Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.104 n.1-2 Pretoria Jan./Feb. 2008
RESEARCH LETTERS
Fish bone sizes as estimators of standard lengths of three southern African freshwater species with application to archaeological samples: a preliminary investigation
Ina Plug
Department of Anthropology and Archaeology, UNISA, Pretoria 0003, South Africa. Address for correspondence: P.O. Box 21022, Valhalla 0137, South Africa. E-mail: plugc@mweb.co.za
ABSTRACT
Estimating standard lengths (SL) from freshwater fish bones found in archaeological samples can provide information on the status of past fish populations, their exploitation and environmental conditions. The bones of three fish species were measured and tested against the SL to determine their accuracy as predictors. A limited number of bones proved useful to determine SL. The results were applied to the fish bones from an archaeological site in Lesotho, to determine the median and maximum size of the prehistoric fishes. Most of these were of breeding size, while the maximum sizes estimated appear to exceed those of current angling records. This study is limited by a small sample of modern fishes with a relatively restricted size range, but nonetheless provides useful insights into the size distribution of ancient fish populations in Lesotho.
Introduction
Using individual bones to estimate fish lengths, mass, or fish ages is common practice in archaeozoology, but different researchers used different bones.1-7 With the exception of Van Neer,6,7 all these studies worked with marine species. Estimating the standard lengths (SL) of fishes from measured archaeological bones can provide insights into the predation patterns of the fishers, for example, seasonal exploitation or over-exploitation, as well as environmental conditions.
In southern Africa no in-depth studies have been conducted on freshwater fishes to estimate SL based on multiple bones of more than one species, though Farquharson8 used the length of the pharyngeal bone of the KwaZulu-Natal yellowfish (Labeobarbus natalensis) and the Tugela mudfish (Labeo rubromaculatus) to estimate SL. The study reported here was undertaken to facilitate the interpretation of freshwater fish remains from Likoaeng, a site on the upper Senqu River, Lesotho, dated between 1310 and 3110 BP.9 Approximately 65 000 fish bones, representing the species Labeobarbus kimberleyensis (largemouth yellowfish), Labeobarbus aeneus (smallmouth yellowfish) and Labeo capensis (Orange river mudfish), were studied. The species found in the archaeological samples are in line with the fish species recorded in the cold waters of the Senqu River at present.10-12
To determine the reproductive maturity of the archaeological fish sample and possible increases or decreases in fish size during the period of site occupation, it was necessary to establish methods for estimating fish sizes from measurements of individual bones. A reference collection of fresh fishes was obtained consisting of 14 specimens of the smallmouth yellowfish, four of the largemouth yellowfish, and five of the Orange river mudfish. The numbers were small because all had to be caught by an angler, the largemouth yellowfish is quite scarce, and the Orange river mudfish, with its mouth placed ventrally,11 is not easily caught. Specimens from the collections of the South African Institute for Aquatic Biodiversity were also examined, but could not be measured as they were mostly articulated.
The purpose of the present study was fourfold. First, to determine how well the SL of the three study species can be estimated from the dimensions of various bones; second, to establish whether the prediction ratios (SL divided by the size of an individual bone) are the same or different for the three species; third, to interpret the ancient size distributions of the three species from the archaeological fish remains at Likoaeng; and fourth, to determine whether the different archaeological bones produce similar reconstructed sizes.
Measuring fish bones
Maximum lengths of the bones were taken to the nearest 0.1 mm using Vernier callipers, following the guidelines of Morales and Rosenlund.13 Terminology used follows Rojo14 and Lepiksaar.15 Vertebra diameters of the archaeological specimens were measured to the nearest 0.5 mm, as the edges were abraded. Pharyngeal bones were measured according to the procedure set out by Farquharson.8 Figure 1 illustrates some measurements taken.
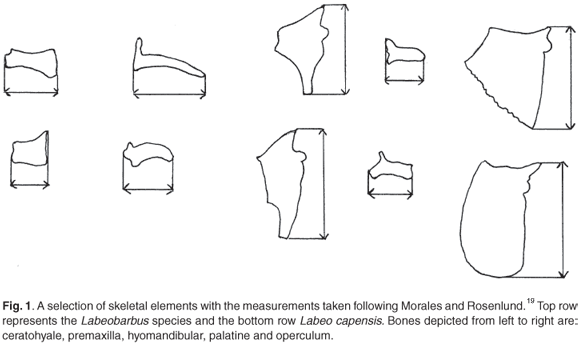
Measurements are most accurate on bones with well-defined measuring points such as the palatine and ceratohyale in all three species, though these are not necessarily the best predictors of SL. Irregularly shaped bones, on which accurate measuring points cannot be defined, have limited value as predictors. Damage on thin bones is common and not always visible, such as the anterior portions of the dentary and the articular in the Orange river mudfish and the processus praeopercularis of the quadrate in all three species. Measurements on such bones are therefore also not reliable. There are no significant size differences between the left and right sides of an individual. The mean of left and right measurements were therefore used where applicable.
Estimating standard lengths
The estimation of SL was investigated by plotting the bone measurements of the comparative sample against the SL of the smallmouth yellowfish (SL 283-390 mm), the largemouth yellowfish (SL 333-542 mm), and the Orange river mudfish (SL 304-408 mm). Within these size ranges the graphs show a linear relationship between the size of each bone and SL, with some scatter of the points around the best-fitting line. The best-fitting lines pass through or close to the origins (zero points) of the graphs. This means that, at least for the limited size ranges encountered in this study, the ratio of bone sizes to SL is the same for all specimen sizes, though different for each bone. This result supports the use of a fixed ratio for estimating fork length of the right maxilla in snappers (Pagrus auratus) by Leach and Davidson.3 Other investigators also found linear relationships between sizes of fish bones and body length, though the best-fitting line did not usually pass through the origin.16-18 Others still used logarithmic linear regression lines to estimate body size from bone sizes.5,19
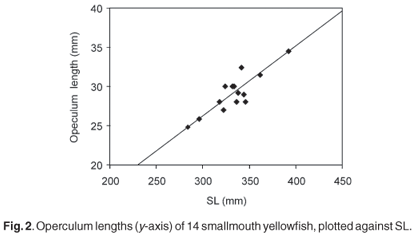
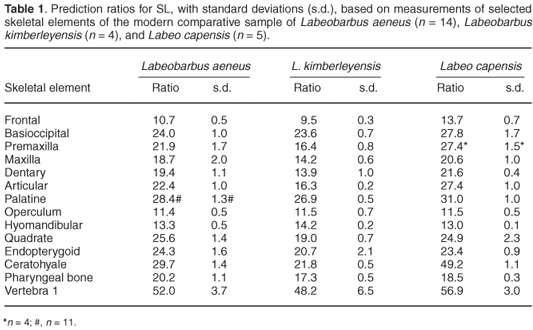
Figure 2 is an example of the relationship found in the present study, based on the operculum of the smallmouth yellowfish. For each species, and each bone, the mean prediction ratio of the available specimens was calculated as the best predictor of SL in the archaeological sample. Table 1 presents these prediction ratios for 14 different bones, selected because they could be measured most accurately in the fresh fish and were the best-preserved skeletal elements in the archaeological sample. The accuracy with which SL can be estimated from different skeletal parts (in fresh fishes) was investigated by calculating, for each species and each bone separately, the standard deviation of the prediction ratios for individual specimens. However, the standard deviations based on the very small numbers of two of the species are unreliable. The prediction ratios for some bones differ markedly between the three species, even between the species belonging to the same genus. Examples are the premaxilla, dentary, articular, and ceratohyale. On the other hand, the prediction ratios for the operculum, hyomandibular and the first vertebra are almost the same for the three species, considering the variability within each species. For some bones (the maxilla, quadrate and endopterygoid) the ratios differ more between the two species of yellowfish than between the smallmouth yellowfish and the Orange river mudfish. The ratios for the pharyngeal bone (20.2, 17.3 and 18.5) are comparable to the 20 of Farquharson8 for the species Labeobarbus natalensis and Labeo rubromaculatus.
Except for vertebra 1, the caudal and precaudal vertebrae of the three species are not species specific. The diameters of the fresh vertebrae were investigated as estimators of SL for the three species individually and combined (23 specimens). Vertebrae sizes varied within the same individual, thus limiting their usefulness as a predictor. The size ranges of caudal and precaudal vertebrae overlap within the same individual, hence no distinction need be made. For each specimen three prediction ratios were calculated: a mean ratio (SL divided by mean vertebra diameter), a minimum ratio (SL divided by the diameter of the largest vertebra) and a maximum ratio (SL divided by the diameter of the smallest vertebra). In all three cases the differences between individuals of the same species are much the same as those between individuals of different species, hence the species can be combined. The combined prediction ratios for the sample of 23 individuals are: mean ratio 56.0, minimum ratio 51.0 and maximum ratio 65.1. Vertebra size can therefore be used to estimate SL only within fairly wide limits.
Measurements of archaeological fish bones
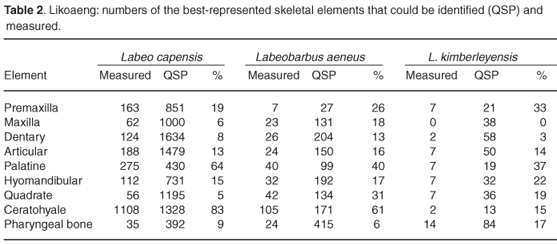
Estimates based on different skeletal elements within a unit of deposit are not completely independent, as a single fish may have given rise to several measurable bones. In layers with small samples, however, there are often marked size differences between left and right elements, indicating that the elements are largely independent where samples are small. Differential preservation has resulted in large differences between the numbers of measurable bones both within and between species (Table 2), and can best be explained by differences in bone structure, morphology and size. The posterior ends of the premaxilla of Orange river mudfish were seldom preserved, and the largest of those of the largemouth yellowfish were often broken. Differential preservation influenced the maxilla, dentary, articular and quadrate bones. The palatine, hyomandibular and ceratohyale were well preserved and many could be measured. Of the ceratohyale of the Orange river mudfish, 83% could be measured, compared to 61% in the smallmouth yellowfish. Few complete specimens were found of the largemouth yellowfish due to breakage of the larger specimens. The pharyngeal bone of the Orange river mudfish did not preserve well, but many specimens could be measured because the anterior arm, where the measuring points lie, were often intact. This bone is thicker and stronger in the two yellowfish species, but tends to break at the angulus inferior or angulus anterior, where the measuring points are.
Estimating standard length
The distributions of estimated SL in different layers of the deposit are irregular and usually contain a 'tail' containing a few very small or very large individuals. The most appropriate indicator of average size is therefore the median SL, rather than the arithmetic mean. Other parameters are the maximum estimated size and the percentage of immature individuals. Table 3 contains estimates of SL based on the skeletal elements of the largemouth yellowfish from Likoaeng. The numbers of bones are small, but suitable to illustrate a methodological point. The estimated median SL based on the sizes of different bones varies. For those bones with sample sizes of at least six, the median ranges from 361 mm (quadrate) to 646 mm (pharyngeal). Despite the small numbers, the differences are partly the result of differential preservation, and illustrate the need to use as many different skeletal elements as possible when estimating SL, particularly with small samples. The largest specimen of this species, as estimated from the size of the pharyngeal bone, could have reached an SL of 977 mm. Furthermore, two estimated SL values of 869 mm are based on the premaxilla. This fish probably grew even larger, as the sample contained broken dentary and pharyngeal fragments markedly larger than those that could be measured.
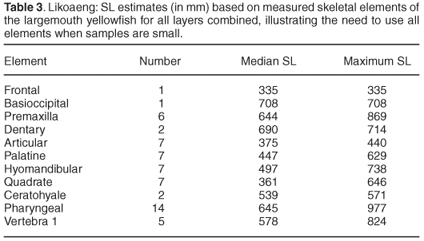
Of the measurable bones of the smallmouth yellowfish, the ceratohyale was the most numerous. Figure 3 illustrates the distribution of estimated SL for this species, based on that element. Most estimates fall between 240 mm and 540 mm SL. The median SL based on the different skeletal elements are very similar. All but one fall between 348 mm and 374 mm, whereas that based on the pharyngeal bone is only 309 mm. The SL estimates based on this element include small specimens under 180 mm. The largest individuals of this species are estimated to have exceeded 600 mm SL, based on all measured bones.
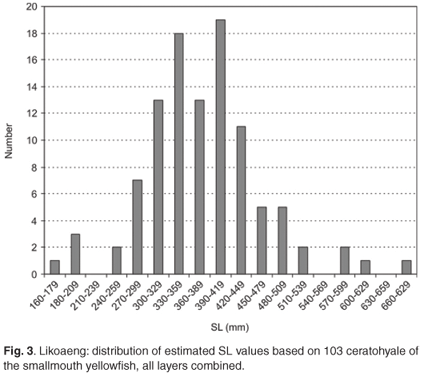
The ceratohyale was also the best-preserved element of the Orange river mudfish and was particularly numerous in Layer VII-IX. The distribution of estimated SL for this layer is shown in Fig. 4 This skeletal element was used to investigate possible differences in the size distribution of the species over time. Frequency distributions of estimated SL (between 54 and 369 specimens per layer) showed that the distributions differed somewhat in the upper and lower tails. Differences between the median estimated SL (between 384 mm and 394 mm) were insignificant and represent differences of a few tenths of a millimetre in bone size. Hence there is no evidence that the average size of fishes of this species changed over a period of several centuries.
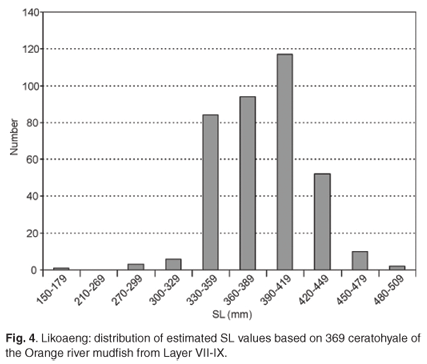
To check the results obtained on the ceratohyale, three other elements, the hyomandibular, premaxilla and palatine, were used in the reconstruction of SL. This exercise was confined to Layer VII-IX, where these elements were the most numerous. The distributions of SL for the different skeletal elements look more or less similar but have different central values. The median estimated SL varies from 389 mm for the ceratohyale to 493 mm for the premaxilla. These differences cannot be ascribed to uncertainties in the prediction factors or small sample sizes and must be the result of differential preservation. The largest estimated SL were derived from the premaxilla, which produced three estimates exceeding 600 mm.
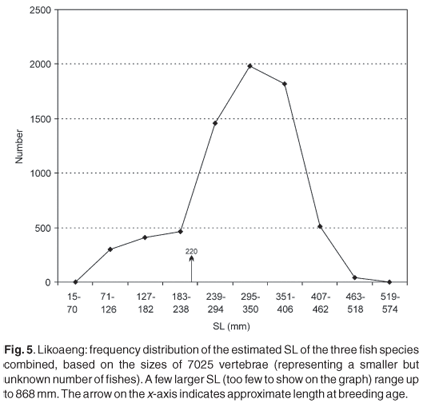
Figure 5 illustrates the frequency distribution of estimated SL of the three species combined, based on the diameters of the vertebrae from all the layers. Of significance is the presence of a substantial number of smaller fish, which are almost totally absent in the size distributions based on other skeletal elements. The two largest measurable vertebrae have a diameter of 15.5 mm, corresponding to an SL of 868 mm (mean prediction ratio), or at least 790 mm (minimum ratio). Furthermore, fragments of even larger vertebrae, probably of the largemouth yellowfish, are present, but could not be measured.
Discussion
Estimating standard length
The measurements obtained on the bodies and skeletal elements of three southern African species indicate that it is possible to estimate the SL of fishes from the dimensions of many of the skeletal elements with acceptable accuracy (Table 1), at least within the limited size ranges available for study. However, the prediction ratio for a given skeletal element may differ widely among species, even among species of the same genus. Prediction ratios therefore need to be determined separately for each species and each skeletal element, preferably using larger samples. Measurements of the vertebrae can be used to predict standard length, though with only limited accuracy.
The fish bones from Likoaeng show that the proportion of measurable bones varies from one element to the next (Table 2). The differences in preservation can to some extent be explained on the basis of the shapes and structures of the bones. Combined with poor preservation of archaeological bone, the measurable specimens of any skeletal element represent a small, and perhaps a quite selective, sample of the total number of that element originally deposited. For some skeletal elements the larger specimens are best preserved, while for other elements the smaller specimens remain unbroken. The pattern is different for each species. This problem is illustrated by the estimates of SL based on the small number of measurable remains of the largemouth yellowfish. The lengths derived from different skeletal elements differ widely (Table 3), and though this is partly the result of the small sample sizes, a contributing cause seems to be differential preservation. To reduce the effects of both small samples and differential preservation, it is important to use as many skeletal elements as possible to reconstruct SL.
More light is thrown on the matter by the measurements on the larger samples of bones of the Orange river mudfish, for which the median SL derived from the sizes of four skeletal elements differs substantially. Possible reasons for these differences, in addition to differential preservation, are: inaccuracies in the prediction ratios (based on only five specimens); the measurements of archaeological bones may differ systematically from those of modern specimens because of taphonomic factors such as warping and differential abrasion patterns of the different skeletal elements. It appears that the ceratohyale, hyomandibular and palatine are most useful to estimate SL for the Orange river mudfish, but that the premaxilla should be used with care.
Maximum fish sizes
Measurable bones of large size are under-represented in the samples of Likoaeng, as these bones are more often fragmented than smaller ones. Similar trends have been observed in mammalian bone preservation.20 The modern record fork length of the largemouth yellowfish is 825 mm,11 which corresponds to an SL of below 800 mm. Even though few remains of this fish could be measured in the archaeological sample, three estimates of SL, based on the premaxilla and the pharyngeal bone, exceed this record, whereas the largest vertebrae also indicate an SL close to or over 800 mm. The same applies to the smallmouth yellowfish, which today reaches a maximum fork length of 500 mm, corresponding to an SL of about 470 mm,11 but estimates of three archaeological specimens exceed an SL of 600 mm, and 16 more exceed 480 mm. The Orange river mudfish can attain a total length of 500 mm,11 or an SL of approximately 430 mm. Many estimates of SL, based on bones in the archaeological sample, exceed this limit, especially those based on the palatine, which yielded an SL of just over 600 mm. It therefore seems that larger specimens of all three species were caught in prehistoric times than at present. Size reductions of fishes from historic and prehistoric sites have been documented from sites in New Zealand,3 Australia,21 Cook Islands,2 the West Indies,4 Egypt,22 Germany23 and the United States.24 In almost all these instances over-exploitation is given as the most likely cause. For the Lesotho sample the reasons for size reduction are unclear. Climate change over the past 3000 years and over-exploitation could be causes, but there is as yet no firm evidence to implicate these.
Utilization of immature fishes
The smallmouth yellowfish reaches breeding size at a standard length of about 200 mm for males and 240 mm for females, but there are variations depending on the locality.25,26 The average breeding size of the Orange river mudfish is an SL of 220 mm for males and 240 mm for females,11,25-28 but in the Caledon River they appear to mature at 150-200 mm fork length for males and 200-250 mm for females.25-29 In this study immature individuals are defined as a vertebra diameter of 4.0 mm or less. This corresponds to an estimated SL of 238 mm. Figure 5 shows that immature fish are represented in the archaeological deposit, though not in large numbers. The percentage of immature individuals changed over time: in the younger layers only 1-7%, but rising abruptly to 28% in layer XIII and to a mean of 55% in the older layers.
Few of the estimated SL values based on the other skeletal elements are small enough to represent individuals below breeding size. There are several possible explanations for this discrepancy: small vertebrae are easier to recognize than other small skeletal elements during excavation; the head and appendicular elements may have been eaten and digested; and fish heads may not have been discarded on the site. The last of these explanations seems least likely, as fish heads of the larger specimens are certainly present. Human digestion of the bones of small fishes is a distinct possibility. Consumption of small fishes was studied by Butler and Schroeder.30 Digestive attrition amounted to a loss of about 74% of the skeletal elements of the fishes consumed,30 while Wheeler and Jones31 found that fish bone loss during digestion could be as high as 90%. Vertebrae were the most numerous skeletal elements present in the retrieved sample of Butler and Schroeder.30 It is possible that the people of Likoaeng consumed some smaller fishes whole. The large number of small fishes in the lower layers may be attributed to several causes. Various lines of evidence suggest that Likoaeng was occupied seasonally during the annual spawning runs of the fish,10 when few small fishes are present.32 Occupation at other times of the year may have resulted in more small fish being caught.
This preliminary investigation of the bone size to SL relationship is based on a small number of modern adult fishes with a limited size range. Further studies on larger samples of all ages should be conducted.33,22,27 This study shows nonetheless that SL can be estimated from archaeological bones of southern African freshwater fishes, and that useful conclusions can be based on the results.
I thank the following persons and institutions for their assistance and/or financial support: the Leverhulme Trust, U.K.; the University of Newcastle upon Tyne, U.K.; G.N. Bailey, University of York, Nick James of Rivendell for arranging the catching and shipment of the fish; Paul Skelton, Roger Bills and staff of the SAIAB, Grahamstown; Cornelis Plug for assistance with the statistical analysis, K. Hamman, Western Cape Nature Conservation, for permission to use Gaigher 1976, and Peter Mitchell for providing the archaeological sample.
1. Butler V. (1993). Natural versus cultural Salmonid remains: origin of the Dalles Roadcut bones, Columbia River, Oregon, USA. J. Archaeol. Sci. 20, 1-24. [ Links ]
2. Butler V. (2001). Changing fish use on Mangaia, southern Cook Islands: resource depression and the prey choice model. Int. J. Osteoarchaeol. 11, 88-100. [ Links ]
3. Leach F. and Davidson J. (2000). Pre-European catches of snapper (Pagrus auratus) in northern New Zealand. J. Archaeol. Sci. 27, 509-522. [ Links ]
4. Wing E.S. (2001). Native American use of animals in the Caribbean. In Biogeography of the West Indies: Patterns and perspectives, eds C.A. Woods and F.E. Sergile, pp. 481-518. CRC Press, Boca Raton, FL. [ Links ]
5. Zohar I., Dayan T., Galili E. and Spanier E. (2001). Fish processing during the early Holocene: a taphonomic case study from coastal Israel. J. Archaeol. Sci. 28, 1041-1053. [ Links ]
6. Van Neer W. (1993). Fish remains from the last interglacial at Bar Tarfawi (eastern Sahara, Egypt). In Egypt During the Last Interglacial: the Middle Paleolithic of Bir Tarfawi and Bir Sahara East, eds F. Wendorf, R. Schild and A. Close, pp. 144-155. Plenum Press, New York. [ Links ]
7. Van Neer W. (1993). Limits of incremental growth in seasonality studies: the example of the Clariid pectoral spines from the Byzantino-Islamic site of Apamea (Syria; sixth to seventh century AD). Int. J. Osteoarchaeol. 3, 119-127. [ Links ]
8. Farquharson F.L. (1980). Appendix 5: Fish remains. Annals of the Natal Museum 24, 68. [ Links ]
9. Plug I., Mitchell P.J. and Bailey G.N. (2003). Animal remains from Likoaeng, an open-air river site, and its place in the post-classic Wilton of Lesotho and the eastern Free State, South Africa. S. Afr. J. Sci. 99, 143-151. [ Links ]
10. Plug I. (in press). The exploitation of freshwater fish during the Later Stone Age of Lesotho: Preliminary results. In Beyond the Affluent Forager, eds J. Kim, C. Grier and J. Uchiyama. Proceedings of the VIth ICAZ Conference, Durham, 2002. Oxbow Press, Oxford. [ Links ]
11. Skelton P. (2001). The Freshwater Fishes of Southern Africa. Struik, Cape Town. [ Links ]
12. Ambrose D. (1999). Lesotho Annotated Bibliography. Section 164 Fish. Institute of Education, National University of Lesotho, Roma. [ Links ]
13. Morales A. and Rosenlund K. (1979). Fish Bone Measurements: An attempt to standardize the measuring of fish bones from archaeological sites. Steenstrupia, Copenhagen. [ Links ]
14. Rojo A.L. (1991). Dictionary of Evolutionary Fish Osteology. CRC Press, Boca Raton, FL. [ Links ]
15. Lepiksaar J. (1983). Some words about fish skeletons for fauna-historical (archaeozoological) studies in my collection. Osteologia I Pisces. Göteborg, privately distributed. [ Links ]
16. Clay D. (1982). A comparison of different methods of age determination in the sharptooth catfish Clarias gariepinus. J. Limnol. Soc. S. Afr. 8, 59-70. [ Links ]
17. Balme J. (1983). Prehistoric fishing in the lower Darling, western New South Wales. In Animals and Archaeology: vol. 2. Shell middens, fishes and birds, eds C. Grigson and J. Clutton-Brock, pp. 23-32. BAR International Series 183, Oxford. [ Links ]
18. Noe-Nygaard N. (1983). Importance of aquatic resources to Mesolithic man. In Animals and Archaeology: vol. 2. Shell middens, fishes and birds, eds C. Grigson and J. Clutton-Brock, pp. 126-141. BAR International Series 183, Oxford. [ Links ]
19. Butler V. (1996). Tui Chub taphonomy and the importance of marsh resources in the Western Great Basin of North America. Am. Antiquity 61(4), 699-717. [ Links ]
20. Plug I. (1988). Hunters and herders: an archaeozoological study of some prehistoric communities in the Kruger National Park. D. Phil. et Litt. dissertation, University of Pretoria, South Africa. [ Links ]
21. Owen J.F. and Merrick J.R. (1994). Analysis of coastal middens in south-eastern Australia: sizing of fish remains in Holocene deposits. J. Archaeol. Sci. 21, 3-10. [ Links ]
22. Luff R.M. and Bailey G.N. (2000). Analysis of size changes and incremental growth structures in African catfish Synodontis schall (schall) from Tel el-Amarna, Middle Egypt. J. Archaeol. Sci. 27, 821-835. [ Links ]
23. Heinrich D. (1985). Die Fischreste aus der Frühgeschichtlichen Marschensiedlung beim Elisenhof in Eiderstedt. Archäologisch-Zoologischen Arbeitsgruppe, Neue Universität, Kiel. [ Links ]
24. Rick T.C. and Erlandson J.M. (2000). Early Holocene fishing strategies on the California coast: evidence from CA-SBA-2057. J. Archaeol. Sci. 27, 621-633. [ Links ]
25. Tómasson T., Cambray J.A. and Jackson P.B.N. (1984). Reproductive biology of four large riverine fishes (Cyprinidae) in a man-made lake, Orange River, South Africa. Hydrobiologia 112, 179-195. [ Links ]
26. Gaigher I.G. (1976). Age determination and growth rate of the Orange River Labeo, Labeo capensis. Internal report. Cape Department of Nature and Environmental Conservation, Cape Town. [ Links ]
27. Nthimo M. (2000). The biology of commercially important fish species and a preliminary assessment of the fisheries potential of the Katse Dam, Lesotho. MSc thesis, Rhodes University, Grahamstown. [ Links ]
28. Baird D.P. (1978). The length/mass relationship and condition of Labeo capensis in the Caledon River. J. Limnol. Soc. S. Afr. 4(1), 53-58. [ Links ]
29. Tómasson T., Bruton M.N. and Hamman K.C.D. (1985). The demography and management of large cyprinids in a reservoir on the Orange River, South Africa. Fisheries Res. 2, 279-308. [ Links ]
30. Butler V. and Schroeder R. (1998). Do digestive processes leave diagnostic traces on fish bones? J. Archaeol. Sci. 25, 957-971. [ Links ]
31. Wheeler A. and Jones A. (1989). Fishes. Cambridge Manuals in Archaeology, Cambridge University Pres, Cambridge. [ Links ]
32. Cambray J.A. (1990). Adaptive significance of a longitudinal migration by juvenile freshwater fish in the Gamtoos river system, South Africa. S. Afr. J. Wildl. Res. 20(4),149-156. [ Links ]
33. Reitz E. and Wing E. (2000). Zooarchaeology. Cambridge University Press, Cambridge. [ Links ]
Received 19 April. Accepted 3 October 2005.














