Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.104 n.1-2 Pretoria Jan./Feb. 2008
CORRESPONDENCE
Toxicity evaluation of synthetic food sweeteners by means of the Weaver Human Cell Test
J.M. Whitcutt; E.M. Bey
Highveld Biological Association, P.O. Box 1456, Lyndhurst 2106, South Africa
Sir, Synthetic food sweeteners are increasingly being used to reduce the calorie content, while still retaining the taste, of foods and beverages that would otherwise contain significant amounts of sugar.
Concerns about the amounts of synthetic food and beverage sweeteners being consumed by the public are reflected in the numbers of web entries currently appearing under the keywords aspartame (2.4 million), cyclamate (0.1 million), saccharin (0.7 million), sucralose (0.6 million), and sweetener (3.3 million).
Health risks arising out of the use of these substances fall into two categories: low-frequency interactions, possibly causing cancer or allergic conditions such as asthma, and high-frequency metabolic disruption leading to a variety of other health problems. The sheer volume of conflicting web-site information, much of it either anecdotal or commercially motivated, makes it virtually impossible to come to meaningful conclusions about the relative safety of any of these products.
The Weaver Human Cell Test (WHCT)1 is designed as a sensitive, low-cost indicator of metabolic disruption. It is a simplified version of the cytotoxicity testing systems that were used in compiling the National Institute of Environmental Health Science's Registry of Cytotoxicity,2 and was recently used to evaluate the relative cytotoxicity of some of these readily available food products.
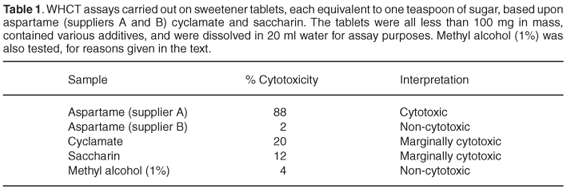
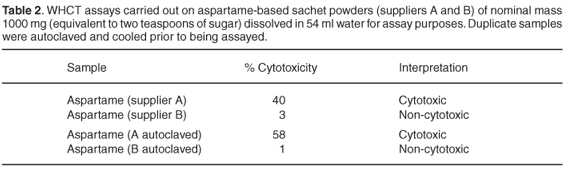
Comments on key components present in the products are given below. Tables 1 and 2 list the results of WHCT assays carried out on some of them.
Aspartame. This is a methyl ester of the dipeptide, aspartyl phenylalanine, both components of which are present in normal food proteins as well as in spoiled food toxins. It is not heat stable, but has been approved by the US Food and Drug Administration for use in hot and cold foods and beverages.
Cyclamate (cyclohexyl sulphamic acid) was extensively used up to 1970, after which it was banned by the FDA for use as a food and beverage additive. The ban has since been lifted, subject to certain provisions.
Saccharin (ortho-sulphobenzoic acid imide) is the oldest of the synthetic sweeteners (first isolated in 1879) and has been used as a sweetener since 1938. It has an undesirable after-taste, which can be masked by adding small amounts of other sweeteners.
Sucralose and neotame are relatively new products and were not included in this study.
The figures given in Table 1 can be interpreted in different ways. First, it is not possible to do a conventional comparison of the IC50 values (concentrations causing a 50% inhibition of the metabolic process being measured) of these sweetener samples because all of the preparations are made up of multiple constituents. It seems that the saccharin and cyclamate-based products are at worst only marginally cytotoxic in the concentrations at which they would normally be used.
The non-toxicity of methyl alcohol (a possible metabolite of aspartame) in this test system reflects the absence in the K-562 detector cells of enzymes that are able to oxidise it to formaldehyde, the active toxicant in comparable whole-body situations. Other cells, which possess such enzyme systems, could be severely damaged. What this result does suggest, however, is that the harmful effects caused by some of the aspartame preparations are not due to the release of methyl alcohol.
It is also clear from the assay results shown in Tables 1 and 2 that all the aspartame preparations from one commercial source were unacceptably cytotoxic at concentrations that could well be encountered in ordinary domestic situations, while corresponding preparations from the other source were not.
The extremely limited sampling procedures underpinning this investigation do not allow any far-reaching conclusions to be drawn. Explanations are nevertheless called for.
The most likely explanation is the presence of an unspecified contaminant, possibly in a single batch of a bulk imported component. This would not be listed in the accompanying documentation nor would it show up in subsequent chemically-based quality control procedures unless specifically tested for. Its detection following our own spot-check sampling procedures can be regarded as purely fortuitous. Recent press reports suggest that there have also been unexpected toxicity problems with imported animal feeds and plant fertilizers.
It is our view that synthetic chemical products destined for subsequent human consumption require more stringent evaluation procedures than appears to be presently the case.
1. Whitcutt J.M. (2005). The Weaver Human Cell Test for water quality. S. Afr. J. Sci. 101, 383-388. [ Links ]
2. National Institute of Environmental Health Sciences (2001). Guidance document on using in vitro data to estimate in vivo starting doses for acute toxicity. NIH Publication no. 01-4500, Washington, D.C. [ Links ]
E-mail: mike@hibi.co.za














