Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.103 n.11-12 Pretoria Nov./Dec. 2007
RESEARCH LETTERS
Fusarium populations in the household food gardens of a peri-urban community
A.M. van der WaltI, *; M.I.M. IbrahimI; H.S. SteynII; C.C. BezuidenhoutI
IMorogo Research Programme, School of Environmental Sciences and Development
IIStatistical Consultation Services, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa
ABSTRACT
Toxigenic Fusarium species, studied mainly for the damage they cause to commercial crops, have received scant research attention in the context of small-scale food production. In this regard home gardens are an important source of nutrition for resource-poor urban families in Africa. We have investigated the presence of Fusarium in household food gardens in a peri-urban setting in the Rustenburg district of South Africa. Standard techniques were employed for the isolation and morphological species identification of Fusarium species from various sources, namely, maize, soil, air and naturally growing morogo vegetables, thepe and lerotho. Nine Fusarium species with mycotic and mycotoxigenic potential were specifically targeted for detection: F. verticillioides, F. proliferatum, F. solani, F. subglutinans and F. oxysporum were predominantly isolated from maize, air, soil and morogo vegetables. All species were isolated in significantly higher numbers from localities in proximity to maize. Fusarium chlamydosporum, F. semitectum and F. equiseti were not retrieved where maize was absent, whereas F. verticillioides and F. proliferatum were predominantly isolated from maize cobs. These results have public-health implications. All nine Fusarium species retrieved from peri-urban food gardens produce toxins and, except for F. poae, have been implicated in opportunistic infections in immune-suppressed individuals.
Introduction
The genus Fusarium includes various phytopathogenic species of economic importance, mainly for the damage they cause to commercial crops.1 Invasion of plant hosts is often linked with the capacity of the fungal pathogen to produce toxins.2 Dietary exposure to fusarial toxins causes irreversible tissue damage through biochemical mechanisms that produce pro-oxidative, pro-inflammatory, carcinogenic and/or immune-suppressive effects at a cellular level.3–6 Some toxigenic Fusarium species have furthermore been implicated as causative agents of life-threatening opportunistic infections in immune-suppressed individuals.7 Mortalities ranged between 50–80% in these cases, mainly because effective treatment of infection was complicated by drug resistance by the Fusarium pathogens and their blood-borne spread to various organs of the body.8
Peri-urban families living on a severely restricted budget in South Africa often resort to growing food at home to augment their food supply. Maize grown as the traditional staple in these situations is supplemented with green leafy vegetables (called morogo), often collected from the field.9 Some morogo species also appear spontaneously in disturbed soils and consequently are found growing among the maize.10,11 Maize ecosystems naturally harbour several toxigenic Fusarium species, however, notably those that produce fumonisin toxins.1,12,13 Fusarium where food is grown has important health implications, particularly for food-insecure populations that, according to Bourne et al.,14 are disproportionately affected by disease because of their unsatisfactory nutritional status.
We conducted a pilot study in a peri-urban community in the Rustenburg district of South Africa to determine the occurrence of nine Fusarium species in the vicinity of household food gardens and the influence of home-grown maize nearby. We considered the factors that may contribute towards sustaining Fusarium species in such environments and discuss the possible health implications for local communities.
Materials and methods
See Appendix.
Results
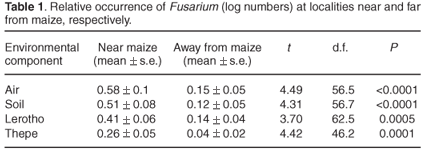
Figure 1 illustrates the number of isolates of each Fusarium species retrieved from samples collected on three sampling occasions from four localities at sites near to, or distant from, maize. All nine species occurred in varying degrees where maize was growing, with the number of isolates ranging from 6 (F. equiseti) to 130 (F. verticillioides). In localities remote from maize, the number of isolates was notably smaller, varying between 3 (F. poae) and 34 (F. verticillioides). Fusarium equiseti, F. chlamydosporum and F. semitectum were not retrieved from maize-free localities. Table 1 shows the evidence for Fusarium isolates retrieved from air, soil, lerotho and thepe as being relatively high from localities near maize. Mean log numbers of Fusarium isolated from where maize grew were highest in air (0.58 ± 0.1) and soil (0.51 ± 0.08) and lower in lerotho (0.04 ± 0.06) and thepe (0.26 ± 0.05). Table 2 shows that, on a 5% level, all species were isolated in significantly higher numbers where maize was nearby. Mean log numbers further indicate that F. verticillioides (0.85 ± 0.9) and F. proliferatum (0.78 ± 0.09) were the predominant species retrieved from localities near maize, followed by F. solani (0.69 ± 0.1), F. oxysporum (0.52 ± 0.1) and F. subglutinans (0.4 ± 0.11). Away from maize, mean log numbers ranged from 0.04 ± 0.04 (F. semitectum) to 0.24 ± 0.07 (F. proliferatum) and 0.3 ± 0.1 (F. verticillioides).


Tukey post hoc comparisons of Fusarium species numbers retrieved from each of the different environmental components are shown in Table 3. Means of log numbers indicate that F. verticillioides (0.83), F. proliferatum (0.66) and F. solani (0.61) were isolated from the air in numbers significantly higher than F. poae (0.16), F. equiseti (0.13) and F. chlamydosporum (0.08). Fusarium semitectum (0) were not retrieved from air samples. Similar results apply to F. verticillioides (0.71) and F. proliferatum (0.68) isolated from soil. Fusarium equiseti were retrieved from neither soil nor lerotho. No significant differences were found between the contamination of lerotho and thepe. In comparison with thepe, F. verticillioides (0.83) and F. oxysporum (0.43) were retrieved in significantly higher numbers from air. The other pathogens were not significantly different from each other in this respect.
In total, 150 Fusarium isolates were retrieved from the silk (69) and kernels (81) of maize cobs sampled from the four localities. Two-way ANOVA was used to determine interaction between maize cob components (leaves, silk and kernels) and Fusarium species (Table 4). No Fusarium was isolated from maize leaves and there was no significant interaction (P = 0.25) between kernels, silk and the Fusarium species isolated from them. In terms of log numbers, however, F. semitectum (0.23), F. equiseti (0.04) and F. chlamydosporum (0.04) were retrieved in significantly smaller numbers from maize kernels and silk than with F. proliferatum (0.79) and F. verticillioides (0.75). Of the maize-associated species, F. subglutinans (0.32) was isolated from kernels and silk in significantly lower numbers than F. proliferatum.
Fusarium isolates were retrieved in significantly higher numbers from localities near maize (Fig. 1). Mean log numbers of isolates retrieved from air, soil, thepe and lerotho respectively reflect this result (Table 1), which is demonstrated also in Table 2 for each of the nine Fusarium species. The number of isolates of F. verticillioides and F. proliferatum was significantly higher in air and soil relative to F. poae, F. equiseti, F. chlamydosporum and F. semitectum (Table 3) and, compared to F. equiseti, F. chlamydosporum and F. semitectum, also in maize cobs (Table 4).
Discussion
With the exception of F. poae, Fusarium species isolated from the environment of peri-urban food gardens have been reported to be capable of causing opportunistic infections in immune-suppressed individuals.21 Moreover, all species targeted for detection in the present survey produce mycotoxins22,23 that could compromise immune functioning.3,24,25 Results illustrated in Fig. 1 suggest these mycotic and mycotoxigenic Fusarium species are common members of autochthonous microbial populations in the environment of peri-urban food gardens. Fusarium species were retrieved in significantly higher numbers from localities in the proximity of home-grown maize than away from maize (Table 1). Though true of all nine Fusarium species, the observation was most pronounced for F. verticillioides, F. proliferatum, F. subglutinans and F. oxysporum (Table 2). Commonly associated with commercial maize ecosystems,13,26,27 the first three species are maize plant pathogens that cause seedling disease, root and crown rot, stalk rot and ear rot.28 Apart from the study of Sreenivasa and co-workers,29 in which F. solani was detected in freshly harvested maize, the presence of this species in maize ecosystems is not often reported.
The isolation of F. solani in significant numbers (P = 0.0002) is of particular interest in view of the high incidence of HIV infection in South Africa.20 Mid-year estimates indicated that 10.9% of the South African population was HIV-positive in 2006.20 Pujol et al.21 describe F. solani as the most dangerous filamentous fungus for immunocompromised patients after Aspergillus fumigatus, whereas Boutati and Anaissie30 report it as the species predominantly isolated from fatal cases of disseminated fusarioses in patients with haematologic malignancies. Fusarium poae, usually associated with destructive diseases of wheat,31,32 was also retrieved in significant numbers (P = 0.02) from localities near maize. F. equiseti and F. chlamydosporum were not isolated from sites away from maize, but in relatively small numbers near these plants. Relative to F. verticillioides, which was isolated as the predominant species, F. equiseti and F. chlamydosporum represented a small percentage of Fusarium isolated from commercial maize in Ghana.26
Figure 1 and Tables 1 and 2 indicate that maize may play a role in maintaining Fusarium species in the environment of peri-urban home gardens. Table 3 reveals significant interaction of maize-associated F. verticillioides and F. proliferatum with air (P = 0.03) and soil (P = 0.05). Cotton and Munkvold28 found F. verticillioides and F. proliferatum multiplied rapidly during the growing season on maize leaf surfaces as well as in rainwater trapped in leaf sheaths, and subsequently survived for up to two years in soil and maize plant residue on the soil surface. Mean log numbers depicted in Table 4 show that F. verticillioides (0.75) and F. proliferatum (0.79) isolated from maize cobs were primarily associated with maize kernels and silk, which might indicate a pathogenic relationship. Fusarium establishes infection when spores in the environment land on the silk, germinate and enter the ear after pollination, according to Cardwell et al.33 Nesci et al.27 attributed the occurrence of F. verticillioides and F. proliferatum in soil of pre-harvest maize ecosystems to the survival of these species in plant debris on the soil surface. Rossi et al.34 found that, under humid field conditions, fusarial spores in crop debris germinate and continue to produce macroconidia. These spores become airborne by splash dispersal during rain showers or irrigation and are disseminated over substantial distances by air currents.35,36
It thus seems likely that in home gardens, F. verticillioides, F. proliferatum and possibly F. subglutinans and F. oxysporum, occurring in association with home-grown maize, are maintained in maize residues and debris on the soil surface. Dispersed from plant debris, and depending on spore and leaf surface characteristics, airborne spores are trapped in differing degree by morogo plants growing in association with maize. This scenario might explain why, in the same environment, F. verticillioides was retrieved in higher numbers from thepe than from lerotho, while F. oxysporum, F. poae and F. semitectum were isolated in greater numbers from lerotho than from thepe (Table 3).
The multiplex PCR method described by Bezuidenhout et al.17,37 confirmed the presence of the FUM 1 gene in F. oxysporum, F. proliferatum, F. solani and F. subglutinans isolated from morogo plants. Furthermore, HPLC analysis detected 44.8 ng g–1 fumonisin B1 in thepe and 58.7 ng g–1 in lerotho.38 Similar findings were reported from maize-based subsistence agriculture in the Limpopo province.39
Fusarium species in home gardens have public-health implications. Urban activities are expected to enhance dissemination of fusarial spores, while human population numbers in urban settings put more individuals at risk of opportunistic fusarial infections. Numerous case studies identified F. verticillioides, F. proliferatum, F. solani and F. oxysporum30,40–43 as causative agents of disseminated fusariosis in immunocompromised individuals, in most instances with fatal consequences.8,44 The prevalence of Fusarium infections of the skin, the upper respiratory tract and eyes suggests that these organs serve as portals of entry, eventually leading to multiple organ infection.30,45 In the present survey these pathogens were isolated in considerable numbers from air, indicating an unavoidable risk for residents of inhalation, skin contact, or eye exposure to fusarial spores. Fusarium spores in the environment enhance the risk of HIV-positive individuals contracting secondary fusarial infections which, according to Dignani and Anaissie8 and Pujol et al.,21 are most difficult to treat. Common features of opportunistic Fusarium infections include the presence of fusaria in the blood stream, the high frequency of skin lesions and a high mortality rate in patients with suppressed immunity as a result of pathogens resistance to drugs.39,46
All the Fusarium species targeted for isolation in the present study are producers of potent toxins including beauvericin,22 fumonisins23 and trichothecenes.47 Dietary mycotoxins may produce a range of biological effects, depending on which of the following properties they possess: antinutritional,48 oxidative,5 pro-mutagenic,6 pro-inflammatory,49 tumorgenic,50 genotoxic51 and/or immune-suppressive.3,25,52
Concluding remarks
Food gardens play a vital role in providing poor families with nutrition.9,10 The present study suggests, however, that home-grown maize in food gardens may play a cardinal role in maintaining harmful Fusarium species in the peri-urban environment. The findings reported here invite further attention, in view of the public-health implications. A database on possible sources and mechanisms of dissemination of toxigenic and mycotic Fusarium in peri-urban food production settings should serve as a basis for future strategies to ensure safe food-production practices among vulnerable populations.
The Morogo Research Programme (MRP) acknowledges the National Research Foundation for financial support (grant FA2004050600064). The MRP is also grateful to the communities of Phokeng, Rustenburg District, for allowing sampling of their morogo, to Johann Denner for assistance with sample collection, and to Saffiya Alli for mycological analysis.
1. Munkvold G.P. and Desjardins A.E. (1997). Fumonisins in maize. Plant Dis. 81, 556–565. [ Links ]
2. Bennett J.W. and Klich M. (2003). Mycotoxins. Clin. Microbiol. Rev. 16(3), 497–516. [ Links ]
3. Baumrucker T. and Prieschl E.E. (2002). Sphingolipids and the regulation of immune response. Sem. Immunol. 14, 57–63. [ Links ]
4. Gelderblom W.C.A., Rheeder J.P., Leggott N., Stockenstrom S., Humphreys J., Shephard G.S. and Marasas W.F.O. (2004). Fumonisin contamination of a corn samples associated with the induction of hepatocarcinogenisis in rats – role of dietary deficiencies. Food Chem. Toxicol. 42, 471–479. [ Links ]
5. Kouadio J.H., Mobio T.A., Maudromont I., Moukha S., Dano S.D. and Creppy E.E. (2005). Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone of fumonisin B1 in human intestinal cell line Caco-2. Toxicology 213, 56–65. [ Links ]
6. Domijan A-M., Želježić D., Milić M. and Peraica M. (2007). Fumonisin B1: Oxidative status and DNA damage in rats. Toxicology 232, 163–169. [ Links ]
7. Nelson P.E., Dignani M.C. and Anaissie E.J. (1994). Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7(4), 479–504. [ Links ]
8. Dignani M.C. and Anaissie E. (2004). Human fusariosis. Clin. Microbiol. Infect. 10(Suppl. 1), 67–75. [ Links ]
9. Jansen van Rensburg W.S., Van Averbeke W., Slabbert R., Faber M., Van Jaarsveld P., Van Heerden I., Wenhold F. and Oelofse A. (2007). African leafy vegetables in South Africa. Water SA 33(3), 317–326. [ Links ]
10. Modi M., Modi A., and Hendricks S. (2006). Potential role for wild vegetables in household food security: a preliminary case study in Kwazulu-Natal, South Africa. Afr. J. Food Agric. Nutr. Dis. 6, 1–13. [ Links ]
11. Odhav B., Beekrum S., Akula U. and Baijnat H. (2006). Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J. Food Comp. Anal. 20, 430–435. [ Links ]
12. Fandohan P., Hell K., Marasas W.F.O. and Wingfield M.J. (2003). Infection of maize by Fusarium species and contamination with fumonisin in Africa. Afr. J. Biotechnol. 2(12), 570–579. [ Links ]
13. Fandohan P., Gnonlonfin B., Hell K., Marasas W.F.O. and Wingfield M.J. (2005). Natural occurrence of Fusarium and subsequent fumonisin contamination in preharvest and stored maize in Benin, West Africa. Int. J. Food Microbiol. 99, 173–178. [ Links ]
14. Bourne L.T., Lambert E. and Steyn K. (2002). Where does the black population of South Africa stand on the nutrition transition? Publ. Health Nutr. 5, 157–162. [ Links ]
15. Nelson P.E., Tousson T.A., and Marasas W.F.O. (1983). Fusarium Species: An illustrated manual for identification. Pennsylvania State University Press, University Park. [ Links ]
16. Medina-Martinez M.S. and Martinez A.J. (2000). Mold occurrence and aflatoxin B1 and fumoniin B1 determination in corn samples in Venezuela. J. Agric. Food Chem. 48(7), 2833–2836. [ Links ]
17. Jivan S.D. (2006). The occurrence of toxigenic moulds in traditional household morogo of Giyani. M.Sc. dissertation, North-West University (Potchefstroom Campus), South Africa. [ Links ]
18. Leslie J.F. and Summerell B.A. (2006). The Fusarium Laboratory Manual. Blackwell, Michigan. [ Links ]
19. Snedecor, G.W. and Cochran, W.G. (1980). Statistical Methods, 7th edn. The Iowa State University Press, Ames, Iowa. [ Links ]
20. Statistics South Africa (2006). Mid-year population estimates, South Africa. Statistical release P0302, 1 August 2006. [ Links ]
21. Pujol I., Guarro J., Gené J. and Sala J. (1997). In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J. Antimicrobial Chemother. 39, 163–167. [ Links ]
22. Logrieco A., Moretti G., Castella G., Kostecki M., Golinski P., Ritieni A. and Chelkowski J. (1998). Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 64(8), 3084–3088. [ Links ]
23. Rheeder J.P., Marasas W.F.O. and Vismer H.F. (2002). Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 68, 2101–2105. [ Links ]
24. Berek L, Petri I.B., Mesterházy Á., Téren J. and Molnár J. (2001). Effects of mycotoxins on human immune functions in vitro. Toxicol. in Vitro 15, 25–30. [ Links ]
25. Hymery N., Sibiril Y. and Parent-Massin D. (2006). In vitro effects of trichothecenes on human dentritic cells. Toxicol. in Vitro 20, 899–909. [ Links ]
26. Kpodo K., Thrane U. and Hald B. (2000). Fusaria and fumonisins in maize from Ghana and their co-occurrence with aflatoxins. Int. J. Food Microbiol. 61, 147–157 [ Links ]
27. Nesci A., Barros G., Castillo C. and Etcheverry M. (2006). Soil fungal population in preharvest maize ecosystem in different tillage practices in Argentina. Soil Tillage Res. 91, 143–149. [ Links ]
28. Cotton T.K., and Munkvold G.P. (1998). Survival of Fusarium moniliforme, F. proliferatum and F. subglutinans in maize stalk residue. Phytopathology 88, 550–555. [ Links ]
29. Sreenivasa M.Y., Dass R.S., Charith Raj A.P. and Janardhana G.R. (2006). Molecular detection of fumonisin producing Fusarium species of freshly harvested maize kernels using polymerase chain reaction (PCR). Taiwania 51(4), 251–257. [ Links ]
30. Boutati E.I. and Anaissie E.J. (1997). Fusarium, a significant emerging pathogen in patients with hematologic malignancies: ten years' experience at a cancer center and implications for management. Blood 90(3), 999–1008. [ Links ]
31. Edwards S.G. (2004). Influence of agricultural practices on fusarium infections of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol. Lett. 153, 29–35. [ Links ]
32. Roháćik T. and Hudec K. (2005). Influence of agro-environmental factors on Fusarium infestation and population structure in wheat kernels. Ann. Agric. Environ, Med. 12, 39–45. [ Links ]
33. Cardwell K.F., Kling J.G., Maziya-Doxon B. and Bosque-Pérez N.A. (2000). Interaction between Fusarium verticillioides, Aspergillus flavus, and insect infestation in four maize genoptypes in Lowland Africa. Phytopathology 90, 276–284. [ Links ]
34. Rossi V., Languasco L., Patorri E. and Giosue S. (2002). Dynamics of airborne Fusarium macroconidia in wheat fields naturally affected by head blight. J. Plant Pathol. 84, 53–64. [ Links ]
35. Hörberg H.M. (2002). Patterns of splash dispersed conidia of Fusarium poae and Fusarium culmorum. Eur. J. Plant Pathol. 108, 73–80. [ Links ]
36. Maiorano A., Blandino M., Teyneri A. and Vanara F. (in press). Effects of maize residues on the Fusarium species infection and deoxynivalenol (DON) contamination of wheat grain. Crop Protection. [ Links ]
37. Bezuidenhout C.C., Prinsloo M, and Van der Walt A.M. (2006). Multiplex PCR-based detection of potential fumonisin-producing Fusarium in traditional African vegetables. Environ. Toxicol. 21(4), 360–366. [ Links ]
38. Alli, S. (2007). The incidence of mycotic and mycotoxigenic Fusarium in a peri-urban food-garden environment. M.Env.Sci. dissertation, North-West University (Potchefstroom Campus), South Africa. [ Links ]
39. Van der Walt A.M., Van der Linde E., Alberts M., Modjadji P., Jivan S.D. and Bezuidenhout C.C. (2006). Fumonisin-producing Fusarium strains and fumonisins in traditional African vegetables (morogo). S. Afr. J. Sci. 102, 151–155. [ Links ]
40. Summerbell R.C., Richardson S.E. and Kane J. (1988). Fusarium proliferatum as an agent of disseminated infection in an immunosuppressed patient. J. Clin. Microbiol. 26(1), 82–87. [ Links ]
41. Segal B.H., Walsch T.J., Liu J.M., Wilson J.D. and Kwon-Chung K.J. (1998). Invasive infection with Fusarium chlamydosporum in a patient with aplastic anemia. J. Clin. Microbiol. 36(6), 1772–1776. [ Links ]
42. Guarro J., Nucci M., Akiti T. and Gené J. (2000). Mixed infection caused by two species of Fusarium in a human immunodeficiency virus-positive patient. J. Clin. Microbiol. 38(9), 3460–3462. [ Links ]
43. Ortoneda M., Guarro J., Madrid M.P., Caracuel Z., Roncero M.I.G., Mayayo E. and Di Pietro A. (2004). Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect. Immunity 72(3), 1760–1766. [ Links ]
44. Nucci M. and Anaissie E. (2002). Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin. Infect. Dis. 35, 909–920. [ Links ]
45. Dornbusch H.J., Buzina W., Summerbell R.C., Lass-Frörl C., Lackener H., Schwinger W., Sovinz P. and Urban C. (2004). Fusarium verticillioides abscess of the nasal septum in an immunosuppressed child: case report and identification of the morphologically atypical fungal strain. J. Clin. Microbiol. 43(4), 1998–2001. [ Links ]
46. Bodey G.P., Boktour M., Maus S., Divic M., Kontoyiannis D., Hachem R. and Raad I. (2002). Skin lesions associated with Fusarium infection. J. Am. Acad. Dermatol. 47, 659–666. [ Links ]
47. Sudakin D.L. (2003). Trichothecenes in the environment: relevance to human health. Toxicol. Lett. 143, 97–107. [ Links ]
48. Carratú M.R., Cassano T., Coluccia P. and Cuomo V. (2003). Antinutritional effects of fumonisin B1 and pathophysiological consequences. Toxicol. Lett. 140–141, 459–463. [ Links ]
49. Osuchowski M.F., Edwards G.L. and Sharma R.P. (2004). Fumonisin B1-induced neurogeneration in mice after intracerebroventricular infusion is concurrent with disruption of sphingolipid metabolism and activation of proinflammatory signaling. NeuroToxicology 26, 211–221. [ Links ]
50. Soriano J.M., González L. and Catalá A.I. (2005). Mechanism of action of sphingolipids and their metabolites in toxicity of fumonisin B1. Progr. Lipid Res. 44, 345–356. [ Links ]
51. Lerda D., Biaggi Bistoni M., Peralta N., Ychari S., Vazquez M. and Bosio G. (2005). Fumonisins in food from Cordoba (Argentina), presence and genotoxicity. Food Chem. Toxicol. 43, 691–698. [ Links ]
52. Theumer M.G., López A.G., Masih D.T., Chulze S.N. and Rubeinstein, H.R. (2003). Immunological effects of AFB1 and AFB2-FB1 mixture in experimental subchronic mycotoxicoses in rats. Toxicology 186, 159–170. [ Links ]
Received 12 October. Accepted 13 November 2007.
* Author for correspondence. E-mail: retha.vanderwalt@nwu.ac.za
Sample collection. Samples were collected from food gardens of selected households at four localities in the township of Phokeng, near Rustenburg, North West province, during the maize-growing season in February and April 2006 as well as in February 2007. Two naturally growing morogo types commonly consumed in the study area, namely thepe (Amaranthus hybridus) and lerotho (Cleome gynandra), were sampled at four localities where morogo vegetables were growing with maize on the same plot of land, and four sites where these plants grew some distance from maize. At each locality, 10 leaves were collected from two separate plants each of thepe and lerotho. Five leaves of a maize plant as well as a maize cob were also sampled. At each locality samples were taken of the top soil layer at three random positions around each of the plants. Three agar plates containing pentachloronitrobenzene (PCNB) medium selective for Fusarium were exposed at three random positions around each plant for three minutes to trap Fusarium spores from the air. Air plates were immediately closed and secured with parafilm. Thepe, lerotho, soil and maize samples were transferred separately to 'ziplock' plastic bags. Samples were transported to the laboratory on ice and immediately processed for mycological analysis upon arrival.
Culture media for isolation and morphological identification of Fusarium. The culture medium, selective for the isolation of Fusarium, contained Peptone PCNB (Terraclor, Sigma, South Africa) to which were added the following antibiotics for inhibition of bacterial growth: benzylpenicillin (Fresenius, South Africa), pendistrep (Virbac Animal Health, South Africa) and chloramphenicol pure (Pharmachemie, South Africa). The following growth media were used for the purification and preparation of single spore cultures of Fusarium isolates: Water Agar plates (WA; Agar Bacteriological, Biolab, Merck, South Africa) and Carnation Leaf Agar (CLA; consisting of a Water Agar plate with a piece of carnation leaf γ-sterilized by Isostar, South Africa, placed on the surface). To identify species, single-spore cultures were subsequently transferred to the following culture media: to Potato Dextrose Agar (PDA; Biolab, Merck, South Africa) to observe colony morphology; to CLA plates and Synthetic Nutrient Agar (SNA; Sigma, South Africa) to examine microscopic structure. Culture media were prepared as described in ref. 15.
Isolation of Fusarium. We used the washing procedure described by Medina-Martinez and Martinez16 to isolate Fusarium that had colonized the external surfaces of morogo leaves, maize leaves and kernels. Each leaf and kernel was separately added to 99 ml sterile 1% peptone water (Biolab, Merck) containing 0.01% Tween 80 and shaken for 10 min on a rotary shaker at room temperature to remove spores from leaf surfaces. Diluents containing the rinsed-off Fusarium spores were subsequently diluted (10–3 to 10–5) and 0.1-ml aliquots of each dilution were used for spread plating onto PCNB agar. Internal colonizing Fusarium was isolated by sterilizing leaf and kernel surfaces in 1% hydrogen peroxide for 1 min followed by aseptical rinses in distilled water three consecutive times.17 Five approximately 1-cm2 squares cut from each morogo leaf, and 10 pieces from each maize leaf, were transferred separately to the surface of a separate PCNB agar plate, employing aseptic procedures. After surface sterilization according to a similar procedure, ten maize kernels from each maize cob were each placed on a separate PCNB agar plate. The three soil samples from each site were thoroughly mixed in the laboratory into a composite soil sample, of which 1 g was carefully weighed and transferred to 9 ml sterile distilled water (10–1 dilution). A dilution series of 10–2 to 10–5 was subsequently prepared aseptically and 0.1-ml aliquots of each dilution used for surface plating onto PCNB agar. All plates (including parafilmed air plates) were subsequently incubated at 25°C for a minimum of 7 days.
Purification of Fusarium colonies. After incubation, plates were examined under a stereo-microscope. Colonies suspected of being Fusarium were selected, and then purified, on the basis of characteristics and procedures described in ref. 15. A 1-cm2 piece of PCNB agar containing the selected colony was aseptically cut from the PCNB plate and transferred to a CLA plate. After incubation for 7–10 days at 25°C, during which time plates were exposed to a 12:12-hour light/dark cycle, single-spore cultures were prepared by flooding each plate with 9 ml sterilized distilled water, and pouring the mixture aseptically over the surface of a WA plate. The WA plates were carefully rotated and the excess water was drained off before being incubated at 25°C in an inclined position. After 16–24 hours of incubation, the WA plates were examined under a stereo-microscope and a piece of agar with a single germinating spore was aseptically transferred to the surface of each of a PDA, CLA and SNA plate. Inoculated plates were incubated at 25°C for 7–14 days.
Morphological identification of Fusarium isolates. After incubation, single-spore cultures of Fusarium isolates growing on the PDA, SNA and CLA were identified from morphology and other features according to illustrated laboratory manuals.15,18 Macroscopic observations of single-spore colonies growing on PDA plates included colony morphology, colony colour, as well as the presence and position of the sporodochia. Using lactophenol for slide preparation, microscopic observations and identifications were based on such characteristics as the position, size, shape, and arrangement of microconidia, macroconidia and chlamydospores of single spore cultures on CLA plates.15,18
Statistical methods. Fusarium isolates from the various sources were subject to two-way and three-way analyses of variance (ANOVA), using Statistica 7.1, in terms of the following: counts for each species from each environmental source in localities near and distant from maize, respectively, were log-transformed (i.e. by taking the logarithm to base 10 of the count + 1) to establish normality of data. This was used as the dependent variable with regard to three factors, namely, species, source and distance from maize. Statistically significant effects at the 5% level for each of these factors could be concluded whenever there were no significant interactions and the P-values were smaller than 0.05. Where appropriate, Tukey post hoc comparisons were performed between mean values of the different components and species. In cases of significant interaction effects with distance from maize, Student's t-tests (assuming unequal variances) were applied to compare means of distance with means for each species or for each source.19














