Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.103 no.11-12 Pretoria nov./dic. 2007
RESEARCH LETTERS
Observations on the population dynamics of the invasive freshwater snail Aplexa marmorata (Pulmonata: Physidae) in Durban, South Africa
School of Biological and Conservation Sciences, Howard College Campus, University of KwaZulu-Natal, Durban 4041, South Africa
ABSTRACT
The invasive freshwater snail Aplexa marmorata was shown to exhibit a univoltine reproductive pattern in two ecologically different habitats, a stable ornamental pond and a small, unstable lake. Generation times and the timing of egg-laying periods did, however, differ between these two sites. It is suggested that the retention of a univoltine pattern in the lake, despite a prolonged drop in water level, may be evidence that A. marmorata is not as reproductively flexible as Physa acuta, the other physid invader in South Africa. Such a lack of reproductive flexibility may explain why A. marmorata has not invaded rivers whereas P. acuta, which is known to adopt a multivoltine pattern following repeated disturbances to its habitat, has done so.
Introduction
Aplexa marmorata (Pulmonata: Physidae) is indigenous to South America and some Caribbean islands. It was first collected in South Africa in Durban in 1986,1 though there is evidence that it may have been in southeastern Africa since at least the 19th century (see Physa mosambiquensis in ref. 2). It has recently been collected in a variety of habitats in KwaZulu-Natal, Limpopo and Mpumalanga provinces and is considered to be South Africa's third-most widespread invasive freshwater snail.3,4 The species was also introduced to West Africa, where it is known to occur in four countries.5
In South Africa, A. marmorata has been found only in lentic waterbodies including reservoirs, swamps, pans, artificial ponds and pools except in a few cases when it was found in intermittent streams and a backwater of the Sabie River in the Kruger National Park.6 Indeed, the recent spread of A. marmorata and a second exotic freshwater snail species, the prosobranch Tarebia granifera, into rivers in the southern part of the park (A. Gerber, pers. comm.), is causing concern to scientists there (H. Sithole, pers. comm.).
Although both these species have clearly become invasive in South Africa, little is known of their biology. This contribution presents preliminary data on the population dynamics of A. marmorata in two habitats in the Durban Metropolitan Area. A knowledge of the species' bionomics is particularly important in view of the success of another physid, Physa acuta, as an invader in Africa and the discovery by Brackenbury & Appleton7 that it could respond to flood disturbances by immediate egg-laying and the production of a new cohort. Where floods occurred repeatedly, P. acuta was shown to produce seven cohorts in a single year.
Methods
Sampling sites
Samples were collected fortnightly at two ecologically different sites within the Durban Metropolitan Area: an ornamental pond in the Durban Botanic Garden and a small natural lake in the Bluff Nature Reserve. The botanic garden site was chosen because it is kept full artificially and does not experience disturbances such as floods or drying-out, whereas the Bluff Nature Reserve site is natural, dependent on rainfall and its water levels fluctuate accordingly. Neither site experiences regular human contact, although people do visit them occasionally.
Botanic garden site
The Durban Botanic Garden (29°15'S, 31°06'E) is about 5 km from the city centre. The actual sampling site is a man-made pond measuring 18.1 m × 1.7 m and an area of 30.8 m2. It is approximately 1.2 m deep with concrete walls and floor and has an estimated volume of 36.9 m3. Water lilies (Nymphaea nouchali) provided surfaces for snail shelter, feeding and egg-laying. The pond is kept full (of municipal water) and the surface cleaned of dead leaves and debris by the garden's staff.
Bluff Nature Reserve site
The sampling site here (29°56'S, 31°59'E) extended along 13 m of the shoreline of the small natural lake in Bluff Nature Reserve to a depth of 0.5 m. All samples were collected within 2 m of the shore and the site thus had an area of 26.0 m2 and a volume of approximately 13 m3. Emergent reeds (Phragmites australis) and bulrushes (Typha capensis) were present in the site and much of the water surface was covered by the floating fern Azolla sp. Aplexa marmorata was first collected from this site by G.B. Wilken in June/July 1989 and it was the first natural waterbody found to harbour the snail in South Africa.8 It has since been found in many habitats in Durban.
Sampling procedure
Samples were collected fortnightly for 30 minutes between 09:00 and 10:00 on each occasion over a period of 13 months from November 1997 to December 1998. A metal scoop net with a 2-mm2 wire mesh fitted into a 300-mm2 frame on a long aluminium handle was used throughout and by the same person each time. Snails were taken, with minimal disturbance, to the laboratory, where their shell heights, that is, distance from the apex of the spire to the ventral margin of the aperture, were measured to the nearest 0.1 mm using a vernier calliper. The number of A. marmorata present in each sample was recorded and densities expressed as the number of snails collected per unit time (30 minutes). All snails were returned to their respective habitats the following day.
During an additional 30-minute period, all egg capsules found attached to the undersides of N. nouchali leaves were counted and clutch sizes recorded. Eggs were never found laid on the shells of A. marmorata by its conspecifics, as was reported by Brackenbury9 for P. acuta in the Umsinduzi River, Pietermaritzburg. No egg production data were available for the Bluff Reserve site. Water temperature, pH and conductivity were measured fortnightly at both sites using a mercury-in-glass thermometer, a Beckman 32 pH meter and a Crison micro CM 2201 conductivity meter, respectively.
Sampling data were analysed with the ELEFAN I program. This program uses the von Bertalanffy Growth Equation to identify the seasonally oscillating growth curves that 'best' fitted the A. marmorata shell height/frequency data.10 Calculations are based on observed values of L∞ derived from shell height-at-age data, allowing the program to trace growth curves through sequentially arranged height/frequency samples.11–13 In this way the number of individual cohorts (generations) of A. marmorata present during the 13-month study period could be determined together with the estimated duration of each. The von Bertalanffy Growth Equation is:
Lt = L∞[1 – exp(–κ(t – t0)] ,
where Lt is the height (mm) at time t, L∞ is the asymptotic height (mean height of oldest individuals) in mm, κ is the exponential rate at which length approaches the asymptotic height, and t0 is the age at height 0. As growth starts at the time of hatching and not at the value of 0 mm, t0 has no significance and is therefore replaced by coordinates of a point through which the curve must pass. The coordinates consist of SS (a starting sample) and SH (a starting height). The Rn value reflects the best fit of the height frequency data (HFD). The values of L∞, C (amplitude), κ and WP (winter point, when growth rate is lowest) are either increased or decreased until Rn reaches its maximum.
Results
Botanic garden site
Physical and chemical characteristics
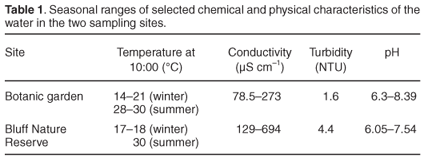
The water was always clear (turbidity = 1.6 nephelometric turbidity units (NTU)) and its temperature at approximately 10:00 ranged from 14–21°C in winter (June–July) to 28–30°C in summer (December–January) (Fig. A in supplementary material online at www.sajs.co.za). Conductivity values ranged from 78.5 µS cm–1 in early January to 273.0 µS cm–1 in June, and pH varied between 6.3 and 8.4 (Table 1).
In addition to A. marmorata, four other gastropod species were found at this site: Pomacea cf. lineata (Ampullariidae), Melanoides tuberculata (Thiaridae), Lymnaea columella (Lymnaeidae) and Helisoma duryi (Planorbidae).
Population fluctuations of A. marmorata
The ELEFAN I model identified three cohorts (generations) during the 13-month sampling period with each partly overlapping both its parent and filial cohorts. Successive cohorts are designated 1–3 in Fig. B in supplementary material online. Each cohort had an estimated life-span of 15 months. In November 1997 when sampling began, cohort no. 1 was present as mature snails with a shell height >8 mm, and had reached 17 mm by early March 1998, after which it disappeared. Cohort no. 2 was present as young snails <8 mm when sampling started in November 1997 and persisted until the sampling stopped in December 1998. A third cohort was detected in May/June 1998, represented by snails of shell height 3–9 mm, and dominated the population at the end of the sampling programme. By this time it had attained an average shell height of >12 mm.
The growth curves in Fig. B suggest that cohorts 2 and 3 both started in autumn, presumably from eggs laid during March and April. Egg capsules were found on 10/23 (44%) sampling occasions (Fig. C online) and these data indicated four periods of oviposition during the year, separated by intervals of up to three months, during which no egg capsules were found. These periods of egg-laying could be allocated to different cohorts as follows: (i) a period with relatively small numbers of capsules per sample from November 1997 to early January 1998 laid by cohort no. 1, (ii) a period of more intense egg-laying from April to May 1998 and another (iii) during July and August 1998. These two periods, one on either side of the coldest winter months (May and June), were attributed to cohort no. 2. Another minor period of egg-laying, October and November 1998, corresponded to cohort no. 3. When considered as an annual cycle, however, the data suggest two prolonged periods of egg-laying during the year, a major one in autumn/winter (May–August) [163 capsules collected] and a minor one in spring/summer (October–January) [70 capsules collected]. The spring/summer period coincided with an increase in the number of young snails in October 1998 but which ELEFAN did not identify as a discrete cohort. It is therefore interpreted here as a rapidly growing late component of cohort no. 2. This is similar to life-cycle pattern 'C' as illustrated for Physa fontinalis in Fig. 29 of ref. 14.
Eggs are laid in gelatinous envelopes covered by a C-shaped capsule with both ends rounded as described by Richards15 and Paraense16 for Physa marmorata (synonymous with A. marmorata following Appleton & Dana,4 but without the sharp terminal tail illustrated by Bondesen for a 'physid' capsule. Individual eggs were more circular in outline than those illustrated by Bondesen17 and the outer covering membrane could not be seen to dissociate into an 'egg string' connecting the eggs within the capsule as shown by both Bondesen17 and Paraense.16 The observed clutch size of A. marmorata ranged from 7 to 35 (mean 15) eggs per capsule (n = 84 capsules).
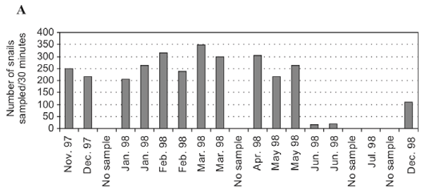
Densities of A. marmorata in the samples ranged from 32/30 minutes to 276/30 minutes with a mean of 169 ± 65 (n = 25). Figure 1B shows that the densities were lowest during summer and spring but increased markedly in late May 1998, remained high during the cool winter months before, and dropped again in early September.
Bluff Nature Reserve site
Physical and chemical characteristics
The lake water was clear (4.4 NTU). Winter temperatures were several degrees higher than in the botanic garden pond, 17–18°C, while summer temperatures were similar, approximately 30°C (Fig. A). Conductivity readings ranged from 129 µS cm–1 to a maximum of 694 µS cm–1 (Table 1). These are considerably higher than in the botanic garden site but the pH range was similar, 6.05 to 7.54. No seasonal pattern in conductivity or pH was evident at either site. The only other freshwater gastropod found at the Bluff site was Lymnaea columella.
Population fluctuations of A. marmorata
As at the botanic garden site, three cohorts were identified with each partly overlapping its parent and filial cohorts (Fig. D online). Cohort no. 1 corresponded to that at the botanic garden site and was present when sampling started with snails greater than 8 mm. Most of these had disappeared by late January 1998, though a few large specimens persisted until April. Cohort no. 2 was also present at the beginning of sampling in November 1997, with snails <4 mm, but these disappeared coincident with a marked recession in water level, which lasted for five-and-a-half months from July to December 1998. After the water level rose again in late December following rains in November, mature snails apparently representing cohort no. 2 were collected. Juvenile snails measuring ~ 4 mm representing cohort no. 3 were also present in this sample. No empty shells were found in the sampling area during the dry season, suggesting that the snails did not die during this period. Rather, because physids are generally not vegetation-dependent, the snails of cohort no. 2 probably followed the receding water level in winter to refuges in parts of the lake that did not dry out. After re-filling, they migrated back to the newly inundated margins.
Figure 1A shows that at the Bluff Nature Reserve site, the density of A. marmorata in samples ranged from zero during the dry period of July to December to peak densities of 264 and 347 snails/30 minutes during January and March 1998, respectively (mean 204 ± 114, n = 15), two months earlier than the highest density was reached at the botanic garden site (Fig. 1B).
Although no egg production data are available for this site, it can be deduced from the growth curves in Fig. D that egg-laying would have occurred each spring (September–October).
Discussion
The ELEFAN I program was designed for the analysis of population studies on fish but is suitable for use on invertebrates with their faster growth rates and shorter generation times.11–13 Davies-Coleman18 successfully used it on the freshwater limpet Burnurpia stenochorias (Ancylidae) in the Bloukrans River near Grahamstown, Eastern Cape, and identified two overlapping cohorts per year in successive samples at two field sites. Their longevity in each case was estimated to be approximately 24 months. In the present study, A. marmorata was found to be univoltine, producing a single cohort per year though with different generation times at the two sites: approximately 15 months at the botanic garden site and 21–22 months at the Bluff lake site. Successive cohorts overlapped for a few months at each site. The data also suggest that the main period of egg-laying differed between the two sites, March/April (autumn) at the botanic garden and September/October (spring) at the Bluff site.
Univoltine life-cycle patterns were thus recorded for A. marmorata in two habitats, which were ecologically very different: one permanent and stable, the other rain-dependent and unstable. Observed differences in generation times and the timing of breeding periods at the two sites may thus be attributed to this fundamental difference, i.e. that the botanic garden site received water regularly and remained full whereas the Bluff Nature Reserve site depended on rainfall, so that its water level fluctuated widely though it never dried completely. Conductivity, turbidity and pH measured at these two sites fluctuated only slightly and are unlikely to have affected the snails' population dynamics.
From data presented by Thomas and McClintock,19 the life-cycle of P. cubensis (synonymous with P. acuta according to Paraense & Pointier20) was similarly influenced by habitat stability such that temperature and rainfall dictated the reproductive pattern in a permanent stream; while episodic drying events did so in a nearby seasonal pond.
Univoltine patterns are well known among the Physidae (see review by Appleton3), notably within the genus Physa, and seem to be adaptive to the ecological conditions of the habitats concerned. In Europe, P. acuta breeds once (univoltine) in natural habitats but may become bivoltine in artificial habitats21 or multivoltine in repeatedly disturbed habitats.7 The present study, in which A. marmorata produced nearly similar generation patterns in two fundamentally different types of habitat, suggests that it is not as plastic in respect of its bionomics as was shown for P. acuta by Brackenbury & Appleton.7
This lack of reproductive plasticity may partly explain why A. marmorata has been found commonly in lentic waterbodies in KwaZulu-Natal but rarely in rivers. By contrast, P. acuta, which does occur in rivers, has been shown to be responsive to habitat disturbances by rapidly repopulating the habitats due to its ability to respond quickly, in terms of gametogenesis.7 These authors showed in a laboratory study that P. acuta kept at high water temperatures (25 and 28°C) reached sexual maturity earlier, i.e. younger and with a smaller shell height, than the indigenous planorbid Bulinus tropicus. As a result, P. acuta not only reaches sexual maturity sooner than B. tropicus but it remains reproductively active for longer.
The rapid re-colonization of the Bluff lake sampling site by adult A. marmorata in November 1998 after five months of drought was not unexpected because some physids are known to be mobile members of the benthos of lakes in their native North America22 and could, as suggested above, migrate to the newly inundated areas from refuges in residual water elsewhere in the habitat.
Aplexa marmorata exhibited a univoltine reproductive pattern in the stable botanic garden pond and retained this pattern in the disturbed Bluff lake site, though the generation times and breeding seasons differed. This apparent rigidity of the reproductive cycle, i.e. a failure to switch to a bivoltine or even a multivoltine pattern in the face of environmental disturbance (see review by Geraerts & Joosse14), may explain why this species is not found in flowing rivers. It may not be able to regain its numbers quickly enough after disturbances such as the droughts and silt-laden floods that are a feature of South African rivers.
We are grateful to H.D. Davies-Coleman (Freshwater Research Unit, Rhodes University) for help with the ELEFAN 1 program and the interpretation of its printouts. We also acknowledge the director of the Durban Botanic Garden and the ranger in charge of Bluff Nature Reserve for permission to sample A. marmorata at the sites under their respective control.
1. Appleton C.C. and Brackenbury T.D. (1998). Introduced freshwater gastropods in Africa with special reference to Physa acuta. In Proc. of Workshop on Medical Malacology in Africa, eds H. Madsen, C.C. Appleton and M. Chimbari, Harare, September 1997, pp. 22–26. [ Links ]
2. Connolly M. (1939). A monographic survey of South African non-marine Mollusca. Annal. S. Afr. Mus. 33, 1–660. [ Links ]
3. Appleton C.C. (2003). Alien and invasive fresh water Gastropoda in South Africa. Afr. J. Aquat. Sci. 28, 69–81. [ Links ]
4. Appleton C.C. and Dana P. (2005). Re-examination of Physa mosambiquensis Clessin, 1886 and its relationship with other Aplexinae (Pulmonata : Physidae) reported from Africa. Afr. Invert. 46, 71–83. [ Links ]
5. Brown D.S. (1994). Freshwater Snails of Africa and their Medical Importance, 2nd edn. Taylor & Francis, London. [ Links ]
6. De Kock K.N. and Wolmarans C.T. (1998). A re-evaluation of the occurrence of freshwater molluscs in the Kruger National Park. Koedoe 41, 1–8. [ Links ]
7. Brackenbury T.D. and Appleton C.C. (1993). Recolonization of the Umsindusi river, Natal, South Africa, by the invasive gastropod, Physa acuta (Basommatophora, Physidae). J. Med. Appl. Malacol. 5, 39–44. [ Links ]
8. Appleton C.C., Brackenbury T.D. and Tonin A.F.G. (1989). Physa mosambiquensis (Clessin, 1886) rediscovered? S. Afr. J. Zool. 24, 340–344. [ Links ]
9. Brackenbury T.D. (1989). Some aspects of the biology of Physa acuta (Gastropoda: Physidae) pertinent to its success as an invader in South African rivers. M.Sc. thesis, University of Natal, Pietermaritzburg. [ Links ]
10. Wetherall J.A. (1986). A new method for estimating growth and mortality parameters from length–frequency data. ICLARM Fishbyte 4, 12–14. [ Links ]
11. Pauly D. (1987). A review of the ELEFAN system for analysis of length–frequency data in fish and aquatic invertebrates. In Proc. International Conference on the Theory and Application of Length-based Methods for Stock Assessment (ICLARM Conf. Proc. 13, 7–34), eds D. Pauly and G.P. Morgan, Mazzara, Italy. [ Links ]
12. Pauly D. and David N. (1981). ELEFAN 1, a BASIC program for the objective extraction of growth parameters from length–frequency data. Meeresforsch 28, 205–211. [ Links ]
13. Pauly D. and Munro J.L. (1984). Once more on the comparison of growth in fish and invertebrates. ICLARM Fishbyte 2, 21. [ Links ]
14. Geraerts W.P.M. and Joosse J. (1984). Freshwater snails (Basommatophora). In: The Mollusca, vol. 7, 141–207, Reproduction. Academic Press, Orlando, FL.mm [ Links ]
15. Richards C.S. (1964). Studies on Puerto Rican Physidae. U.S. Publ. Hlth Rep. 79, 1025–1029. [ Links ]
16. Paraense W.L. (1986). Physa marmorata Guilding, 1828 (Pulmonata: Physidae). Memorias do Instituto Oswaldo Cruz 81, 459–469. [ Links ]
17. Bondesen P. (1950). A Comparative Morphological-biological Analysis of the Egg Capsules of Freshwater Pulmonate Gastropods. Naturhistorisk Museum, Aarhus, Denmark. [ Links ]
18. Davies-Coleman H.D. (2001). The growth and reproduction of the freshwater limpet Burnupia stenochorias (Pulmonata, Ancylidae), and an evaluation of its use as an ecotoxicology indicator in whole effluent testing. Ph.D. thesis, Rhodes University, Grahamstown. [ Links ]
19. Thomas D.L. and McClintock J.B. (1996). Aspects of the population dynamics and physiological ecology of the gastropod Physella cubensis (Pulmonata: Physidae) living in a warm-temperate stream and ephemeral pond habitat. Malacologia 37, 333–348. [ Links ]
20. Paraense W.L. and Pointier J-P. (2003). Physa acuta Draparnaud, 1805 (Gastropoda: Physidae): a study of topotypic specimens. Memorias do Instituto Oswaldo Cruz 98, 513–517. [ Links ]
21. Duncan C.J. (1959). The life cycle and ecology of the freshwater snail Physa fontinalis (L.). J. Anim. Ecol. 28, 97–117. [ Links ]
22. Clampitt P.T. (1970). Comparative ecology of the snails Physa gyrina and Physa integra. (Basommatophora: Physidae). Malacologia 10, 113–151. [ Links ]
Received 16 August 2004. Accepted 18 June 2005.
This article is accompanied by supplementary material online at www.sajs.co.za
† Present address: Human Sciences Research Council, P.O. Box X9182, Cape Town 8000, South Africa.
§ Author for correspondence. E-mail: appletonc@ukzn.ac.za
Supplementary material to:
Dana P. and Appleton C.C. (2007). Observations on the population dynamics of the invasive freshwater snail Aplexa marmorata (Pulmonata: Physidae) in Durban, South Africa. S. Afr. J. Sci. 103, 493–496.