Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.104 no.3-4 Pretoria Mar./Abr. 2008
RESEARCH ARTICLES
The feasibility and implications of nuclear georeactors in Earth's core–mantle boundary region
R.J. de MeijerI, II, *; W. van WestrenenIII
IStichting EARTH, de Weehorst, 9321 XS Peize, The Netherlands
IIDepartment of Physics, University of the Western Cape, Private Bag X17, Bellville 7535, South Africa
IIIFaculty of Earth and Life Sciences, Vrije Universiteit Amsterdam, De Boelelaan 1085, 1081 HV Amsterdam, The Netherlands
ABSTRACT
We examine the likelihood and geochemical consequences of the existence of nuclear georeactors in the core–mantle boundary region (CMB) between the Earth's silicate mantle and metallic core. Current geochemical models for the Earth's interior predict that U and Th in the CMB are concentrated exclusively in the mineral calcium silicate perovskite (CaPv), leading to predictable concentration levels of approximately 12 ppm U + Th, 4.5 Ga ago assuming that CaPv is distributed evenly throughout the CMB. Assumption of a similar behaviour for primordial 244Pu provides a substantial flux of neutrons from spontaneous fission. We show that an additional concentration factor of only an order of magnitude is required both to initiate and maintain self-sustaining nuclear georeactors based on fast fission. Continuously operating georeactors with a power of 5 TW (1 TW = 1012 W) can account for the observed isotopic compositions of helium and xenon in the Earth's mantle. Our hypothesis requires the presence of elevated concentrations of U and Th in the CMB, and is amenable to testing by direction-sensitive geoneutrino tomography.
Introduction
Accurate knowledge of the concentrations and distribution of the main heat-producing elements uranium, thorium and potassium in the interior of the Earth is required to account for our planet's thermal evolution and dynamics. Classically, these elements are considered to be concentrated predominantly in the Earth's crust,1 with significantly lower concentrations in the underlying mantle and core.2 Recent measurements of small but significant excesses of 142Nd (formed by α-decay of 146Sm, t1/2 = 103 Myr) in terrestrial rocks, compared with undifferentiated meteorites,3,4 have dramatically altered this notion.
These 142Nd excesses indicate that all terrestrial samples have formed from a reservoir with an initial Sm/Nd ratio that is higher than found in the meteorites that are likely to have formed the building blocks of Earth. This in turn requires the presence of an ancient (>4.52 Ga) isolated geochemical reservoir in the Earth's interior that has a complementary lower initial Sm/Nd ratio. Mass-balance calculations show that this early-enriched hidden reservoir must also be enriched in other lithophile elements, including K, U and Th. The current view is that up to 40% of the mantle's budget of U and Th (equivalent to 20% of the total terrestrial inventory) is located in this isolated reservoir.5
Seismic observations show that the Earth's mantle convects,6 and numerical models demonstrate that this convection severely limits the possibility of material remaining completely isolated in the bulk mantle for 4.5 billion years (Ga).7 As a result, the only likely locus of an isolated geochemical reservoir is in the lowermost part of the mantle, the core–mantle boundary (CMB) region directly on top of Earth's metallic liquid outer core. Material that resides at this lower boundary layer for mantle convection has limited interaction with the convection cells. According to Tolstikhin et al.,5 only 6% of the initial isolated reservoir mixes back into the remainder of the mantle after formation.
These significant findings have prompted our re-examination of the probability and consequences of the presence of active natural nuclear reactors (so-called georeactors) in the CMB region. Georeactors in the Earth's metallic core, as significant heat-producing entities in its interior, have been proposed at various times in the last fifty years.8–13 Various authors10–13 have estimated their present-day power to be between 4 and 30 TW, which is significant, compared to the observed present-day heat flux at Earth's surface (31–44 TW).14,15 Uncertainties in the size of the Earth's various internal heat sources can allow for additional heat sources of 0–15 TW, such as from georeactors.16 Analysis of the recent KamLAND data on antineutrino fluxes has also set an upper limit of 18 TW arising from georeactors.17
Previous studies that have postulated active georeactors invoke a gravitationally-induced concentration of actinides to supercritical values in the metallic core of the Earth, either in the centre11 or on top of the progressively crystallizing solid inner core.13 To date, these studies have been largely rejected by Earth-science researchers, mostly for geochemical reasons. Although laboratory experiments show that it is possible to incorporate uranium in iron-rich metallic melts under highly reducing conditions at high pressures and temperatures,18,19 these conditions also lead to incorporation of significant amounts of trace and even major elements (among them Mg, Ca, and the lanthanides) in the metallic phase. This is inconsistent with most geochemical models for the composition of the Earth,2,20 as they show that Mg, Ca, and the lanthanides cannot be present in the core at concentration levels that would accompany significant U incorporation, if undifferentiated meteorites formed the basic building blocks of our planet.
We first combine experimental results in this paper, quantifying the distribution of U and Th between the main silicate minerals present in Earth's lower mantle, with geochemical estimates for the U and Th content of the CMB region. We also assess the CMB concentration of Pu, because of the potentially important role of its isotope 244Pu (t1/2 = 80 Myr) in fission processes in the early Earth. Secondly, we calculate the concentrations of U, Th, and Pu that are required for a georeactor to operate spontaneously and we assess the feasibility of U/Th/Pu concentrations in the CMB reaching these levels.
After concluding that both starting and sustaining georeactors appear to be feasible, we analyse some critical geochemical consequences of the presence of georeactors in the CMB. We show that the observed isotopic compositions of Earth's noble gas inventory for helium, krypton and xenon can be explained through a time-integrated georeactor energy production of 7 × 1029 J, equivalent to continuous activity of a single reactor for a period of 4.5 Ga with a power of 5 TW. We also conclude that the fission products of georeactors can be traced from the He and Xe isotopic composition of the Earth's subsurface. Geoneutrino tomography provides the best and, at present, the only way to test the georeactor hypothesis, and may find an answer to the question whether nuclear reactors presently exist in the interior of the Earth.
Constraints on U, Th, and Pu distribution in the CMB
Addressed in our introduction, current models of the evolution of the early Earth, based on geochemical analyses of terrestrial material and meteorites, require the presence of a 'hidden', deep-Earth geochemical reservoir that has remained isolated from convective processes in Earth's mantle for >4.52 Ga.3 Tolstikhin et al.5 state that this reservoir, necessarily located in the CMB, contains up to 20% of the total terrestrial inventory of radiogenic heat-producing elements K, U and Th. We assume that Pu behaves similarly, and that the Pu concentration in the BSE equalled 0.19 ppb at 50 Myr after the creation of the solar system.21 Table 1 presents the masses of 232Th, 235U, 238U and 244Pu present in the bulk silicate Earth (BSE, equivalent to continental crust + mantle) today and 4.55 Ga ago, using the BSE model of McDonough.2 Assuming the BSE mass of 4.0 × 1024 kg, and CMB mass of approximately 2.0 × 1023 kg, the concentrations of U and Th in the CMB are calculable to be 80 and 320 ppb at present, respectively. When the isolated reservoir was formed, these values (corrected for nuclear decay) would have been 210 ppb U, 400 ppb Th and 0.8 ppb Pu. It should be noted that these concentrations depend inversely on the assumed mass of the CMB, which is not well constrained.

These concentrations are averages for the CMB as a whole. However, high-pressure experiments on putative mantle rocks, in combination with geophysical observations of the density structure of Earth's interior, show that virtually all of Earth's lower mantle (at depths > 660 km) consists of three main minerals:65 vol% magnesium silicate perovskite [MgPv, nominal chemical composition (Mg,Fe)SiO3], 30 vol% ferropericlase (FP, nominal composition (Mg,Fe)O) and 5 vol% calcium silicate perovskite (CaPv, nominal composition CaSiO3).
Experimental studies have also quantified U and Th distribution between these phases at high pressures and temperatures.22 Although it is currently impossible to obtain distribution data at CMB conditions (i.e. pressures of approximately 125 GPa and temperatures of 2500–4000 K), experiments at 25 GPa and 2600 K indicate that U and Th concentrations in calcium silicate perovskite are 3–4 orders of magnitude greater than concentrations in co-existing MgPv.22 The recently discovered new high-pressure form of MgPv, named postperovskite,23 which may be stable in the CMB, is unlikely to influence this distribution. Ferropericlase in turn generally incorporates even lower concentrations of trace elements than MgPv.24 No experimental data on Pu distribution are available, but trends in distribution as a function of ionic radius indicate that Pu favours CaPv over the other lower mantle minerals by two orders of magnitude.
Combining this information and assuming that 5 vol% of the fully-mixed CMB consists of CaPv, we conclude that concentrations of U, Th and Pu in CaPv were 4.3 ppm, 7.9 ppm and 23 ppb, respectively, upon formation of the isolated reservoir. Domains with lower concentrations of CaPv and/or higher concentrations of U, Th, and Pu may have even greater concentrations of these fissile nuclei.
Concentration requirements for the initiation and operation of georeactors
In this section we consider the conditions required for initiation and progression of georeactors in the CMB 4.5 Ga ago. Fission of heavy nuclei such as thorium and uranium produces approximately 200 MeV (3.2 × 10–11 J) energy, and releases about three fast neutrons. Several nuclei fission spontaneously; the probability is very small compared with α-decay for nuclei such as 235U, 238U, and 244Pu, but is significant for initiating georeactors. The initiation is dependent upon whether criticality conditions for a georeactor can be met, while sustainable conditions are related to the availability of sufficient reactants. Criticality is achieved if the neutron-multiplication factor keff exceeds unity.
Fission simulations by Ravnik and Jeraj25 show that a local concentration of U of 1 wt% is required to have initiated a georeactor 4.5 Ga ago, and that keff cannot exceed unity for Th/U ratios larger than 1.1. The availability of Pu has not been taken into account by their calculations that relate to probability of present-day reactors. The ratio between spontaneous fission (sf) and α-decay of 244Pu is a million times larger than for 238U. So, despite three orders of magnitude lower concentration than 238U, spontaneous fission of 244Pu, if present, will be a more important source of neutrons than spontaneous fission of 238U, by about three orders of magnitude.
Every neutron may start a chain reaction, but as long keff < 1, all chains will extinguish, and the reactor will remain subcritical. Nevertheless, under these conditions, fissile materials such as 233U, 239,240Pu are produced, and under the conditions described above, it is probable that a georeactor will become critical. This will first be at low power density, but with an increase in fuel production or concentration of the geological processes, the power density will increase.
Our calculations show that the presence of 244Pu in the early Earth plays a crucial role in raising keff to supercritical levels. In any mass or volume, the value of k is the difference between the number of neutrons released by fission events and the numbers lost due to absorption. Power production is directly related to the rate of production of neutrons, which in turn is related to the specific spontaneous fission rate of nuclei. The mass fractions of 235U and 238U were 0.24 and 0.76, respectively, at time = –4.5 Ga ago (see Table 1). For U, this amounts to a fission rate of 8.6 × 10–17 per atom per year, and 2.8 × 10–11 per atom per year for 244Pu. A concentration of 1 wt% U corresponds to 3.6 × 10–18 × NA kg–1 yr–1, NA being Avogadro's number. Interestingly, the fission rate for 4.3 ppm U and 19 ppb Pu (estimated concentrations for CaPv in the CMB) amounts to 2.1 × 10–18 × NA kg–1 yr–1, approximating the value required for criticality in an environment without Th. The presence of Th reduces the value of keff to 0.8 at a Th/U ratio of 1.8 in the absence of 244Pu.25 A local concentration factor for Pu in the CMB of only an order of magnitude (compared to a homogeneous distribution of CaPv) is required to raise keff sufficiently and initiate a georeactor. Seismic observations show that at present the CMB region is extensively heterogeneous, as assessed through seismic wave velocity measurements.26 Volumes exhibiting both higher-than-average and lower-than-average wave propagation speeds, with diameters as small as 30 km, are now resolvable. Although the precise nature of these heterogeneities remains unresolved, we propose that significant local concentration factors are currently plausible. The dynamics of the CMB 4.5 Ga ago are poorly explored. The higher rotation rate of Earth at that time, and higher interior temperatures, are likely to have facilitated local concentration of density heterogeneities to levels that exceed those currently observed, due to centrifugal forces and buoyancy effects associated with local heating.
Assuming that one or more georeactors were initiated in the CMB shortly after the formation of an isolated reservoir, we now consider the conditions that are required for a reactor to sustain operation. In the likely absence of large amounts of hydrogen in the CMB, neutrons produced by fission will be thermalized only gradually, and will have a mean free path on the order of a hundred metres. Apart from generating fission, they will be captured mainly by 238U and 232Th, producing 239Pu and 233U, respectively, which in turn are fissile nuclides. In addition, 239Pu decays through α-particle emission to 235U. Although Th, in particular, may hamper the initiation of a georeactor, capture will eventually lead to an increase in fuel for a potential georeactor. The potential georeactor, after initiation, will sustain itself as a breeder reactor and will be self-perpetuating due to generation of its own fuel by the processes that have been described.10,27,28
The question remains as to whether U and Th concentrations of 4.3 and 7.9 ppm in CaPv in the CMB are adequate for sustainable georeactors 4.5 Ga ago (note that the 19 ppb of 244Pu plays no role). One of the three neutrons produced in fission must lead to a new fission process to maintain a nuclear reaction, keff = 1; the two others are expected to be absorbed by the CMB materials. This leads to the following simplified relationship between the masses of fissionable, mf, and non-fissionable material, mr:

where σa and σf are the atomic cross sections for neutron absorption in the non-fissionable material and the cross section for fission, respectively, and molr and molf represent the average molecular weights. According to Rinard,29 the cross-section ratio at 1 MeV neutron energy is about 2000–3000, indicating that the homogeneously mixed CaPv and a homogeneous mixture of U and Th constitute concentrations that are a factor of twenty too low for the assumed mass of the CMB. Such an additional concentration factor is probable for a dynamic system such as the CMB. For example, concentration factors of several orders of magnitude could easily occur during local partial melting. In the case of CaPv, cyclic flow due to radiogenic heating, followed by cooling, could arise from the interaction of gravitational and thermal stabilities. In a functioning georeactor, such a mechanism could 'clear' the reactor of its less dense fission products.
A concentration factor of twenty is even necessary to prevent the total CMB becoming a georeactor. We point out that the concentration factors for initiation and sustained operation are independently comparable.
Geochemical consequences of georeactors
Concluding that georeactors in the CMB are feasible, we consider some of their geochemical consequences. We limit ourselves to a first-order approximation, which implies (1) binary fission of 232Th, 235U and 238U is considered alone (no Pu fission is incorporated); (2) initial concentrations remain constant over the full period of activity; (3) thermal and fast fission contribute equally; (4) all three fuel nuclei have the same probability of fission; (5) the probabilities of production of the nuclides are taken from the code SCALE;30 (6) the fission products are assumed to mix homogeneously with the material in the CMB; (7) a fraction of the fission products (10 wt%) enters the overlying mantle and is homogeneously mixed by mantle convection.
We focus on the noble gases helium, krypton and xenon. Neon and argon are disregarded, as they are minimal products of binary fission. In addition, argon consists mainly of 40Ar, a decay product of 40K. We also present calculations for Se, Mo, Ru and Pd. These elements could be of interest in testing the georeactor hypothesis, because of their low abundance in the silicate Earth, combined with high probabilities for production of some of their isotopes by binary fission.
Helium
The 3He/4He atomic ratio in Earth's atmosphere31 is 1.37 × 10–6. α-Particles are emitted during the radioactive decay series of uranium and thorium, becoming 4He atoms. Spontaneous fission of 235U and 238U generates 3H (tritium), which decays with a half-life of 12.3 yr to 3He. This conversion is assumed to take place instantaneously and with 100% efficiency. Table 2 presents the half-life for radioactive decay of the Th and U nuclides, the number of α-particles produced in the decay, the half-life for spontaneous fission, the relative probability of fission in the decay, the probabilities per fission for the formation30 of 3H and 4He, and the expected 3He/4He ratios. The 3He value in this ratio is the production probability via spontaneous fission only. For 4He, we use the sum of production in natural decay and in spontaneous fission. Examining the last column of Table 2, it is clear that the 3He/4He ratio in the atmosphere cannot be accounted for solely by natural decay of U, Th and Pu, including spontaneous fission. A ratio of 6.6 × 10–10 is obtained, when taking into account the decayed masses of the various nuclides.
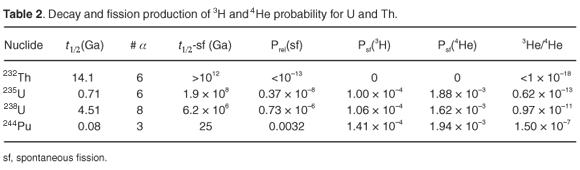
The probabilities for thermal and fast fission are given in Table 3,together with the 3He/4He ratios for pure fission. From comparison of the last columns in Tables 2 and 3, it is evident that fission in a georeactor, followed by transport of He to the atmosphere, will lead to a significant change in the atmospheric 3He/4He ratio.

We estimate the 3He and 4He production in the CMB from radioactive decay and fission processes and assess 4He production in the remaining mantle. The U and Th content is calculated for the remaining mantle from the concentration in the upper mantle according to the BSE model (0.0065 ppm U and 0.0173 ppm Th). No primordial concentrations of He are assumed to be present.
Table 4 presents the masses of Th and U 4.55 Ga ago and currently in the CMB and the remaining mantle, the changes in Th and U masses due to natural decay, and the resulting production of 4He for the two compartments. Pu does not play a significant role in this and following comparisons of isotopic ratios, due to its relatively small mass, and has therefore been omitted. The amounts of 4He produced by spontaneous fission can be neglected and have also been omitted. The values in the two last columns of Table 4 are given by:

In this equation the masses m and Δm are in kilograms, Nα is the number of α-particles produced in the decay-chain per nucleus and A is the atomic number of the fissile nucleus.
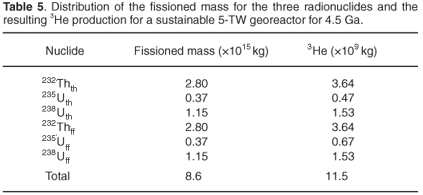
Assessing the products of a georeactor, we assume that the georeactor generates an energy of 7.1 × 1029 J, equivalent to continuous operation at a power of 5 TW during the past 4.5 Ga. Since one fission event produces about 200 MeV or 3.2 × 10–11 J, one can calculate that at the U and Th composition 4.5 Ga ago, 8.2 × 1013 J was released by the fission of 1 kg of mixture. Thus, 8.6 × 1015 kg of mixture fissioned in the georeactor.
Assuming all helium, produced either by natural decay or by fission, remains inside the CMB, the combination of the data in Tables 4 and 5 gives an 3He/4He atomic ratio of 3.14 × 10–6. An atomic ratio of 1.28 × 10–6 is obtained if the 3He combines with all the 4He in the mantle and is distributed homogeneously. Higher ratios are obtainable by assuming inhomogeneities, a larger time-integrated power delivered by georeactors or a smaller U and Th content of the CMB.
Krypton and xenon
Fission in general will lead to a preferential population of neutron-rich isotopes of elements. An additional consequence of georeactors is observable in the isotope ratios of elements with a low abundance but a high probability of being formed by fission. We discuss krypton and xenon, which could play an essential role in testing a CMB-georeactor hypothesis. The probability of fission is obtained as the sum of the probabilities for all isotones decaying to a particular isotope of an element, for each of these fission chains. For example, during formation of 81Kr, the sum extends over the production of the nuclei 81Kr, 81Br, 81Se, 81As, 81Ge and 81Ga, but for 80Kr only the production of 80Kr and 80Br contribute, because 80Se is stable.
We have to know the natural abundances of these two elements in various compartments of the Earth to be able to estimate the possible modifications in isotope ratios for Kr and Xe. The abundances for Kr and Xe are well-established as 1.14 ppm and 87 ppb by volume, respectively, in our atmosphere. We take the abundances in C-chondrites, according to Lodders,32 to estimate the abundances of these elements in the mantle and the CMB. Lodders32 states that these abundances could be altered due to outgassing. The uncertainty is particularly large for the lighter noble gases. The chondritic values of 5.22 × 10–11 and 1.74 × 10–10 for Kr and Xe, respectively, compared to the atmospheric concentrations, show evidence that outgassing of Kr is still relevant. We start from the chondritic value for xenon (i.e. assuming no significant outgassing for this heavy noble gas), and scale the abundance of krypton in proportion to the atmospheric ratio. This assumes implicitly that the outgassing of the meteorites at low temperatures over 4.55 Ga is of the same order of magnitude as the outgassing on Earth at high temperatures in the first 0.1 Ga. This gives a corrected Kr abundance of 2.28 × 10–9 in both the mantle and the CMB, leading to calculated masses of Kr in the CMB and the mantle of 5.4 × 1014 and 9.1 × 1015 kg, respectively. Moreover, we assume that the isotopic ratios in primordial Kr and Xe are identical to the present values in air.
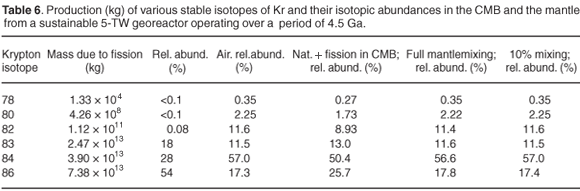
Table 6 presents the mass of the various stable isotopes of krypton and the derived isotopic abundances. The ratios as present in our atmosphere have been listed in column 4. The remaining columns list data, as isotopic ratios, for a number of scenarios in which the fission product krypton is fully mixed with the CMB (column 5) and the mantle (column 6), as well as a 10% mixture of the fission products with the mantle (column 7). From Table 6 one can note that the isotopic abundances in the fission products are strongly shifted towards the heavier isotopes. The effect is already strongly diminished in the CMB due to mixing with the naturally present Kr. This leads to a hardly noticeable effect in the case of full mixing with the mantle, and no noticeable effect, if the hidden reservoir is mixed only to an extent of 10 wt% with the entire mantle.
A similar approach has been taken for xenon. From the chondritic abundance32 of 1.74 × 10–10, we can estimate the mass of xenon in the CMB and mantle to be 4.14 × 1013 and 6.96 × 1014 kg, respectively. Data are presented in Table 7 in a similar way to Table 6. Comparison between rows 3 and 4 again shows a shift of the abundances towards the heavier isotopes. With Xe, the maximum occurs at the second-heaviest isotope (134Xe), in contrast to Kr, where the heaviest isotope is most abundantly produced. On the one hand, the production of xenon in fission is stronger than krypton production, and on the other hand, the natural elemental abundances for xenon are lower than for krypton. Fission products have a larger influence on the xenon isotopic ratios after mixture. Thus, the changes in isotopic composition of mantle-derived xenon gases are expected to be more obvious than for krypton. Another noticeable feature is the 'trapping' of fission products by stable Te isotopes (128Te and 130Te). This trapping boosts the relative abundance of 129Xe, relative to its neighbours.

Other elements
We discuss the elements Se, Mo, Ru and Pd, selected on the basis of their relatively low abundances (75, 50, 5 and 4 ppb, respectively) in the Earth's silicate mantle, and their relatively large probability of formation through fission.
For Se, although the isotopic ratios in the fission products deviate significantly from natural ratios, the mass of selenium in the CMB is for each isotope about two orders of magnitude larger than the mass produced in fission. As a result, there are only minute changes (<0.1%) in the mixed CMB material.
The production cross sections in fission for Mo isotopes are considerably larger than for Se, so that one would expect a larger effect of georeactors on the mantle Mo isotopic composition. Again, fission favours production of heavier isotopes, but the difference with respect to the natural abundances is not as large as for Kr, Xe and Se. This feature significantly reduces the geochemical signal of a 5-TW georeactor in the CMB. Full mixing of the fission products with the entire mantle creates only small effects (<1%) and a 10 wt% mixing of the fission products with the mantle results in an even smaller effect (<0.1%).
Palladium (Pd) and ruthenium (Ru) are elements with a low abundance in the BSE (3.9 and 5.0 ppb, respectively). They are thus likely candidates for which the consequences of a possible georeactor in the CMB will be clearly detectable. For Ru, in contrast to the previously discussed elements, the highest fission yields are not obtained for the heaviest isotopes, but are similar to the natural ratios in the middle group. A consequence is that the differences between the isotopic abundances in the mixtures and in the BSE rapidly diminish on mixing with CMB or mantle material. The differences become <1% with a leak of 10 wt% of the fission products into a well-mixed mantle.
For Pd, the highest fission yield is obtained for 105Pd and 106Pd, being isotopes that also have a high natural abundance. A consequence is that the differences in abundance for the mixtures and for the BSE rapidly diminish on mixing with CMB or mantle material. Moreover, the fission yields are one to two orders of magnitude smaller than, for instance, Mo. The differences are insignificant (<0.1%) when mixing with the overlying mantle is included.
Assessment of the noble gases
Helium
The measured abundance of 3He relative to 4He in terrestrial reservoirs is a stringent test for the georeactor hypothesis. Appendix 1 gives the observed 3He/4He ratios in various terrestrial compartments, and attempts to identify the main sources for the two helium isotopes. The natural decay of U and Pu has a small branch of spontaneous fission, resulting in a fixed 3He/4He ratio <10–9; several orders of magnitude smaller than found anywhere on Earth. This apparent excess in 3He has been termed the helium paradox.33,34
Primordial 3He, as a significant source for this 'excess', is unlikely, because of the high temperatures in the early ages of the Earth, resulting in elements that are far less volatile than He (e.g. Na, K, and Cl) to be partially ejected from Earth's mantle. Currently, 3He concentrations in wells and plumes can range over several orders of magnitude34 also arguing against thoroughly-mixed primordial 3He as a source. As detailed above, the persistent presence of a 5-TW georeactor in the CMB will lead to 3He/4He ratios of 3.14 × 10–6 for a homogeneously mixed CMB, and 1.28 × 10–6 for a thoroughly-mixed mantle. These estimates of 3He/4He ratios are based on an even distribution of the helium isotopes in the various compartments of the Earth's mantle. Isolated georeactors would lead to an expected 3He/4He ratio of 0.5–1 × 10–1 (Table 3). As georeactors are characteristically localized, a range of ratios between 1.28 × 10–6 and 1 × 10–1 would be expected for samples coming from the deep Earth. Observed values in deep wells and mantle plumes, in practice, range from 1–50 times the atmospheric value of 1.37 × 10–6, easily within the range obtainable from localized georeactors. Georeactors thus provide a natural source of 3He, quantitatively resolving the He paradox.
Krypton and xenon
The Kr isotopic composition of mantle samples is typically identical to that of the atmosphere. Spontaneous fission of 238U leads to minor production of 83,84,85Kr, but these fission products have not been detected in basalts, to date, because of the small yields in a mass region where Kr nuclides are relatively abundant. Based on our calculations, no significant deviations from the atmospheric ratios would be expected in mantle material, in good agreement with the data.
On the other hand, the xenon isotopic composition of samples from Earth's mantle differs significantly from that of Earth's atmosphere.35 Most notably, excesses in 129Xe and 136Xe in mantle rocks have been identified.36,37 Our current atmosphere has a 129Xe/130Xe ratio of 6.48, and a 136Xe/130Xe ratio of 2.17, but estimates for the current mantle source for mid-ocean ridge basalts show 129Xe/130Xe of 8.2 and 136Xe/130Xe of 2.7.
129Xe excesses have been interpreted as being derived from extinct radioactivity of 129I, while excesses in 136Xe are supposedly derived from spontaneous fission of 238U and 244Pu. It can be deduced from Table 7 that 129Xe/130Xe and 136Xe/130 ratios are 8.7 and 12.1 for a complete mixing of the fission products with the mantle, decreasing to 6.7 and 3.2 for a 10 vol% mixing of georeactor fission products in the mantle.
The latter is in close agreement with observation. Closer agreement is achievable by considering some of the assumptions in our first-order model. In particular, fast and thermal fission are assumed to contribute equally to the fission processes, and the Xe concentrations of the mantle are assumed to be equal to the concentrations found in chondritic meteorites,32 namely 1.74 × 10–10. These assumptions have a significant impact on the predicted xenon isotopic ratios.
Our calculations show that the chondritic concentrations dominate by three orders of magnitude, due to trapping by 130Te, 130Xe in the CMB. Considering 129Xe in the CMB, the mass from fission is an order of magnitude larger than the chondritic mass, and the contributions from Th and U are 0.8 and 0.2, respectively. For 136Xe, the mass in the CMB due to fission is two orders of magnitude larger than the chondritic mass. But the contribution of U increases to one third. The contribution by fission to the 129Xe isotope mass is only 3%, but for 136Xe, the contribution remains 30% for a mixture of 10% of the fission-produced Xe isotopes with those present in the mantle, in accordance with the chondritic abundances.
In summary, minor changes of the contributions could lead to exact agreement between predictions with respect to georeactor products and observed xenon isotopic ratios in the Earth's mantle. We are aware that manipulations of this kind are controversial, in view of our crude, first-order approximation, nevertheless we conclude that the Xe isotopic composition resulting from the georeactor hypothesis is consistent with the Xe isotopic record of mantle samples.
Proposed test of the georeactor hypothesis
Our model assumes that regions of elevated concentrations of U and Th continue to be present within the CMB even today. Such locations produce heat by either natural decay or possibly through georeactors.10,27 Both with natural decay and during fission, antineutrinos will be produced, reaching the Earth's surface without noticeable absorption. The two processes can be distinguished by the antineutrino energy spectrum. In the case of natural decay, the antineutrinos of U and Th have distinctly different energies of up to 3 MeV. In the case of fission, the antineutrinos will have a bell-shaped distribution ranging from 2 MeV up to 8 MeV, similar to the antineutrino emission from nuclear-power reactors.
The KamLAND collaboration38 presented the first evidence for geoneutrinos (antineutrinos produced within the Earth) in July 2005. Development of direction-sensitive antineutrino detectors39,40 should provide a means for a critical test of our hypothesis. The first step in the development of this type of detector has recently been completed with success.41,42 Measurements with an array of these detectors could reveal whether elevated concentrations of U and Th are present in the CMB, and if so, could identify the process by which heat is produced.
Conclusions
Geochemical models for the Earth's interior allow for concentration levels of U + Th in the mineral calcium silicate perovskite present in the CMB, of approximately 12 ppm 4.5 Ga ago, requiring an additional concentration factor of only about 20 to maintain a georeactor that is based on fast fission. A similar concentration factor is required to initiate the georeactor. The main source of neutrons under these conditions is 244Pu. This nuclide is estimated to be present in calcium perovskites at a concentration of 19 ppb. Sustainable georeactors with a power of 5 TW and natural abundances based on chondritic and/or BSE values can explain the observed deviations of the isotopic compositions of helium and xenon in the Earth's mantle. For krypton, selenium, molybdenum, palladium and ruthenium, the absence of measured isotopic anomalies in mantle samples has also been accounted for. Our hypothesis is amenable to testing by direction-sensitive geoneutrino tomography.
We thank Jan Leen Kloosterman, Technical University Delft, for providing results of the SCALE calculations, and for discussions regarding initiation and sustainability of georeactors. We are indebted also to W.F. McDonough, University of Maryland, for valuable discussion, and to an anonymous reviewer for valuable suggestions.
1. Rudnick R.L . and Gao, S. (2003). The composition of the continental crust. Treatise Geochem. 3, 1–64. [ Links ]
2. McDonough W.F. (2003). Compositional models for the Earth's core. Treatise Geochem. 2, 547–568. [ Links ]
3. Boyet M. and Carlson R.W. (2005). 142Nd Evidence for early (>4.53 Ga) global differentiation of the Silicate Earth. Science 309, 576–581. [ Links ]
4. Carlson R.W., Boyet M. and Horan M. (2007). Chondrite barium, neodymium, and samarium isotopic heterogeneity and Early Earth differentiation. Science 316, 1175–1178. [ Links ]
5. Tolstikhin I., Kramers J.D. and Hofmann A.W. (2006). A chemical Earth model with whole mantle convection: the importance of a core-mantle boundary layer (D") and its early formation. Chem. Geol. 226, 79–99. [ Links ]
6. Van der Hilst R.D., Widiyantoro S. and Engdahl E.R. (1997). Evidence for deep mantle circulation from global tomography. Nature 386, 578–584. [ Links ]
7. Van Keken P.E., Ballentine C.J. and Hauri E.H. (2003). Convective mixing in the Earth's mantle. Treatise Geochem. 2, 471–491. [ Links ]
8. Kuroda P.K. (1956). On the nuclear physical stability of the uranium minerals. J. Chem. Phys. 4, 781–782. [ Links ]
9. Driscoll R.B. (1988). Nuclear disruption of a planet with convective outer core. Bull. Am. Phys. Soc. Ser. II 33, 1031–1037. [ Links ]
10. Herndon J.M. (1992). Nuclear fission reactors as energy sources for the giant outer planets. Naturwissenschaften 79, 7–14. [ Links ]
11. Herndon J.M. (1993). Feasibility of a nuclear fission reactor at the center of the earth as the energy source for the geomagnetic field. J. Geomagn. Geoelectr. 45, 423–437. [ Links ]
12. Herndon J.M. (2003). Nuclear georeactor origin of the oceanic basalt, 3He/4He, evidence and implications. Proc. Natl Acad. Sci. USA 100, 3047–3050. [ Links ]
13. Rusov V.D., Pavlovich V.N., Vaschenko V.N., Tarasov V.A., Zelentsova T.N., Bolshakov V.N., Litvinov D.A., Kosenko S.I. and Byegunova O.A. (2007). Geoantineutrino spectrum and slow nuclear burning on the boundary of the liquid and solid phases of the Earth's core. J. Geophys. Res. 112, B09203, doi:10.1029/2005JB004212. [ Links ]
14. Pollack H.N., Hurter S.J. and Johnson J.R. (1993). Heat flow from the Earth's interior: analysis of the global data set. Rev. Geophys. 31, 267–280. [ Links ]
15. Hofmeister A.M. and Criss R.E. (2005). Earth's heat flux revised and linked to chemistry. Tectonophysics 395, 159–177. [ Links ]
16. Schuiling R.D. (2006). Is there a nuclear reactor at the center of the Earth? Earth, Moon, and Planets 99, 33–49. [ Links ]
17. Maracic J. and KamLAND Collaboration (2006). Experimental status of geo-reactor search with the KamLAND detector. Earth, Moon, and Planets 99, 147–153. [ Links ]
18. Malavergne V., Tarrida M., Combes R., Bureau H. (2005). Uranium and lead in the early planetary core formation: new insights given by high pressure and temperature experiments. Lunar Planet. Sci. XXXVI., 1823. [ Links ]
19. Bao X., Secco R.A., Gagnon J.E., Fryer B.J. (2005). Experiments of U solubility in Earth's core. AGU Spring Meeting V13B-06 (abstract). [ Links ]
20. Javoy M. (1995). The integral enstatie chondrite model of the Earth. Geophys. Res. Lett. 22, 2219–2222. [ Links ]
21. Porcelli D. and Ballentine C.J. (2002). Models for the distribution of terrestrial noble gases and the evolution of the atmosphere. Rev. Mineral. Geochem. 47, 411–480. [ Links ]
22. Corgne A., Liebske C., Wood B.J., Rubie D.C. and Frost D.J. (2005). Silicate perovskite-melt partitioning of trace elements and geochemical signature of a deep perovskitic reservoir. Geochim. Cosmochim. Acta 69, 485–496. [ Links ]
23. Murakami M., Hirose K. and Kawamura K. (2004). Post-perovskite phase transition in MgSiO3. Science 304, 855–858. [ Links ]
24. Walter M.J., Nakamura E., Tronnes R.G. and Frost D.J. (2004). Experimental constraints on crystallization differentiation in a deep magma ocean. Geochim. Cosmochim. Acta 68, 4267–4284. [ Links ]
25. Ravnik M. and Jeraj R. (2005). Criticality analyses of regions containing uranium in the Earth history. Kerntechnik 70, 146–152. [ Links ]
26. Van der Hilst R.D., De Hoop M.V., Wang P., Shim S-H., Ma P., Tenorio L. (2007). Seismostratigraphy and thermal structure of Earth's core-mantle boundary region. Science 315, 1813–1817. [ Links ]
27. Hollenbach D.F. and Herdon J.M. (2001). Deep-earth reactor: nuclear fission, helium and the geomagnetic field. Proc. Natl Acad. Sci.USA 98, 11085–11090. [ Links ]
28. Seifritz W. (2003). Some comments on Herdon's nuclear georeactor. Kerntechnik 68, 193–196. [ Links ]
29. Rinard P.M. (1991). Neutron interactions with matter. In Passive Nondestructive Assay of Nuclear Material, Los Alamos Technical Report NUREG/CR-5550, LA-UR-90-732, 357–377. [ Links ]
30. SCALE: NUREG/CR-0200, Version 4.4A, (2000). Oak Ridge National Laboratory, TN. [ Links ]
31. Pfennig G., Klewe-Nebenius H. and Seelman-Eggebert W. (1998). Karlsruhe Nuklidkarte. Forschungszentrum Karlsruhe GmbH. [ Links ]
32. Lodders K. (2003). Solar system abundances and condensation temperatures of the elements. Astrophys. J. 591, 1220–1247. [ Links ]
33. O'Nions R.K. and Oxburgh E.R. (1983). Heat and helium in the Earth. Nature 306, 429–431. [ Links ]
34. Anderson D.L. (1998). The helium paradox. Proc. Natl Acad. Sci. USA 95, 4822–4827. [ Links ]
35. Graham D.W. (2002). Noble gas isotope geochemistry of mod-ocean ridge and ocean island basalts: characterization of mantle source reservoirs. Rev. Mineral. Geochem. 47, 247–318. [ Links ]
36. Staudacher T. and Allègre C.J. (1982). Terrestrial xenology. Earth Planet. Sci. Lett. 60, 389–406. [ Links ]
37. Moreira M., Kunz J. and Allègre C.J. (1998) Rare gas systematics in popping rock: isotopic and elemental compositions in the upper mantle. Science 279, 1178–1181. [ Links ]
38. Araki T. et al. (2005). Experimental investigation of geologically produced antineutrinos with KamLAND. Nature 436, 499–503. [ Links ]
39. de Meijer R.J., van der Graaf E.R. and Jungmann K.P. (2004). Quest for a nuclear georeactor. Nuclear Phys. News 14, 20–25. [ Links ]
40. de Meijer R.J., van der Graaf E.R. and Jungmann K.P. (2004). Quest for a nuclear georeactor. Rad. Phys. Chem. 71, 769–744. [ Links ]
41. de Meijer R.J., Smit F.D., Brooks F.D., Fearick R.W., Wörtche H.J. and Mantovani F. (2006). Towards Earth AntineutRino TomograpHy (EARTH). Earth, Moon and Planets 99, 193–206. [ Links ]
42. Smit F.D., de Meijer R.J., Fearick R.W. and Wörtche H.J. (2006). In Proc. Fast Neutron Detection and Applications, Cape Town, April 2006, Proceedings of Science, paper 96. [ Links ]
43. Grimberg A. et al. (2006). Solar wind neon from Genesis: implications for the lunar noble gas record. Science 314, 1133–1135. [ Links ]
44. MacDonald G.J.F. (1963). The escape of helium from the earth's atmosphere. Rev. Geophys. Space Phys. 1, 305–349. [ Links ]
45. Tolstikhin I. and Hofmann A.W. (2005). Early crust on top of the Earth's core. Phys. Earth Planet. Int. 148, 109–130. [ Links ]
Received 12 February. Accepted 20 April 2008.
* Author for correspondence. E-mail rmeijer@geoneutrino.nl
Appendix 1. Earth's helium inventory
This appendix addresses the observed 3He/4He ratios in various compartments of the Earth and its atmosphere and proposes the main sources for these two helium isotopes.
Solar wind
The helium content of the solar wind has been measured43 by analysing metallic glass aliquots flown on the satellite Genesis, collecting data for 887 days. The results are a helium particle flux of 9.72 × 106 cm–2 s–1 and a 3He/4He ratio of (4.62 ± 0.04) × 10–4, corresponding to a flux of 2.59 × 106 kg yr–1 4He and about 900 kg yr–1 3He at the outer atmosphere. Only about 1% enters the Earth's atmosphere due to the shielding by the geomagnetic field.
Atmosphere
The Earth's atmosphere has a mass of about 5.1 × 1018 kg. The helium content is about 5 × 10–4 vol%, corresponding to a mass of 3.71 × 1012 kg. 3He/4He ratio reported31 is 1.37 × 10–6. Estimated fluxes in and out of the Earth's atmosphere44 are in listed in Table A1.

Data in Table A1 show that the 3He and 4He concentrations are not in equilibrium. ? indicates unknown value. These could be estimated from either the 3He/4He ratio in the solar wind for the extra-terrestrial influx of 4He, or from the atmospheric ratio for the 3He ejection from the Earth's surface. The system hardly changes in either case; the discrepancy between influx and escape only increases. Table A1 shows that if the 3He/4He ratio in the Earth's continental and oceanic crust and sediments is similar to that in the Earth's atmosphere, then the 3He concentration in the atmosphere is dominated by the extra-terrestrial influx and the 4He concentration by the influx from the Earth's surface.
In a simple compartment model one can define removal rates of 3He and 4He. The values in Table A1 correspond to half-life times of ~1 and ~40 Myr, respectively. This difference is considerably larger than predicted from the mass difference between the two isotopes. In a similar way the increase can be expressed as a net rate of increase. Data in Table A1 indicate that the total mass of 3He is likely to increase at a faster rate than 4He, but inaccuracies are too large to make a definitive statement.
It can be concluded from these data that, in the atmosphere, the 3He concentration is primarily determined by extra-terrestrial input and the 4He concentration by ejection from the continental and oceanic crusts and sediments. The atmosphere's 3He/4He ratio is therefore not a consequence of 3He in the Earth's interior.
The continental and oceanic crusts
For the assessment of the helium inventory of the Earth's interior, we consider subdivision into upper and lower mantle, and adapt best estimates for the U and Th contents using recent bulk silicate Earth (BSE) models. We add the CMB to the usual division, and assume that half of the U and Th in the lower mantle is concentrated in the CMB, in approximate agreement with Tolstikhin et al.45 It gives approximately similar values for U and Th concentrations in the upper and lower mantle.
In addition to the volumes of the upper and lower mantle, Table A2 gives the mass of thorium, as well as of the two uranium isotopes. The production rate of 4He in the various compartments follows predictably from the half-life time, and that each decaying 238U atom gives 8 atoms of 4He, and both 235U and 232Th generate 6 4He atoms each. It can be concluded from the 4He value in the crust, within accuracy, that this number does not differ significantly from the 4He ejection rate at the Earth's surface of 2 × 106 kg yr–1. We conclude that the crust may be considered as a closed system for 4He. Consequently, we can consider transport of helium from the deeper Earth to the crust to be insignificant.

The mantle and the CMB
Without significant transport from the mantle to the crust, 4He will build up over time, due to natural decay of U and Th. The amount is calculated from the present U and Th content, using the radioactive decay constants to derive the amount of each of the uranium isotopes that have decayed in the last 4.5 Gy. The total amount of the 4He isotope can be calculated using the number of 4He atoms produced in each of the decay chains.
Table A3 lists the mass of U isotopes and Th that has decayed over the last 4.5 Ga. The decay of 238U makes the largest contribution to the 4He production, but despite the small abundance of 235U at present, this uranium isotope makes a larger contribution than 232Th.















