (1995). Sequence of RNA2 of the Helicoverpa armigera stunt virus (Tetraviridae) and bacterial expression of its genes. J. Virol. 76, 799–811. [ Links ]
9. du Plessis L., Hendry D.A., Dorrington R.A., Hanzlik T.N., Johnson J.E. and Appel M. (2005). Revised sequence of the tetravirus Nudaurelia capensis ω virus (NωV). Arch. Virol. 150, 2397–2402. [ Links ]
10. Agrawal D.K. and Johnson J.E. (1995). Assembly of the T=4 Nudaurelia capensis ω virus capsid protein, post-translational cleavage and specific encapsidation of its mRNA in a baculovirus expression system. Virology 207, 89–97. [ Links ]
11. Tomasicchio, M., Venter, P.A., Gordon, K.H.J., Hanzlik, T.N. and Dorrington R.A. (2007). The induction of apoptosis results in spontaneous maturation of tetravirus procapsids in vivo. J. Gen. Virol. 88, 1576–1582. [ Links ]
12. Munshi S., Liljas L., Cavarelli J., Bomu W., McKinney B., Reddy V. and Johnson, J.E. (1996). The 2.8Å Structure of a T=4 animal virus and its implications for membrane translocation of RNA. J. Mol. Biol. 261, 1–10. [ Links ]
13. Taylor D.J., Krishna N.K., Canady M.A., Schneemann A. and Johnson J.E. (2002). Large-scale, pH-dependant, quaternary structure changes in an RNA virus capsid are reversible in the absence of subunit autoproteolysis. J. Virol. 76, 9972–9980. [ Links ]
14. Helgstrand C., Munshi S., Johnson J.E. and Liljas L. (2004). The refined structure of Nudaurelia capensis omega virus reveals control elements for a T=4 capsid maturation. Virology 318,192–203. [ Links ]
15. Pringle F.M., Kalmakoff J. and Ward V. (2001). Analysis of the capsid processing strategy of Thosea asigna virus using baculovirus expression of virus-like particles. J. Gen. Virol. 82, 259–266. [ Links ]
16. Juckes I.R.M. (1970). Viruses of the pine emperor moth. Bull. S. Afr. Soc. Plant Pathol. Microbiol. 4, 18. [ Links ]
17. Struthers J.K. and Hendry D.A. (1974). Studies of the protein and nucleic acid components of Nudaurelia capensis β virus. J. Gen. Virol. 22, 355–362. [ Links ]
18. Olson N.H., Baker T.S., Johnson J.E. and Hendry D.A. (1990). The three-dimensional structure of frozen-hydrated Nudaurelia capensis β virus, a T=4 insect virus. J. Struct. Biol. 105, 111–122. [ Links ]
19. Hendry D.A., Hodgson V., Clark R. and Newman J. (1985). Small RNA viruses coinfecting the pine emperor moth (Nudaurelia cytherea capensis). J. Gen. Virol. 6, 627–632. [ Links ]
20. Canady M.A., Tihova M., Hanzlik T.N., Johnson J.E. and Yeager M. (2000). Large conformational changes in the maturation of simple RNA virus, Nudaurelia capensis omega virus (NωV). J. Mol. Biol. 299, 573–584. [ Links ]
21. Canady M.A., Tsuruta H. and Johnson J.E. (2001). Analysis of rapid, large-scale protein quaternary structural changes: time resolved X-ray solution scattering of Nudaurelia capensis omega virus (NωV) maturation. J. Mol. Biol. 311, 803–814. [ Links ]
22. Bothner B., Taylor D.A., Jun B., Lee K.K., Suizdak G., Schultz C.P., and Johnson J.E. (2005). Maturation of a tetravirus capsid alters the dynamic properties and creates a metastable complex. Virology 207, 17–27. [ Links ]
23. Morris T.J., Hess R.T. and Pinnock D.E. (1979). Physiochemical characterization of a small RNA virus associated with baculovirus infection in Trichoplusia ni. Intervirology 11, 238–247. [ Links ]
24. Pringle F.M., Gordon K.H.J., Hanzlik T.N., Kalmakoff J. Scotti P.D. and Ward V. (1999). A novel capsid expression strategy for Thosea asigna virus (Tetraviridae). J. Gen. Virol. 80, 1855–1863. [ Links ]
25. Laemmli U. (1970). Cleavage of structural proteins during assembly of the head of a bacteriophage T4. Nature 227, 680–685. [ Links ]
26. Dong X.F., Natarajan P., Tihova M., Johnson J.E. and Schneemann A. (1998). Particle polymorphism caused by deletion of a peptide molecular switch in a quasi-equivalent virus. J. Virol. 72, 6024–6033. [ Links ]
27. Agrawal D.K. and Johnson J.E. (1992). Sequence and analysis of the capsid protein of Nudaurelia capensis omega virus, an insect virus with T = 4 icosahedral symmetry. Virology 190, 806–814. [ Links ]
28. Drake J.W. and Holland J.J. (1999). Mutation rates among RNA viruses. Proc. Natl Acad. Sci. USA 96, 13910–13913. [ Links ]
29. Domingo E., Baranowski E., Ruiz-Jarabo C.M., Martin-Hernandez A.M., Saiz J.C. and Escarmis C. (1998). Quasispecies structure and persistence of RNA viruses. Emerg. Infect. Dis. 4, 521–527. [ Links ]
30. Hendry D.A., Becker M.F. and van Regenmortel M.V.H. (1968). A non-inclusion virus of the pine emperor moth Nudaurelia cytherea Stoll. S. Afr. Med. J. 42, 117. [ Links ]
31. Juckes I.R.M. (1979). Comparison of some biophysical properties of the Nudaurelia β and ε viruses. J. Gen. Virol. 42, 89–94. [ Links ]
Received 12 June 2007. Accepted 12 April 2008.
* Author for correspondence. E-mail: r.dorrington@ru.ac.za
Virus purification
The virus purification protocol was modified from Morris et al.23 In the case of larvae collected from the wild, approximately 200 g larvae was blended using a Waring homogenizer in extraction buffer (0.5 M Tris-HCl at pH 7.5, 0.01 M Na2EDTA, 0.2% β-mercaptoethanol, 250 ml of 98% butan-1-ol made up to 1 litre in distilled water) at 4°C. For virus purification from virus feed bioassay experiments, volumes were adjusted proportional to the mass of the larvae. The supernatant was clarified by centrifugation at 11 325 × g for 15 minutes at 4°C and the pellet resuspended and pelleted in extraction buffer. The supernatants were pooled and the virus precipitated by the addition of 8% PEG 6000 and 0.1 M NaCl. The precipitated proteins were pelleted by centrifugation at 11 325 × g for 15 minutes at 4°C and the pellet resuspended in one-tenth volume of TE buffer (0.1 M Tris-HCl, 0.01 M Na2EDTA, pH 7.5) followed by centrifugation at 11 325 × g for 10 minutes at 4°C to pellet insoluble protein. The virus particles were pelleted from the supernatant through a 1.5 ml 30% sucrose cushion (w/v in 50 mM Tris-HCl at pH 7.5) in a Beckmann SW 41Ti at 40 000 rpm for 1.25 hours at 4°C. The pellet was left at 4ºC overnight to resuspend in 50–100 µl 50 mM Tris-HCl (pH 7.5). Virus samples used for RNA extraction were subjected to discontinuous CsCl density gradient ultracentrifugation as described by Hendry et al.19 Virus samples used for virus feed bioassays were subjected to a linear 10–40% sucrose (w/v in 50 mM Tris-HCl, pH 7.5) gradient as described by Pringle et al.24 Wild-type HaSV virus particles were obtained from Terry Hanzlik, CSIRO Division of Entomology, Canberra, Australia.
SDS–PAGE and Western analysis
Diluted virus samples were analysed by SDS–PAGE according to Laemmli,25 and proteins visualized by staining with Coomassie Brilliant Blue. Western analysis was conducted using the Roche Chemiluminescence Western Blotting system with polyclonal antibodies raised against NβV and NωV.17,19
Virus-feed bioassays
N. capensis eggs were collected from the wild and allowed to hatch at room temperature or stored at 4°C up to six weeks until needed. Neonates were dehydrated overnight and then fed a droplet containing 1:100 purified virus diluted in water (approximately 107 particles per µl). The larvae were then placed (in groups of three) in sterile 50-ml centrifuge tubes containing sprigs of Pinus radiata needles and sealed with muslin cloth. Fresh, hydrated pine needles were replaced as needed and dead larvae were collected on a daily basis and stored at –80°C until further use.
Transmission electron microscopy
Virus particles were prepared and analysed using the methods of Dong et al.26 or Taylor et al.13 For estimation of particle size, the diameters of between 500 and 1000 particles were measured.
RNA extraction
A protocol modified from Hendry et al.19 and Agrawal and Johnson27 was used for extraction of viral RNA. Next, 300 µl of CsCl fractions containing virus was mixed with 15 µl of 10% SDS, heated to 80°C for 50 seconds and then cooled rapidly on ice for 5 minutes, after which 15 µl of 1 × TAE buffer (0.04 M Tris-HCl, pH 8.5; 0.02 M acetic acid; 0.05 M Na2EDTA) was added. An equal volume of phenol (equilibrated with 50 mM Tris-Cl pH 8.0; 10 mM Na2EDTA) was added and the tube vortexed for 1 minute, followed by the addition of 300 µl of chloroform and further vortexing for 1 minute. The sample was centrifuged at 14 000 × g for 10 minutes at room temperature, the aqueous phase removed and subjected to two further phenol/chloroform extractions. Next, 300 µl chloroform was added to the aqueous phase followed by vortexing for 1 minute and centrifugation at 14 000 × g at room temperature for 10 minutes. The aqueous layer was the subjected to a further two chloroform extractions and the RNA precipitated by adding 15 µl of 2 M sodium acetate (pH 5.2), 30 µl of 2 M acetic acid and 600 µl of ice-cold 95% ethanol to the aqueous phase. The solution was left to precipitate at –20°C overnight, microfuged at 14 000 × g for 15 minutes at 4°C, and the pellet washed with 600 µl ice-cold 70% ethanol. The pellet was then dried, resuspended in 40 µl DEPC-treated water and stored at –80°C. RNA was analysed by formaldehyde denaturing electrophoresis and the relative sizes of RNAs determined using the Sigma-Aldrich 280–6600 nt RNA molecular weight marker.
cDNA synthesis and sequence analysis
The Expand Reverse Transcriptase kit (Roche Applied Sciences) was used for cDNA synthesis. NβV RNA was treated with DNAse (MBI Fermentas) and subjected to RT–PCR using primers CapF (gga tcc aat ATG GAT GCC AAC GTG CAG ATA AC) and CapR (GGG AAC CTT AGC TTG TAG GTT CGA A) corresponding to nucleotides 4030–4061 and 5885–5860 as well as Rep2 (GAA TGG GCA GTC AGA CCG) and Cap2 (CGG GTC TAG ATA GTC GTG) or NBV4050 (TGG CAT CCA TCT TGT CCG) corresponding to nucleotides 3248–3265, 4300–4317 and 4050–4032 of the NβV genome, respectively. The amplicons were ligated into the pGEM-T Easy vector and two recombinant clones of each were used to determine the cDNA sequence.