Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.103 n.9-10 Pretoria Sep./Oct. 2007
RESEARCH IN ACTION
Anti-viral effects of aqueous extracts of Aloe ferox and Withania somnifera on herpes simplex virus type 1 in cell culture
L. KambiziI; B.M. GoosenII; M.B. TaylorIII; A.J. AfolayanI, *
IBotany Programme Unit, University of Fort Hare, Private Bag X1314, Alice 5700, South Africa
IIDepartment of Medical Virology, University of Pretoria, P.O. Box 2034, Pretoria 0001, South Africa
IIIDepartment of Medical Virology, University of Pretoria, and National Health Laboratory Service, P.O. Box 2034, Pretoria 0001, South Africa
ABSTRACT
ALOE FEROX AND WITHANIA SOMNIFERA are among southern African plants commonly used for the treatment of sexually transmitted infections (STIs). Aqueous extracts from both species, together with aloin, isolated from A. ferox, were evaluated for antiviral activity against herpes simplex virus type 1 (HSV-1) in vitro. The aqueous extracts showed detectable activity at a concentration of 1000 µg/ml against the virus in monolayers of the Vero African green monkey cell cultures, whereas aloin showed significant activity at 62 µg/ml. HSV-1 is usually associated with mucocutaneous infections of the oropharynx but can also cause genital herpes. Herpes simplex virus type 2 (HSV-2) is the classical genital herpes pathogen, but with current sexual habits, can give rise to both oral and genito-anal infections. Our results indicate that the use of these two plant species for the treatment of STIs could have a scientific rationale.
Introduction
The indigenous people of the Eastern Cape province of South Africa have a long history of traditional plant usage for the treatment of various diseases and ailments.1,2 This includes the use of plants for the treatment of sexually transmitted infections (STIs). STIs are common among young adults in the rural communities of the province. Herbalists take advantage of the biodiversity of plant species to treat these infections.3
In our previous survey of plants used for the treatment of STIs,3 we found that the traditional healers and other knowledgeable rural dwellers of the study area listed Aloe ferox and Withania somnifera as two of the commonest species used for the treatment of genital herpes, a disease commonly caused by herpes simplex virus type 2 (HSV-2). Herpes simplex virus type 1 (HSV-1) has also been implicated in genital infections. Aloe ferox (Asphodelaceae) is widely used in traditional healing. According to several authors, the species is used for the treatment of leukaemia, as an anti-inflammation and anticancer agent and against neuroectodermal tumours.1,4,5 Withania somnifera (Solanaceae) grows in the Eastern Cape. It is used for the treatment of arthritis, tuberculosis, cancer and STIs.1,6,7
The antimicrobial properties of these two plant species have been widely reported in the literature.8–10 However, the reports have focused mainly on their antifungal and antibacterial properties, whereas information on their antiviral activity is scanty.11 We examined the antiviral effects of A. ferox and W. somnifera on HSV-1 in vitro in this study.
Materials and methods
See Appendix.
Results and discussion
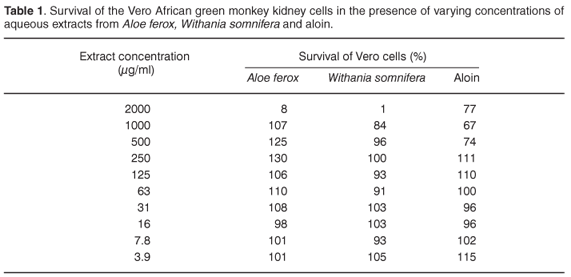
Vero cell monolayers treated with water extracts from A. ferox or W. somnifera, including the stock solution of aloin, exhibited altered morphology after one week. Their integrity, treated with concentrations from 3.9 µg/ml to 2000 µg/ml of these extracts, was maintained, although morphological changes and/or cell death, indicative of cytotoxic effects (CPE), were observed at a concentration of 2000 µg/ml. These observations were confirmed by the MTT assay (Table 1).
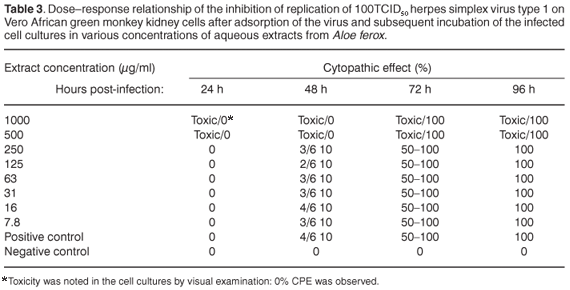
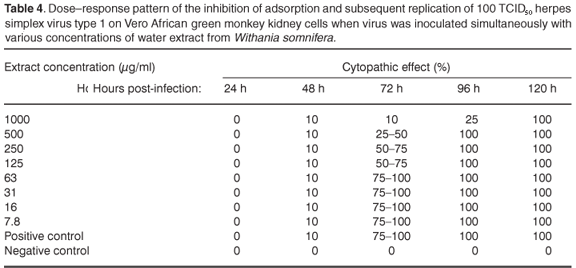
In the assay to assess the possible antiviral properties of the plants, aqueous extract from A. ferox exhibited partial activity against HSV-1 at a concentration of 1000 µg/ml when the virus was inoculated onto the cell cultures simultaneously with the plant extract. The infected cells showed 50–75% CPE 96 h after infection at 1000 µg/ml (see Table 2 in supplementary material online at www.sajs.co.za). Exposure to an aqueous extract from W. somnifera, on the other hand, delayed the appearance of CPE at 1000 µg/ml. The infected cells showed 25% CPE 96 h after infection (see Table 3 online). However, 100% CPE 96 h post-infection was noted at concentrations ranging from 7.8 µg/ml to 500 µg/ml. Water extract from A. ferox exhibited no activity against the replication of HSV-1 after viral adsorption had taken place at concentrations ranging from 7.8 µg/ml to 1000 µg/ml (see Table 4 online).
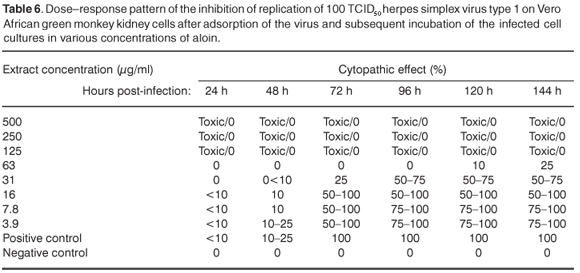
The extracts showed some activity, with no CPE, at a concentration of 1000 µg/ml. At this concentration, the extracts may contain compounds that are either true antivirals, but are present in quantities that are insufficient to inactivate all infectious viral particles, or they may be compounds that retard the replication and spread of the virus. 17,18 Aloin exhibited activity against HSV-1 at a concentration of 63 µg/ml, and the infected cells showed 25–50% CPE 120 h after infection (see Table 5 online). It is important to note that at a concentration of 63 µg/ml, aloin delayed the appearance of CPE, possibly due to interference in the replication cycle of the virus (see Table 6 online).
The activity of aloin and the partial activity of the aqueous extracts of the experimental plants could validate the use of A. ferox and W. somnifera by traditional healers, as these practitioners use water for extraction, and prescribe the extracts to their patients with no maximum concentration limit.
This research was supported by the National Research Foundation.
1. Van Wyk B.E., Van Oudtshoorn B. and Gericke N. (1997). In Medicinal Plants of South Africa, Briza, Pretoria. [ Links ]
2. Grierson D.S. and Afolayan A.J. (1999). Antibacterial activity of some indigenous plants used for the treatment of wounds in the Eastern Cape, South Africa. J. Ethnopharmacol. 66, 103–106. [ Links ]
3. Kambizi L. and Afolayan A.J. (2003). Phytomedicinal studies of four selected medicinal plants used for the treatment of STIs in the Eastern Cape. Fort Hare Papers 12,10–24. [ Links ]
4. Capasso F., Borrelli F. and Capasso R. (1998). Aloe and its therapeutic use. Phytotherapy Res. 12, 124–127. [ Links ]
5. Pecere T., Gazzola M.V., Mucignat C., Parolin C., Vecchia F.D., Cavaggion A., Baso G., Diaspro A., Salvato B., Carli M. and Palù G. (2000). Aloe emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 60, 2800–2804. [ Links ]
6. Devi P.U. (1996). Withania somnifera Dunal (Ashwagandha): Potential plant source of a promising drug for cancer chemotherapy and radiosensitization. Indian J. Exp. Biol. 34, 927–932. [ Links ]
7. Singh S. and Kumar S. (1998). In Withania somnifera. The Indian ginseng ashwagandha. Central Institute of Medicinal and Aromatic Plants: 293. Lucknow. [ Links ]
8. Sarath N., Arseculeratne A.A., Gunatilaka L. and Panabokke R.G. (1985). Studies on medicinal plants of Sri Lanka. Part 14: Toxicity of some traditional medicinal herbs. J. Ethnopharmacol. 13, 323–335. [ Links ]
9. Afolayan A.J., Grierson D.S., Kambizi L., Madamombe I. and Masika P.J. (2002). In vitro antifungal activity of some South African medicinal plants. S. Afr. J. Bot. 68, 72–76. [ Links ]
10. Arora S., Dhillon S., Rani G. and Nagpal A. (2004). The in vitro antibacterial/synergistic activities of Withania somnifera extracts. Fitoterapia 75, 385–388. [ Links ]
11. Scartezzini P. and Speroni E. (2000). Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 71, 23–43. [ Links ]
12. Grist N.R., Bell E.J., Follette E.A.C. and Urquhart G.E.D. (1979). In Diagnostic Methods in Clinical Virology, 3rd edn, pp. 60–79. Blackwell Scientific Publications, Oxford. [ Links ]
13. Van Rensburg C.E.J., Anderson R., Myer M.S., Jooné G.K. and O'Sullivan J.F. 1994). The riminophenazine agents clofazimine and B669 reverse acquired multidrug resistance in a human lung cancer cell line. Cancer Lett. 85, 59–63. [ Links ]
14. Hussain R.F., Nouri A.M.E. and Oliver R.T.D. (1993). A new approach for measurement of cytotoxicity using colorimetric assay. J. Immunol. Methods 160, 89–96. [ Links ]
15. Kambizi L. Sultana N. and Afolayan A.J. (in press). Bioactive compounds isolated from Aloe ferox: A plant traditionally used for the treatment of sexually transmitted infections in the Eastern Cape, South Africa. J. Pharmaceut. Biol. [ Links ]
16. Taylor R.S.L., Manandhar N.P., Hudson J.B. and Towers G.H.N. (1996). Antiviral activities of Nepalese medicinal plants. J. Ethnopharmacol. 52,157–163. [ Links ]
17. Kupin V.I. and Polevaia E.B. (1986). Stimulation of the immunological reactivity of cancer patients by Eleutherococcus extract. Vopr. Onkol. 7, 21–26. [ Links ]
18. Kudi A.C. and Myint S.H. (1999). Antiviral activity of some Nigerian medicinal plant extracts. J. Ethnopharmacol. 68, 289–294 [ Links ]
This article is accompanied by supplementary material online at www.sajs.co.za
* Author for correspondence. E-mail: aafolayan@ufh.ac.za
Plant collection
Leaves of A. ferox and the roots of W. somnifera were collected from the Eastern Cape. Voucher specimens (Kambizi Med.2003/1, Kambizi Med.2003/2) were prepared, formally identified and deposited at the University of Fort Hare Botany Department. According to the traditional healers in the study area, only the roots of W. somnifera are used for the treatment of STIs.
Preparation of the extracts
Air-dried plant materials (100 g each) were pulverized and extracted separately by shaking for 30 min in 800 ml of water. The extracts were filtered through Whatman No. 1 filter paper and evaporated to dryness under reduced pressure. Each dry extract was dissolved in nuclease-free water to a final concentration of 2000 µg/ml (w/v). A stock solution (2000 µg/ml) of pure dried aloin, similarly isolated from A. ferox, was also prepared. Solutions were stored at 4°C and used within 5 days.
Sterile stock solutions of the extracts were obtained by filtration through a 0.45-µm membrane (Ministart® filter unit, Sartorius, Göttingen). Dilutions of extracts and aloin, in serum-free Eagle's minimum essential medium (MEM) (Highveld Biological, Johannesburg), were tested for cytotoxicity at concentrations from 3.9 µg/ml to 2000 µg/ml. These dilutions were then tested for anti-viral activity.
Cell cultures
Standard cell culture techniques12 were used for all procedures using cell cultures. Monolayers of the Vero African green monkey cell line (ACACC No. 84113001, European Collection of Cell Cultures) were prepared by seeding 96-well microtitre trays with 105 cells/ml. MEM supplemented with 5% heat-inactivated fetal calf serum (FCS) (Delta Bioproducts, Kempton Park, South Africa) and containing 100 U/ml penicillin and 100 µg/ml streptomycin was used for the propagation of the cells. Cell cultures were incubated at 37°C in 5% CO2 in air in a humidified atmosphere. Maintenance medium was the same as the propagation medium, except that the former contained only 2% FCS.
Virus stock
A stock suspension of HSV-1 with titres of 4.7 × 106 TCID50/ml, was prepared from a clinical isolate of HSV-1 (Diagnostics Laboratory, Department of Medical Virology, National Health Laboratory Service, Pretoria). The virus was diluted in serum-free MEM and used at a final concentration of 100 TCID50 per microtitre tray well.
Cytotoxicity assay
The extracts were tested for cytotoxicity by exposing monolayers of the Vero cell cultures to dilutions of the sterile extracts. Doubling dilutions of the extracts, in serum-free MEM, from a concentration of 3.9 µg/ml to 2000 µg/ml, were used for testing on 24-hour-old monolayers of Vero cells. The cells were monitored visually, by light microscopy, daily for seven days and on the seventh day tested for cytotoxicity using a tetrazolium salt reduction (MTT) assay,13 based on the method of Hussain et al.14 Monolayers of cells exposed to serum-free MEM alone were used as a control. The same procedure was repeated for aloin isolated from A. ferox.15 Due to the inadequate quantity of aloin isolated from our plant extract, the aloin used in this experiment was from a commercial source (Sigma Chemical Co., St Louis, MO).
Antiviral assay
Dilutions of the plant extract and aloin were tested for antiviral activity at the final concentrations of 7.8, 16, 31, 63, 125, 250, 500 and 1000 µg/ml. The 24-hour-old monolayers of Vero cells in 96-well microtitre trays were starved in serum-free MEM for 1 h in a humidified CO2 atmosphere (5% CO2/95% filtered air) at 37°C. After starvation, the serum-free MEM was withdrawn and 100 µl (100 TCID50) of virus was added to the wells and allowed to adsorb to the cell cultures for 1 h at 37°C in 5% CO2 in air in a humidified atmosphere. After adsorption for 1 h, the unbound virus was withdrawn, and the cells rinsed once with serum-free MEM, after which 200 µl of the appropriate dilution of extracts in serum-free MEM was added to each of six wells. The cell cultures were incubated at 37°C in a humidified CO2 atmosphere (5% CO2/95% filtered air). As positive control, cells infected with virus were maintained in serum-free MEM, and cells mock-infected with 100 µl serum-free MEM and maintained in serum-free MEM, serving as negative controls. Cells were examined daily for seven days, by light microscopy, for the appearance of any cytopathic effect (CPE). The absence of CPE at a specific concentration of the extracts or aloin was considered to be indicative of antiviral activity.
Supplementary material to:
Kambizi L., Goosen B.M., Taylor M.B. and Afolayan A.J. (2007). Anti-viral effects of aqueous extracts of Aloe ferox and Withania somnifera on herpes simplex virus type 1 in cell culture. S. Afr. J. Sci. 103, 359–360.