Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.104 no.3-4 Pretoria mar./abr. 2008
RESEARCH ARTICLES
Sexual dimorphism in the mandible of indigenous South Africans: a geometric morphometric approach
D. FranklinI, *; P. O'HigginsII; C.E. OxnardI, III
ICentre for Forensic Science, The University of Western Australia, 35 Stirling Hwy, Crawley, Western Australia
IIFunctional Morphology and Evolution Research Unit, The Hull York Medical School, Heslington, York, Y010 5DD, U.K.
IIISchool of Anatomy and Human Biology, The University of Western Australia
ABSTRACT
We report here on analyses of new landmark data which capture the range of variation in the expression and magnitude of mandibular sexual dimorphism in adult South African Bantu-speaking individuals. The sample examined, separately and pooled, comprises 225 (120 male and 105 female) individuals of known sex representing five local populations: Zulu, Swazi, Xhosa, Sotho and Tswana. Thirty-eight bilateral three-dimensional landmarks were acquired using a Microscribe G2X digitizer and were analysed using geometric morphometric methods. Multivariate statistics were applied to visualize the pattern, and assess the significance, of shape variation between the sexes. All samples demonstrate highly significant size and shape dimorphism, the condyle and ramus consistently being the most dimorphic region of the mandible. Our results also indicate that the mandible of individuals from this population is just as dimorphic, if not more so, than the cranium alone.
Introduction
Much of our understanding of modern human skeletal variation in southern Africa is derived from anthropological techniques based on the traditional linear metrical system. With specific reference to either the whole skull or its functional components, there is a long history of such research (e.g. refs 1–8). Recent research has also focused on using various skeletal elements to quantify variation related to sexual dimorphism, both cranial9–12 and postcranial,13–15 primarily because the determination of sex in unknown skeletal remains is one of the key biological characteristics (alongside age, ethnicity and stature) used to facilitate forensic identification.16 The need for research of this nature is driven by the increasing incidence of violent activities resulting in large numbers of unidentified bodies being referred to the forensic investigator.5,17
Among the largest and most comprehensive of the anthropological studies carried out on southern African populations was that of de Villiers,3 who examined 745 (586 male, 159 female) South African crania, 648 associated mandibles, and published data on more than 2000 other African male individuals. The data were analysed without computer assistance using the relatively simple uni- and multivariate statistics then available. This work was regarded as having established a new set of anthropological norms, which changed the ' purely morphological study of anthropology to a discipline involving not only morphology, but also computerized statistics and genetics' (ref. 18, p. ix). Her research demonstrated that sexual dimorphism in the skull of indigenous South Africans appeared to be 'associated mainly with the mandible' (ref. 19, p. 122).
Innovative analytical techniques, such as those drawn from the discipline of geometric morphometrics, are now commonly used in physical anthropology.20,21 Geometric morphometric methods use anatomical landmark data for the quantitative analysis of biological form.22,23 They are statistically powerful and offer great potential in visualization of results in terms of anatomy; as such they prompt re-investigation of material that may have been studied many years ago using techniques appropriate to that era. In the search for new perspectives, we recently applied geometric morphometric methods to the study of variation in the crania of indigenous southern Africans24,25 and were able to both clarify the nature of previously identified anatomical variations and identify additional features of sexual dimorphism and population variation. These studies proved useful in understanding past population movements and interactions better, and also highlighted new criteria for the identification of sex, able to be easily applied by forensic and medical practitioners alike.
The focus of the present study is to use geometric morphometric methods to investigate sexual dimorphism in the mandibles of a documented series of adult South African Bantu-speaking peoples. To appreciate better the range of variation in the expression and magnitude of sexual dimorphism in this population, the material was examined at two levels—the pooled sample and the individual local populations separately; the analyses relating to those studies are referred to as steps 1 and 2, respectively. Although some degree of apparent morphological variation has been demonstrated between these local populations,25 we validated the pooling of groups by performing a series of regression analyses designed to assess the significance of sexual dimorphism and population variation in the sample; no significant interaction effects were found.
Materials and methods
We examined the mandibles of 225 (120 male, 105 female) South African Bantu-speaking adult individuals drawn from the following local populations: Zulu [30♂, 29♀], Swazi [208, 119], Xhosa [25♂, 19♀], Sotho [28♂, 25♀], and Tswana [17♂, 21♀]. The stated age ranges are: male 18–69 years (mean 37 years); female 18–70 years (mean 36.5 years). All individuals were derived from the Raymond A. Dart Collection of Human Skeletons, housed in the School of Anatomical Sciences of the University of the Witwatersrand, Johannesburg. As this collection is prepared from dissection hall subjects, the sex, local population ('tribal origin') and a statement of age is documented for each skeleton.27,28 Only non-pathological specimens (as assessed on the basis of macroscopic examination) were included; mandibles presenting significant alveolar resorption due to excessive tooth loss were also excluded from the study.
Data acquisition
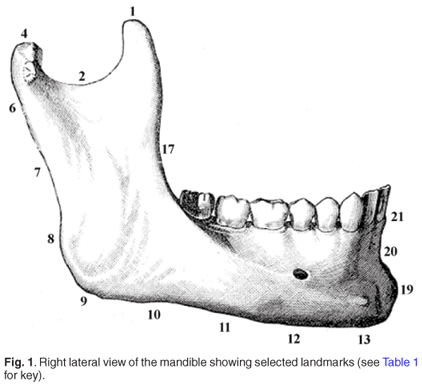
Data were collected in the form of 38 bilateral three-dimensional landmark coordinates acquired using a Microscribe G2X digitizer (Immersion Corporation, San Jose, CA). To ensure familiarity of reference to physical and forensic anthropologists alike, landmarks were selected to correspond closely to those commonly employed in both traditional and geometric morphometric studies. In addition, a new series of mandibular landmarks was designed to characterize the shape of the posterior ramus, lateral body and symphysis; see Table 1 and Fig. 1 for complete description and illustration. Selected landmarks (6–8 & 27–29; 10–12 & 31–33, Table 1) were first determined instrumentally using vernier calipers; the bone was marked at the appropriate location, and then following mounting on a stable platform, the coordinate data were acquired.

Shape analysis
Data were analysed using geometric morphometric methods;22,23 we used the shape analysis software morphologika2 (www.york.ac.uk/ res/fme) to analyse the three-dimensional coordinates of the landmarks. This software, which is now well established in the literature,24–26,29–33 encompasses standard geometric morphometric procedures for analysing three-dimensional data. Further statistical analyses were carried out using SPSS 15.0.
The form of each mandible is represented by the Cartesian coordinates of the three-dimensional configuration of anatomical landmarks described and illustrated in Table 1 and Fig. 1, respectively. To eliminate the non-shape variation in the sample, generalized Procrustes analysis (GPA) was used, whereby differences in landmark coordinates due to the position of specimens during the digitization process were minimized and size was standardized. This process involved optimally superimposing landmark configurations based on a least-squares algorithm;34 the scaling procedure adjusted the landmark coordinates such that each mandible had unit centroid size, which was computed as the square root of the sum of squared distances of all landmarks from the centroid.23,35
The new Cartesian coordinates obtained after the superimposition were the shape coordinates used for statistical comparisons of individuals and they can be summarized using a standard principal components analysis (PCA) on the covariance matrix; mean male and female configurations were then visualized.23
Statistical comparisons
To assess the significance of sexual dimorphism in mandibular size, analysis of variance (ANOVA) was used to compare mean male and female centroid size values. To assess the significance of sexual dimorphism in mandibular shape, a series of permutation tests were used, in which the true difference between means (Procrustes distance) was compared with the distribution of differences between means obtained by randomly permuting group membership 1000 times.36 Multivariate regression analyses were used to assess the significance of sexual dimorphism in the pooled sample and separately for each of the five local populations; plots of fitted values against standardized residuals showed that the assumptions of regression were met. Cross-validated discriminant functions were then used to assess sex classification accuracy; to assess size effects on sex classification accuracy, log transformed centroid size values were also included in a separate series of discriminant analyses.
Both the regression and discriminant analyses used the PC scores (PCs) from the GPA/PCA of the sample because, for computational and statistical reasons, the number of variables is required to be somewhat less than the number of specimens. Selection of PCs was on the basis of optimization of discrimination. To achieve optimal group discrimination, we examined plots of discriminant classification accuracy against the number of variables used; this approach allowed us to remove redundant higher order variables.37 As this approach has effectively reduced the dimensionality of the sample, it was important to test how well the relative positions of specimens were summarized in a space of reduced dimensionality relative to the shape space of all variables. Euclidean distance matrices were calculated using both the PCs selected on the basis of discrimination (reduced shape information) and all PCs (all shape information); the matrices were tested for significance of correlation using a Mantel test (incorporating 10 000 random permutations). A large and significant correlation justifies reducing the total number of shape variables in the analysis without losing relevant information.
Intra-observer error
As the degree of error in the acquisition of this landmark series has been fully described elsewhere (see ref. 26), we do not repeat the results here but state that measurement error was exceedingly small on all significant PCs.
Results
Step 1 – analysis of the pooled population sample
Mean male and female centroid size values in the pooled sample were shown by ANOVA to be highly significantly different: P < 0.0001, F = 172.33; ♂ 349.8 (s.d. 11.1); ♀ 330.7 (s.d. 10.6). This indicates strong size dimorphism in the mandibles of South African Bantu-speakers. The permutation test (1000 random permutations) indicates that the Procrustes distance between sex means was significantly different (P = 0.018), indicating that males and females were significantly different in mean mandibular shape.
Multivariate regressions of shape against sex (performed using PCs 1–3 [optimal PCs for classification as assessed by discriminant function plots – see below]—accounting for 42.9% of the total variance) indicated significant sexual dimorphism in the pooled sample (Wilks' Λ = 0.77, corresponding to an F-statistic of 22.0 with 3 and 221 d.f. [P < 0.0001]). Classification accuracy of the cross-validated discriminant analysis (performed using PCs 1–3) was 73.8% (♂ 83/120; ♀ 83/105); when the same analysis was performed incorporating log transformed values of centroid size, the accuracy was 83.1% (♂ 97/120; ♀ 90/105). The correlation between the reduced and full dimensionality shape spaces was strong and significant (r = 0.87; P < 0.001), justifying using the reduced data set.
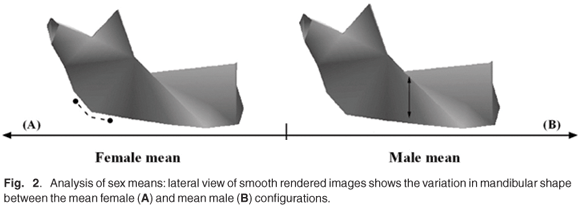
The main shape differences visualized by warping between the mean male and female configurations are shown in Fig. 2. It is evident from the lateral rendered images that the male mandible (Fig. 2B) has a relatively more acute gonial angle; this is associated with, in the male compared with the female, anterior relocation of the condyle relative to other parts of the mandible (Fig. 2A). Relative to the condyle, the coronoid process is displaced anteriorly and inferiorly in the female mandible; this appears to be associated with a relatively broader and shallower sigmoid notch (Fig. 2A). In the male mandible there is also an increase in relative ramus height and breadth, and also the height of the posterior corpus (Fig. 2B). In viewing the mandible from its superior aspect (not shown), it is apparent that the heads of the condyles are relatively (but not absolutely) more medially relocated in the male; this shape feature appears to be associated with an acute gonial angle (see above). The opposite configuration is evident in the female mandible.
Step 2 – analysis of the individual local populations
For brevity of description, results pertaining to the statistical significance of sexual dimorphism in mandibular size and shape, in each of the local populations, are summarized in Table 2. ANOVA and permutation tests demonstrated that each of the local populations exhibited significant size and shape dimorphism (Table 2). Multivariate regressions of shape against sex similarly demonstrated significant sexual dimorphism in each of the local populations. Classification accuracy of cross-validated discriminant functions of shape ranged from 83.1% in the Zulu to 68.2% in the Xhosa. Discriminant analyses incorporating log centroid size showed an improvement in classification accuracy across all populations, although relative improvements were considerably variable: +16.1% in the Swazi to only +1.9% in the Sotho (Table 2). The PCs used in the regression and discriminant analyses are listed in Table 2; matrix correlations of shape distances in the full and reduced shape spaces are high in each instance (0.91 to 0.95; all P < 0.001).

With regard to the quantification of sexually dimorphic shape features, it was found that the individual populations generally expressed the same features observed in the pooled population sample (see above); thus further visualizations are not presented. It was evident, however, that there was some variation in the degree to which some dimorphic features are expressed. For example, each population showed a relatively more acute gonial angle in the mean male configuration, but in the Swazi this difference was the least pronounced. Further, the Swazi males expressed considerable bilateral gonial eversion compared with the females of the same population; this feature was not observed in any other group.
Discussion
In this study we have used a documented skeletal collection to investigate mandibular sexual dimorphism in adult Bantu-speaking South Africans. The techniques applied were drawn from the discipline of geometric morphometrics, by which highly significant size and shape dimorphism were shown both in the pooled and in the individual local populations. With regard to the pooled population sample, the following discussion considers the sex-based variation in mandibular morphology in relation to developmental and functional requirements. The sex classification accuracy achieved using the geometric morphometric technique was also considered and compared with traditional morphometric approaches. Aspects of sexual variation in the mandibular morphology of the individual local populations are also discussed.
Pooled population sample
In considering shape variation between the sexes, it was evident that the most dimorphic regions of the mandible were the condyle and ramus, and to a lesser extent the lateral body (Fig. 2). The aetiology of such variation is likely attributable to differential growth trajectories and functional adaptations (see refs 41–43). For example, it has been shown that the male mandible continues to grow steadily after puberty (relative to the maxilla and nasion), whereas the female mandible tends not to exhibit the same growth pattern.44 This was demonstrated in the morphometric study of Hunter & Garn,45 who showed the male ramus to be on average 14% longer that that of the female, whereas other facial dimensions approximated only an 8% difference.
In a more recent morphometric study of variation in great ape and modern human mandibles, Humphrey et al.46 found that almost any site of mandibular bone deposition, or resorption, appeared to have the potential to become sexually dimorphic. In that study it was concluded that ' the sites associated with the greatest morphological changes in size and remodelling during growth, those that involve the mandibular condyle and ramus in particular, are generally the most sexually dimorphic in the species examined here' (ref. 46, p. 510). In this respect, the results of the present study appear to support previous research; in particular that sexual dimorphism in this bone is differentially expressed across its functional units, and mostly apparent in the condyle and ramus.
In this study the mandibular angles were also quite dimorphic, with the female configuration being more obtuse, and vice versa for the male (Fig. 2). The functional significance of the mandibular angle has been shown in animal models to be directly related to the functions of the muscles of mastication (e.g. masseter and pterygoid) which attach to its surface.47 When those muscles were excised in male rats, the angular process was reduced in all dimensions;47 a similar relationship was demonstrated for the temporalis muscle.48 Comparable results expressed quantitatively came from the less invasive dietary difference experiments of Moore.49 It would thus appear plausible to suggest that sex-specific mechanical forces involving the masticatory apparatus (e.g. whether cultural or dietary in origin) could directly influence the development of the muscles of the lower jaw and by consequence their underlying skeletal structures (see also Ricketts,50 Petrovic et al.51). This is expected to be population specific (see below).
Cross-validated sex classification accuracy in the pooled sample was a respectable 83.1%; this figure is almost identical to the accuracy we achieved using nine linear measurements on the same sample.12 We also previously applied geometric morphometric methods to a large documented sample of southern African crania; we correctly assigned 87% of individuals according to sex.24 With subsequent cross-validation of those data, however, the figure falls to 81.3%. In general, the classification accuracy of the present study was similar to results obtained using traditional morphometric approaches on a variety of different populations; e.g. Steyn & İşcan9 81.5%; Giles52 83–87%; Hanihara53 85%. Our results, however, offer anatomically informative visualizations of sexual dimorphism as well as statistical findings.
The above statistics, in addition to a plethora of similar studies available in the literature, demonstrate that different populations generally show some variation in the level of sex classification accuracy achieved. This is related to populational variability in the magnitude of the expression of sexual dimorphism, which is inherently influenced by the complex inter-relationship of numerous factors, including, but not limited to, access to adequate nutrition, sexual division of labour, and underlying genetic adaptations and selective forces.54 Sex-specific functional requirements and adaptations similarly affect the sex discriminatory power of individual skeletal elements. For example, there is good evidence in the literature that the hip bone (os coxa) is a very reliable element for sex determination,55 an obvious consequence of female adaptations for parturition.56
So, with regard to dimorphism in the skull (cranium and mandible) of Bantu-speaking South Africans, we have good evidence to suggest that we have reached the upper limit of sex classification accuracy for the pooled sample, seemingly irrespective of our methodological approaches (see above). Clearly, however, both the present and our previous research efforts11,12 have demonstrated that some of the individual local populations can be classified with even higher accuracy (this point is discussed in further detail below). This research also highlights another important point; the mandible of individuals from this population is just as dimorphic as, if not more so, than the cranium alone, supporting the much earlier research of de Villiers.3,19
Individual local populations
This analysis has demonstrated some apparent variation in the pattern and degree of mandibular sexual dimorphism in the local populations. It appears that the variation observed is not due to mandibular features unique to a single population, but rather to differences in the relative development and expression of a suite of morphological features common to the five closely related local populations. The Zulu and Swazi exhibited particularly strong mandibular dimorphism (Table 2); the Sotho appeared least dimorphic, which supports our previous research using cranial geometric morphometric data.24
The effect of size on discrimination clearly varies among the five local populations, suggesting some variation in the degree of mandibular size dimorphism (Table 2). The two populations with the largest centroid size dimorphism showed the greatest increase in classification accuracy when log centroid size was incorporated into the discriminant analyses: Zulu +15.2%; Swazi +16.1%.
Interestingly, the Xhosa also showed a marked increase in classification accuracy (+11.3%) despite having one of the lowest F-values (Table 2). The relatively small increase in the Tswana (+5.3%) and Sotho (+1.9%) populations implies that shape parameters (which doubtless include allometric shape) have the greatest influence on discrimination.
Although the local populations share a close ancestral relationship, the differences observed in the expression and magnitude of mandibular sexual dimorphism may have been influenced by several factors, including differential admixture with adjacent populations (particularly the Khoisan – see Franklin et al.25). There is also good evidence showing that dietary factors can influence the expression of sexual dimorphism.57 It has been proposed that males are more susceptible to fluctuations in nutritional quality, hence growth is reduced to a greater degree than in females, thus resulting in a more equal body size and reduced dimorphism.52,58,59 Basically, this means that under conditions of inadequate nutrition, males are achieving a lower percentage of their genetic growth potential than females.60
Further, it is also known that the nature of the division of labour and other cultural activities result in differential mechanical loading and musculoskeletal development, which can contribute to variation in the pattern and degree of sexual dimorphism in a population.58,61,62 There is a considerable literature demonstrating how functional loading affects hominid mandibular morphology (e.g. refs 63–65). Moss66 demonstrated that hypertrophy of the masseter muscle is associated with expansion and flaring of the angular process. Cultural activities, such as daily chewing of a tough material (pine resin), has been shown to increase facial height, with other flow-on effects to the mandible, including, but not limited to, increased prognathism and a reduction in gonial angle.67 It is clearly evident that populational variability in the expression of sexual dimorphism is likely to be influenced by the complex interaction of many biological, environmental and cultural factors.
Lastly, it is well known in the literature that the most accurate sex determinations are made using population-specific data.10,68 Although we demonstrated a small degree of variation in the expression of sexually dimorphic features in the local populations, these differences are unlikely to have great forensic utility, simply because the subdivisions within South African populations are rapidly diminishing—the result of increasing admixture and the dissolution of once prohibitive cultural barriers. In forensic cases, furthermore, the local population of a recovered individual is generally unknown, thus the pooled sample data are the most appropriate to consult.
Conclusions
This study further demonstrates that geometric morphometric methods are a valuable tool for elucidating morphological differences related to sexual dimorphism. Our analyses showed highly significant size and shape dimorphism in the samples examined; the condyle and ramus were consistently the most dimorphic regions of the mandible. The forensic investigator in South Africa can use the sex-specific shape features identified in this study as a potential source of population-specific data useful for assigning sex in unknown remains. Finally, after reconciling the results of the present study with our earlier research on the cranium of individuals from the same South African populations,10,24 we can confidently assert that the mandible is an equally suitable element in terms of potential sex classification accuracy.
We thank P.V. Tobias of the Department of Anatomical Sciences, University of the Witwatersrand, for access to the Raymond A. Dart Collection of Human Skeletons. Technical assistance with the Dart collection was provided by E. Mofokeng and was much appreciated. Funding was provided by Australian Research Council Discovery Grant DP0557157.
1. Shrubsall F.C. (1899). A study of A-Bantu skulls and crania. J. Roy. Anthropol. Inst. Great Britain & Ireland 28, 55–94. [ Links ]
2. Tobias P.V. (1959). Studies on the occipital bone in Africa: I Pearson's occipital index and the chord-arc index in modern crania: means, minimum values, and variability. J. Roy. Anthropol. Inst. Great Britain & Ireland 89, 233–52. [ Links ]
3. de Villiers H. (1968). The Skull of the South African Negro: A Biometrical and Morphological Study. Witwatersrand University Press, Johannesburg. [ Links ]
4. Rightmire G.P. (1970). Bushman, Hottentot and South African Negro crania studied by distance and discrimination. Am. J. Phys. Anthropol. 33, 169–196. [ Links ]
5. Morris A.G. (1992). The Skeletons of Contact: a study of protohistoric burials from the lower Orange River Valley, South Africa. Witwatersrand University Press, Johannesburg. [ Links ]
6. Franklin D., Freedman L. and Milne N. (2005). Three-dimensional technology for linear morphological studies: a re-examination of cranial variation in four southern African indigenous populations. HOMO 56, 17–34. [ Links ]
7. L'Abbe E.N., Ribot I. and Steyn M. (2006). A craniometric study of the 20th century venda. S. Afr. Archaeol. Bull. 61, 19–25. [ Links ]
8. Morris A.G. and Ribot I. (2006). Morphometric cranial identity of prehistoric Malawians in the light of sub-Saharan African diversity. Am. J. Phys. Anthropol. 130, 10–25. [ Links ]
9. Steyn M. and İşcan M.Y. (1998). Sexual dimorphism in the crania and mandibles of South African whites. Forensic Sci. Int. 98, 9-16. [ Links ]
10. Franklin D., Freedman L. and Milne N. (2005). Sexual dimorphism and discriminant function sexing in indigenous South African crania. HOMO 55, 213–228. [ Links ]
11. Franklin D., O'Higgins P., Oxnard C.E. and Dadour I. (2006). Determination of sex in South African Blacks by discriminant function analysis of mandibular linear dimensions: a preliminary investigation using the Zulu local population. Forensic Sci. Med. Pathol. 2, 263–268. [ Links ]
12. Franklin D., O'Higgins P., Oxnard C.E. and Dadour I. (2008). Discriminant function sexing of the mandible of indigenous South Africans. Forensic Sci. Int. doi:10.1016/j.forsiint.2008.03.014. [ Links ]
13. Steyn M. and İşcan M.Y. (1999). Osteometric variation in the humerus: sexual dimorphism in South Africans. Forensic Sci. Int. 106, 77-85. [ Links ]
14. Patriquin M.L., Steyn M. and Loth S.R. (2005) Metric analysis of sex differences in South African black and white pelves. Forensic Sci. Int. 147, 119–27. [ Links ]
15. Dayal M.R. and Bidmos M.A. (2005). Discriminating sex in South African blacks using patella dimensions. J. Forensic Sci. 50, 1294–1297. [ Links ]
16. Ratbun T.A. and Buikstra J.E. (1984). The role of the forensic anthropologist: the case for cooperative research. In Human Identification: Case studies in forensic anthropology, eds R.A. Rathbun and J.E. Buikstra, pp. 5–14. Charles C Thomas, Springfield, IL. [ Links ]
17. L'Abbe E.N., Loots M. and Meiring J.H. (2005). The Pretoria bone collection: a modern South African skeletal sample. HOMO 56, 197–205. [ Links ]
18. Trevor-Jones R. (1986). A tribute. In Variation, Culture and Evolution in African Populations: Papers in honour of Dr Hertha de Villiers, eds R. Singer and J.K. Lundy, pp. ix. Witwatersrand University Press, Johannesburg. [ Links ]
19. de Villiers H. (1968). Sexual dimorphism of the skull of the South African Bantu-speaking Negro. S. Afr. J. Sci. 64, 118–124. [ Links ]
20. Richtsmeier J.T., Deleon V.B. and Lele S.R. (2002). The promise of geometric morphometrics. Yrbk Phys. Anthropol. 45, 63–91. [ Links ]
21. Adams D.C., Rohlf F.J. and Slice D.E. (2004). Geometric morphometrics: Ten years of progress following the 'Reolution'. Ital. J. Zool. 71, 5–16. [ Links ]
22. Rohlf F.J. and Marcus L.F. (1993). A revolution in morphometrics. Trends Ecol. Evol. 8, 129–32. [ Links ]
23. Bookstein F.L. (1991). Morphometric Tools for Landmark Data: Geometry and biology. Cambridge University Press, Cambridge. [ Links ]
24. Franklin D., Freedman L., Milne N. and Oxnard C.E. (2006). A geometric morphometric study of sexual dimorphism in the crania of indigenous southern Africans. S. Afr. J. Sci. 102, 229–238. [ Links ]
25. Franklin D., Freedman L., Milne N. and Oxnard C.E. (2007). Geometric morphometric study of population variation in indigenous southern African crania. Am. J. Hum. Biol. 19, 20–33. [ Links ]
26. Franklin D., O'Higgins P., Oxnard C.E. and Dadour I. (2007). Sexual dimorphism and population variation in the adult mandible: forensic applications of geometric morphometrics. Forensic Sci. Med. Pathol. 3, 15–22. [ Links ]
27. Tobias PV. (1987). Memories of Robert James Terry (1871–1966) and the genesis of the Terry and Dart collections of human skeletons. Adler Mus. Bull. 13, 31–34. [ Links ]
28. Tobias P.V. (1991). On the scientific, medical, dental and educational value of collections of human skeletons. Int. J. Anthropol. 6, 277–280. [ Links ]
29. Lockwood C.A., Lynch J.M. and Kimbel W.H. (2002). Quantifying temporal bone morphology of great apes and humans: an approach using geometric morphometrics. J. Anat. 201, 447–464. [ Links ]
30. Jeffery N. and Spoor F. (2004). Ossification and midline shape changes of the human fetal cranial base. Am. J. Phys. Anthropol. 123, 78–90. [ Links ]
31. Cobb S.N. and O'Higgins P. (2004). Hominins do not share a common postnatal facial ontogenetic shape trajectory. J. Exp. Zool. (Mol. Dev. Evol.) 302B, 302–321. [ Links ]
32. Franklin D., Oxnard C.E., O'Higgins P. and Dadour I. (2007). Sexual dimorphism in the subadult mandible: quantification using geometric morphometrics. J. Forensic Sci. 52, 6–10. [ Links ]
33. Cardini A., Jansson A. and Elton S. (2007). A geometric morphometric approach to the study of ecogeographical and clinal variation in vervet monkeys. J. Biogeogr. doi:10.1111/j.1365–2699.2007.01731.x [ Links ]
34. Rohlf F.J. and Slice D.E. (1990). Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 39, 40–59. [ Links ]
35. Dryden I.L. and Mardia K.V. (1993). Multivariate shape analysis. Sankhya 55(A), 460–80. [ Links ]
36. Good P. (2000). Permutation Tests: A Practical Guide to Resampling Methods for Testing Hypotheses. Springer Series in Statistics, New York. [ Links ]
37. Baylac M., Villemant C. and Simbolotti G. (2003). Combining geometric morphometrics with pattern recognition for the investigation of species complexes. Biol. J. Linn. Soc. Lond. 80, 89–98. [ Links ]
38. O'Higgins P. and Jones N. (1998). Facial growth in Cercocebus torquatus: An application of three-dimensional geometric morphometric techniques to the study of morphological variation. J. Anat. 193, 251–272. [ Links ]
39. Vixxoxarsdóttir U.S., O'Higgins P. and Stringer C. (2002). A geometric morphometric study of regional differences in the ontogeny of the modern human facial skeleton. J. Anat. 201, 211–229. [ Links ]
40. Harmon E.H. (2007). The shape of the hominoid proximal femur: a geometric morphometric analysis. J. Anat. 210, 170–185. [ Links ]
41. Enlow D.H. and Hans M.G. (1996). Essentials of Facial Growth. W.B. Saunders, New York. [ Links ]
42. O'Higgins P., Johnson D.R., Moore W.J. and Flinn R.M. (1990) The variability of patterns of sexual dimorphism in the hominoid skull. Experientia 46, 670–672. [ Links ]
43. Bulygina E., Mitteroecker P. and Aiello L. (2006). Ontogeny of facial dimorphism and patterns of individual development within one human population. Am. J. Phys. Anthropol. 131, 432–443. [ Links ]
44. Walker G.F. and Kowalski C.J. (1972). On the growth of the mandible. Am. J. Phys. Anthropol. 36, 111–118. [ Links ]
45. Hunter W.S., and Garn S.M. (1972). Disproportionate sexual dimorphism in the human face. Am. J. Phys. Anthropol. 36, 133–138. [ Links ]
46. Humphrey L.T., Dean M.C., and Stringer C.B. (1999). Morphological variation in great ape and modern human mandibles. J. Anat. 195, 491–513. [ Links ]
47. Avis V. (1961). The significance of the angle of the mandible: an experimental and comparative study. Am. J. Phys. Anthropol. 19, 55–61. [ Links ]
48. Washburn S.L. (1947). The relationship of the temporal muscle to the form of the skull. Anat. Rec. 99, 239–248. [ Links ]
49. Moore W.J. (1965). Masticatory function and skull growth. J. Zool. 146, 123–131. [ Links ]
50. Ricketts R.M. (1975). Mechanisms of mandibular growth: a series of inquiries on the growth of the mandible. In Determinants of Mandibular Form and Growth, ed. J.A. McNamara, pp. 77–100. Center for Human Growth and Development: Ann Arbor, Michigan. [ Links ]
51. Petrovic A.G., Stutzmann J.J. and Oudet C.L. (1975). Control processes in the postnatal growth of the condylar cartilage of the mandible. In Determinants of Mandibular Form and Growth, ed. J.A. McNamara, pp. 101–153. Center for Human Growth and Development: Ann Arbor, Michigan. [ Links ]
52. Giles E. (1964). Sex determination by discriminant function analysis of the mandible. Am. J. Phys. Anthropol. 22, 129–136. [ Links ]
53. Hanihara K. (1959). Sex diagnosis of Japanese skulls and scapulae by means of discriminant functions. J. Anthropol. Soc. Nippon 67, 21–27. [ Links ]
54. Frayer D.W. and Wolpoff M.H. (1985). Sexual dimorphism. Ann. Rev. Anthropol. 14, 429–473. [ Links ]
55. Bruzek J. (2002). A method for visual determination of sex, using the human hip bone. Am. J. Phys. Anthropol. 117, 157–168. [ Links ]
56. Rosenberg K.R. (1992). The evolution of modern human childbirth. Yrbk Phys. Anthropol. 35, 89–124. [ Links ]
57. Gray J.P. and Wolfe L.E. (1980). Height and sexual dimorphism of stature among human societies. Am. J. Phys. Anthropol. 53, 441–456. [ Links ]
58. Lazenby R.A. (2002). Population variation in second metacarpal sexual size dimorphism. Am. J. Phys. Anthropol. 118, 378–384. [ Links ]
59. Stini W.A. (1969). Nutritional stress and growth: sex difference in adaptive response. Am. J. Phys. Anthropol. 31, 417–426. [ Links ]
60. Tobias P.V. (1975). Anthropometry among disadvantaged peoples: studies in Southern Africa. In Biosocial Interrelations in Population Adaptation, eds E.S. Watts, F.E. Johnston and G.W. Lasker, pp. 287–305. Mouton, The Hague. [ Links ]
61. Borogognini-Tarli S.M. and Repetto E. (1986). Methodological considerations on the study of sexual dimorphism in past human populations. Hum. Evol. 1, 51–66. [ Links ]
62. Ruff C.B. (1987). Sexual dimorphism in human lower limb bone structure: relationship to subsistence strategy and sexual division of labor. J. Hum. Evol. 16, 391–416. [ Links ]
63. Moss M.L., and Simon M.R. (1968). Growth of the human mandibular angular process: a functional cranial analysis. Am. J. Phys. Anthropol. 28, 127–138. [ Links ]
64. Hylander W.L. (1979). The functional significance of primate mandibular form. J. Morph. 160, 223–229. [ Links ]
65. Herring S.W. (1993). Epigenetic and functional influences on skull growth. In The Skull, vol. 1: Development, eds J. Hanken and B.K. Hall, pp. 153–206. The University of Chicago Press, Chicago. [ Links ]
66. Moss M.L. (1968). The primacy of functional matrices in orofacial growth. Dent. Pract. 19, 65–73. [ Links ]
67. Ingervall B. and Britsanis, E. (1987). A pilot study of the effect of masticatory muscle training on facial growth in long-face children. Eur. J. Orthodont. 9, 15–23. [ Links ]
68. Giles E. and Elliot O. (1963). Sex determination by discriminant function analysis of crania. Am. J. Phys. Anthropol. 21, 53–68. [ Links ]
Received 7 November 2007. Accepted 18 March 2008.
* Author for correspondence. E-mail: daniel.franklin@uwa.edu.au














