Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.103 no.7-8 Pretoria jul./ago. 2007
COMMENTARY
Monitoring effects of anthropogenic climate change on ecosystems: a role for systematic ecological observation?
Guy F. MidgleyI, *; Steven L. ChownII; Barney S. KgopeI
IGlobal Change Research Group, Ecology and Conservation, Kirstenbosch Research Centre, South African National Biodiversity Institute, Private Bag X7, Claremont 7735, South Africa
IIDST-NRF Centre of Excellence for Invasion Biology, University of Stellenbosch, Private Bag X1, Matieland 7602, South Africa
ABSTRACT
We consider here the opportunities and challenges for South Africa in long-term ecological research (LTER) to detect the impacts of anthropogenic climate change on biota (as one of several competing objectives of long-term monitoring). The LTER approach has high potential for this purpose in South Africa because of a wealth of historical climate data relative to much of the African continent, and good representation of many African ecosystem types. However, there are substantial challenges to the identification and attribution of climate change impacts on African ecosystems. These are posed by climate variability at a range of time scales, the importance of rainfall rather than temperature as an ecological driver, and the significance of fire as a stochastic disturbance. An awareness of environmental and climate history will be crucial to interpreting data on trends, and sites with established historical data should be preferred for this reason. The placement of LTER sites to provide representivity of ecosystem types may unintentionally limit the detectability of climate change impacts, because change might best be detected in ecotonal or azonal environments. This could be overcome by additional experimental manipulations at LTER sites to 'force' anticipated changes and characterize species and ecosystem responses. A focus on the detection of climate change would sharpen an LTER programme's emphasis over time and provide policy advice, and science training rationales for the long term. It should especially interpret key information to decision-makers as a priority.
Introduction
At the heart of the UN Framework Convention on Climate Change, Article 2 describes its objective as 'stabilization of greenhouse gas concentrations in the atmosphere at a level that would prevent dangerous anthropogenic interference with the climate system', and goes further to define this as a level that would not compromise food production, would allow sustainable economic development, and should be 'achieved within a time-frame sufficient to allow ecosystems to adapt naturally to climate change' (see http://unfccc.int/resource/docs/convkp/ conveng.pdf). It is not surprising, therefore, that some of the most powerful data that have influenced policy debate in the climate change arena derive from longitudinal studies of wild organisms and ecosystems that reveal their responses to climate trends.1–3 Indeed, in the development of reports of the Intergovernmental Panel on Climate Change (IPCC, the premier policy-relevant compendium of climate change information), the first powerful compilation of such recorded responses was carried out for the Third Assessment Report.4 The success and influence of this contribution has ensured that, in the Fourth Assessment Report, an entire chapter has been devoted to this topic (Working Group 2, Chapter 1, Assessment of Observed Changes and Responses in Natural and Managed Systems; see http://www.ipcc.ch/activity/ wg2outlines.pdf).
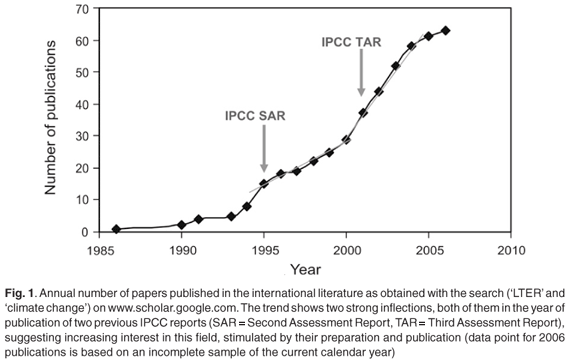
Systematic environmental monitoring has long been recognized as a key support for rational policy-making, and an important early-warning mechanism.5 It is interesting to note that the publication of reports relating to climate change and long-term ecological research (LTER) seems to have been stimulated by the release of IPCC reports (Fig. 1), possibly indicating a growing appreciation of the policy value of such studies, and prompting further publication of data sets and conceptual designs for more efficient and policy-relevant monitoring systems.6 We briefly review here the results and influence of some key LTER studies for their relevance to interpreting climate change impacts, which is arguably one of the most significant roles for the South African Environmental Observation Network (SAEON) initiative in South Africa.46
Studies using systematic site-based observation
The longest-running records of systematic observation most often are of phenological phenomena (the timing of growth and development events), generally of plants, e.g. ref. 7. Many such studies were summarized by Hughes8 in one of the first attempts at a comprehensive assessment. It is clear from this and subsequent studies that reliable, long-running biological response data sets are concentrated in the northern hemisphere.9 A main reason for the successful extraction of trends from these data sets is that growth stages monitored (e.g. bud-break, leafing-out, flowering) are generally limited by low temperatures, and may show a threshold-type response.10 It comes as no surprise, therefore, that a large majority of studies successfully identifying climate change impacts on these processes11 are situated in parts of the world where ecological processes are limited by low temperatures,12 enabling detection of consistent trends even in marine environments.13 This is not to say that temperature responses are always simple to interpret, as evidenced, for example, by complex changes in bird populations as a function of brood activity.14
Studies involving changes in low-temperature limitations have also identified consistent shifts in species' range, such as in altitude15 or tree-lines16 and even in shifts in geographic range polewards over large regions.9,11 Significant range shifts have emerged generally in vagile organisms such as butterflies,1 with a few exceptions.17 Incipient range shifts in plants may be detected as changes in population growth rate, especially in long-lived plants such as the kokerboom (Aloe dichotoma)18 or in forest trees.19 Studies of range shifts most positively identify such changes through records of new appearances, indicating range expansion.20 Range contraction21 is more difficult to demonstrate directly. Local species extinction and even global extinction such as recorded for the Costa Rican golden toad22 indicate a range loss. Attributing such an effect to local climatic changes may be complex, however, and requires explanations that tease apart ancillary and synergistic stresses, such as the impact of disease on climate-stressed populations.23
Complex multi-species studies that provide insight into ecosystem characteristics and processes such as community composition and biomass production24 have been carried out by re-analysing long-term data sets from studies that were originally established for reasons not related to climate change at all. Some studies (unfortunately, often of shorter duration) have identified strong predator–host relationships that operate either independently of or in addition to climate regimes.25 Such studies underline the importance of flexible, integrated and sufficiently complete sampling regimes that are robust enough to allow trends to be detected or positively refuted.
As the policy imperative for LTER has grown, e.g. ref. 26, a need for an integrated view of anthropogenic impacts on natural ecosystems and biogeochemical cycling has emerged. The first major studies to monitor the global biogeochemical cycles were those of the carbon cycle, established in the late 1950s on Mauna Loa, Hawaii,27 and these remain relevant.28 Today, large spatial scale studies involving cooperative groups and consortia have been initiated, e.g. ref. 29, and these now involve established long-term monitoring sites for both changes in ecosystem composition and productivity, e.g. ref. 30, and measurement of ecosystem-level physiological characteristics, e.g. ref. 31.
Significant concerns have been expressed, however, about blocks between monitoring efforts and policy making. For example, despite the annual investment of hundreds of millions of dollars in the United States in environmental monitoring, access to data and information for policy and decision makers has been lacking. This limits the extent to which policies may be updated in order to meet desired objectives, and suggests careful attention to the production of annual statistical reports and interpretational summaries from LTER efforts.32 This is a significant challenge for LTER projects that might be addressed, for example, by clear linkages with national programmes such as State of the Environment reporting (http://www.ngo.grida.no/soesa/ nsoer/).
In the southern hemisphere, LTER research has a narrower scope and a shorter history than north of the equator. Indeed, recent reviews have highlighted the comparative dearth of long-term research in the southern hemisphere.3,9 In the south, LTER efforts to detect climate change impacts may be complicated by the hemispheric difference in environmental controls of ecosystem processes,33 namely energy-related factors in the north in contrast to water-related factors in the south. Nonetheless, significant effects related to climate change have been detected through LTER, such as in marine communities,34 and in studies of island environments of the southern oceans.35 Long-term monitoring of seabird populations has been a critical policy input for marine resource management.36
Some terrestrial monitoring studies have also detected trends in the southern hemisphere, such as in forest trees of the South American tropics, where significant increases in individual plant turnover and biomass have been documented.30 In South Africa, game counts and other forms of wildlife monitoring in terrestrial ecosystems have provided early insights into the potential influence of climate change on wildlife populations, such as in the Kruger National Park.37
Looking to the future – SAEON-based opportunities and potential limitations
Many modelling studies based on empirical correlations of species relationships with climate (the so-called bioclimatic niche approach) suggest that species ranges and abundance should be sensitive to climate change.8,38–41 Systematic observations could therefore provide a sensitive test of these projections by monitoring species turnover in time, with the added benefit of revealing the demographic processes that accompany range shifts. Recent developments in detecting climate change effects on ecosystems and wild species have begun to invoke the stringent requirement of 'joint attribution'42 – that is, a statistical match between modelled expectations of climate changes and their impacts on the ecosystem as predicted and observed. Early work of this type on Aloe dichotoma has achieved some success.18
The likelihood of future joint attribution in southern Africa remains slim, nonetheless, given the few studies in this area under way. A lack of credible evidence that climate change is affecting biodiversity and ecosystems, either positively or negatively, or that predicted effects are being realized, will ultimately undermine a key voice for climate policy and adaptation measures in South Africa. A pressing goal of SAEON should be the development of coupled modelling and empirical studies over a range of time scales that can further the achievement of joint attribution.
Given the wealth of biodiversity in southern Africa, and the wide array of plant and animal 'functional types', this region seems to offer excellent opportunities to use a national network of systematically observed sites for changes in abundance and/or species distributions that may confirm or refute expected responses to climate trends. The region has also developed a significant store of species distribution databases (though recognizing the considerable taxonomic bias thereof and the poor quality of invertebrate databases),43 and fine-grained climate and substrate spatial information. Such resources have fuelled a productive effort in modelling the potential responses of many hundreds of species, including invasive aliens, to potential anthropogenic climate changes.38–41 This represents a powerful data set to guide focused monitoring efforts at the national scale, especially if combined with future modelling efforts that should develop projections for changes in ecosystem processes (such as fire and biological invasions), and range shifts for a broader range of vagile functional types such as birds and insects, under a broader set of climate change scenarios.
It is not only climate trends that may force ecosystem modifications—changes in atmospheric CO2 alone are believed to be able to alter the relative success of plant functional types by favouring, for example, woody plants coexisting with grasses in savanna-type ecosystems.44 Long-term monitoring of permanent plots with controlled fire frequency in key sites around the country has been crucial in developing an early understanding of how this trend may alter southern African ecosystem structure and function.45 More recent modelling of CO2 fertilization effects suggest that even semi-desert regions may experience some increase in vegetation cover as CO2 continues to rise and possibly ameliorate or counteract the potentially adverse effects of warming and increasing soil water stress.46 Many large-scale experiments in the northern hemisphere have simulated this atmospheric change and discovered some ameliorating impacts,47 but only one such experiment48 has been conducted in the southern hemisphere.
While the potential role of LTER in detecting the effect of climate change is promising, significant barriers remain to establishing credible links between climate change trends and ecological responses in South Africa, and indeed in broader regions of the southern hemisphere. There are three main reasons for this:
• Species ranges and ecosystem function may be controlled by water availability and drought, rather than linear and/or threshold-type temperature effects.49,50 This implies that climates in South Africa are typical of parts of the world where few ecological changes have been attributed to climate change, and stochastic events may trigger changes in ecosystem states. Teasing apart water and temperature control on plant species composition is critical in projecting the effect of climate change on tree success in South African grasslands, for example, where frost has been proposed as a primary limiting factor.51 Several other factors may, however, be dominant drivers.45
• Species occurrence and ecological success and ecosystem function are significantly affected by fire regime in many parts of the region,45,52 obscuring or complicating the attribution of climate change impacts.
• LTER sites are selected to represent 'typical' biomes or ecosystem types, a strategy that possibly limits the sensitivity of their component species to climate change, as sites may be positioned far from the 'edge' of biome and species ranges.
To counter these limitations, LTER efforts need careful strategizing to avoid obvious pitfalls.
Extreme events: It will be important to account carefully for inherently variable southern African rainfall,53 due especially to an apparent quasi-cyclical pattern on roughly decadal time scales,54 often associated with remote changes in sea-surface temperatures and global oscillations such as the El Niño–Southern Oscillation.55 An awareness of environmental and climate history will be crucial to interpreting data on trends – observations beginning after the severe drought of 1925–1934 would likely yield rather different trends from observations started after the good rainfall seasons of the 1970s. Sites with already established historical data should be preferred for this reason. Importantly, LTER efforts should avoid poor integration between observations made on different aspects of the environments at different sites that may result from a narrow focus of individual projects. The independent monitoring of different environmental features has previously been noted as an unintended weakness in LTER.6
Many challenges are associated with long-term monitoring of extreme climatic events, and the maintenance of consistency of observational methods is key.56 In order to define an event statistically, the use of multiple variables to define events is advisable, and should facilitate the identification of trends, especially if events can be monitored over spatial scales broader than that of a single site. Appropriate statistical methods for defining extreme events should also be carefully considered.57 Given that there are substantial difficulties associated even with identifying extreme climate events and detecting their trends,56 there will be an even greater onus on careful and reproducible observations of biota. Indeed, such transient-type responses have been suggested as the key to understanding long-term ecological change.52 Investigations of the changing variance in systems may likewise provide a key indicator of the likely imminence of ecological transition.58,59
Fire impacts: While fire is widely recognized as a driver of ecosystem structure, function and species composition, most work in this arena has focused on plant species, vegetation and ecosystem nutrient cycling. Rather little is known about the effects of fire on fauna, e.g. ref. 60. Unfortunately, fire frequency and intensity are functions of climatic factors, and may therefore be expected to change as climate alters,61 as has been observed in temperate ecosystems.62 A key decision on LTER sites will centre on fire management – the maintenance of a range of fire regimes in fire-prone environments will be desirable, while the possible introduction of fire as a novel ecosystem disturbance in non-fire-prone sites will increasingly become relevant if warming trends continue. This is an issue that needs careful deliberation.
Site representivity: In LTER focused on the detection of climate change effects, placement of observation sites can be optimized to increase the probability of detecting trends. This may be carried out in one way by assessing where climate changes themselves may be more likely, or by positioning sites at points where change may be inherently more detectible. The importance of ecotonal vegetation types as areas that may be sensitive to changes in environmental conditions has long been recognized, and they may therefore be useful in change detection. For example, Stohlgren63 described the arrangement of forty-two 1000-m2 plots in characteristic forest types and intervening ecotones, on 14 vegetation transects. Permanent monitoring transects such as these should be designed and implemented to detect species compositional change over time, and especially to detect increasing prevalence of alien organisms, e.g. ref. 64.
What is clear is that a strategy of selecting representative ecosystem types for long-term monitoring may result in site placement well within the geographical borders of target biomes or ecosystem types. Such a strategy reduces the chances of detecting species and ecosystem responses to climate changes, as these 'typical' sites may be far from ecotonal regions where component species are more likely to experience limiting climatic drivers. This shortcoming might be overcome by using experimental treatments that drive climatic conditions to more extreme levels,65,66 or simulate anticipated human impacts such as eutrophication, e.g. ref. 67. In addition, altitudinal transects, and transects which span the boundaries of one or more biomes, may be of particular significance for detecting the effects of change, especially where such change is predicted to be rapid, as is the case in some areas of the Grassland/Savanna, Succulent Karoo and Fynbos biomes.45,64,68
Taken together, the use of altitudinal transects and experimental treatments (such as drought, warming or CO2 fertilization) to 'force' responses or control disturbance regimes would be a useful addition to LTER and would help to address all three challenges identified above. Such treatments may also provide insights into anticipated responses in biota69 that may guide ongoing detection of change by the LTER programme.
In summary, while the detection of the effects of climate change is only one of several primary objectives, SAEON provides a rare opportunity to coordinate this important objective countrywide, to maximize the chance for ecological work to guide policy. The cost of coordinating observations, as suggested above, is not likely to be much higher than that of programmes of independent observations. The cost of implementing experimental treatments replicated between SAEON sites will be high, however, but will provide useful opportunities to train and employ ecologists in a policy-relevant field that is urgently needed in this country. A focus on detecting climate change through SAEON would likely both sharpen the emphasis of monitoring protocols over time, and provide a rationale for maintaining funding inputs, science training and capacity building for the long haul, especially if the information generated were interpreted for the benefit of policy-making.
1. Parmesan, C., Ryrholm N., Stefanescu C., Hill J.K., Thomas C.D. et al. (1999). Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399, 579–583. [ Links ]
2. Parmesan C. and Yohe G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. [ Links ]
3. Root T.L., Price J.T., Hall K.R., Schneider S.H., Rosenzweig C. and Pounds J.A. (2003). Fingerprints of global warming on wild animals and plants. Nature 421, 57–60. [ Links ]
4. Gitay H., Brown S., Easterling W. and Jallow B. (2001). In Climate Change 2001 – Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel of Climate Change (IPCC), eds J.J. McCarthy, O.F. Canziani, N.A. Leary, D.J. Dokken and K.S. White, pp. 237–342. Cambridge University Press, Cambridge. [ Links ]
5. Vaughan H., Brydges T., Fenech A. and Lumb A. (2001). Monitoring long-term ecological changes through the ecological monitoring and assessment network: science-based and policy relevant. Environmental Monitoring and Assessment 67, 3–28. [ Links ]
6. Parr T.W., Ferretti M., Simpson I.C., Forsius M. and Kovács-Láng E. (2002). Towards a long-term integrated monitoring programme in Europe: network design in theory and practice. Environmental Monitoring and Assessment 78, 253–290. [ Links ]
7. Sparks T.H. and Carey P.D. (1995). The responses of species to climate over two centuries: an analysis of the Marsham Phenological Record, 1736– 1947. J. Ecol. 83, 321–329. [ Links ]
8. Hughes L. (2000). Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol. 15, 56–61. [ Links ]
9. Rosenzweig C., Casassa G., Karoly D.J., Imeson A., Liu C. et al. (2007). In Intergovernmental Panel on Climate Change. Working Group II: Climate Change Impacts, Adaptation and Vulnerability, eds O.F. Canziani and M. Parry. Cambridge University Press, Cambridge. [ Links ]
10. Schwartz M.D. (1994). Monitoring global change with phenology: the case of the spring green wave. Int. J. Biometeorol. 38, 18–22. [ Links ]
11. Walther G.R., Burga C.A. and Edwards P.J. (eds) (2001). 'Fingerprints' of Climate Change – Adaptative behavior and shifting species ranges. Kluwer, New York. [ Links ]
12. Nemani R.R., Keeling C.D., Hashimoto H., Jolly W.M., Piper S.C., Tucker C.J., Myneni R.B. and Running S.W. (2003). Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300, 1560–1563. [ Links ]
13. Southward A.J., Hawkins S.J. and Burrows M.T. (1995). Seventy years' observations of changes in distribution and abundance of zooplankton and intertidal organisms in the western English Channel in relation to rising sea temperature. J. Thermal Biol. 20, 127–155. [ Links ]
14. Visser M.E., Adriaensen F., van Balen J.H., Blondel J., Dhondt A.A. et al. (2003). Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. B 270, 367–372. [ Links ]
15. Pauli H., Gottfried M. and Grabherr G. (2001). In 'Fingerprints' of Climate Change – Adapted behaviour and shifting species ranges, eds G.R. Walther, C.A. Burga and P.J. Edwards, pp. 139–149. Kluwer, New York. [ Links ]
16. Millar C.I., Westfall R.D., Delany D.L., King J.C. and Graumlichc L.J. (2004). Response of subalpine conifers in the Sierra Nevada, California, U.S.A., to 20th-century warming and decadal climate variability. Arctic, Antarctic, and Alpine Research 36, 181–200. [ Links ]
17. Sturm M., Racine C. and Tape K. (2001). Climate change: increasing shrub abundance in the Arctic. Nature 411, 546–547. [ Links ]
18. Foden W., Midgley G.F., Hughes G., Bond W.J., Thuiller W., Hoffman M.T., Kaleme P., Rebelo A. and Hannah L. (2007). A changing climate is eroding the geographical range of the Namib Desert tree Aloe through population declines and dispersal lags. Divers. Distrib. 13, 645–653. [ Links ]
19. Mantgem P.J. v. and Stephenson N.L. (2007). Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol. Lett. 10, 909–916. [ Links ]
20. Thomas C.D., Bodsworth E.J., Wilson R.J., Simmons A.D., Davies Z.G., Musche M. and Conradt L. (2001). Ecological and evolutionary processes at expanding range margins. Nature 411, 577–581. [ Links ]
21. Hampe A., Petit R.J. and Cortufo F. (2005). Conserving biodiversity under climate change: the rear edge matters. Ecol. Lett. 8, 461–467. [ Links ]
22. Pounds J.A., Fogden M.P.L. and Campbell J.H. (1999). Biological response to climate change on a tropical mountain. Nature 398, 611–615. [ Links ]
23. Pounds J.A., Bustamante M.R., Coloma L.A., Consuegra J.A. et al. (2006). Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167. [ Links ]
24. Silvertown J., Dodd M.E., McConway K., Potts J. and Crawley M. (1994). Rainfall, biomass variation, and community composition in the Park Grass Experiment. Ecology 75, 2430–2437. [ Links ]
25. Fleming R.A. and Candau J-N. (1998). Influences of climatic change on some ecological processes of an insect outbreak system in Canada's boreal forests and the implications for biodiversity. Environmental Monitoring and Assessment 49, 235–249. [ Links ]
26. Baer P., Harte J., Haya B., Herzog A.V., Holdren J., Hultman N.E., Kammen D.M., Norgaard R.B. and Raymond L. (2000). Equity and greenhouse gas responsibility. Science 289, 2287. [ Links ]
27. Keeling R.F., Chin J.F.S. and Whorf T. (1996). Increased activity of northern vegetation inferred from atmospheric CO2 measurements. Nature 382, 146–149. [ Links ]
28. Zeng N., Mariotti A. and Wetzel P. (2005). Terrestrial mechanisms of interannual CO2 variability. Global Biogeochemical Cycles 19, GB1016. [ Links ]
29. Ingram J.S.I., Canadell J., Elliott T., Hunt L.A., Linder S., Murdiyarso D., Stafford Smith M. and Valentin C. (1999). In The Terrestrial Biosphere and Global Change, eds B. Walker, W. Steffen, J. Canadell and J. Ingram, pp. 45–65. Cambridge University Press, Cambridge. [ Links ]
30. Lewis S.L., Phillips O.L., Baker T.R., Lloyd J. et al. (2004). Concerted changes in tropical forest structure and dynamics: evidence from 50 South American long term plots. Phil. Trans. R. Soc.: Biol. Sci. 359, 421–436. [ Links ]
31. Baldocchi D., Falge E., Gu L. and Olson R.A.E. (2001). FLUXNET: a new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull. Am. Met. Soc. 82, 2415–2434. [ Links ]
32. Messer J.J., Linthurst R.A. and Overton W.S. (1991). An EPA program for monitoring ecological status and trends. Environmental Monitoring and Assessment 17, 67–78. [ Links ]
33. Chown S.L., Sinclair B.J., Leinaas H.P. and Gaston K.J. (2004). Hemispheric asymmetries in biodiversity – a serious matter for ecology. PLoS Biol. 2, 1701–1707. [ Links ]
34. Weimerskirch H., Inchausti P., Guinet C. and Barbraud C. (2003). Trends in bird and seal populations as indicators of a system shift in the Southern Ocean. Antarctic Sci. 15, 249–256. [ Links ]
35. Bergstrom D. and Chown S.L. (1999). Life at the front: history, ecology and change on southern ocean islands. Trends Ecol. Evol. 14, 472–477. [ Links ]
36. Forcada J., Trathan P.N., Reid K., Murphy E.J. and Croxall J.P. (2006). Contrasting population changes in sympatric penguin species in association with climate warming. Global Change Biology 12, 411–423. [ Links ]
37. Ogutu J.O. and Owen-Smith N. (2003). ENSO, rainfall and temperature influences on extreme population declines among African savanna ungulates. Ecol. Lett. 6, 412–419. [ Links ]
38. Erasmus B.F.N., Van Jaarsveld A.S., Chown S.L., Kshatriya M. and Wessels K.J. (2002). Vulnerability of South African animal taxa to climate change. Global Change Biology 8, 679–693. [ Links ]
39. Midgley G.F., Hannah L., Millar D., Thuiller W. and Booth A. (2003). Developing regional and species-level assessments of climate change impacts on biodiversity in the Cape Floristic Region. Biol. Cons. 112, 87–97. [ Links ]
40. Thuiller W., Broenniman O., Hughes G., Alkemades J.R.M., Midgley G.F. and Corsi F. (2006). Vulnerability of African mammals to anthropogenic climate change under conservative land transformation assumptions. Global Change Biology 12, 424–440. [ Links ]
41. Thuiller W., Midgley G.F., Hughes G.O., Bomhard B., Drew G., Rutherford M.C. and Woodward F.I. (2006). Endemic species and ecosystem sensitivity to climate change in Namibia. Global Change Biology 12, 759–776. [ Links ]
42. Root T.L., MacMynowski D.P., Mastrandrea M.D. and Schneider S.H. (2005). Human-modified temperatures induce species changes: joint attribution. Proc. Natl Acad. Sci. USA 102, 7465–7469. [ Links ]
43. Koch S.O., Chown S.L., Davis A.L.V., Endrödy-Younga S. and Van Jaarsveld A.S. (2000). Conservation strategies for poorly surveyed taxa: a dung beetle (Coleoptera, Scarabaeidae) case study form southern Africa. J. Insect Conserv. 4, 45–56. [ Links ]
44. Bond W.J. and Midgley G.F. (2000). A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Global Change Biology 6, 865–869. [ Links ]
45. Bond W.J., Midgley G.F. and Woodward F.I. (2003). What controls South African vegetation – climate or fire? S. Afr. J. Bot. 69, 79–91. [ Links ]
46. Van Jaarsveld A.S., Pauw J.C., Mundree S., Mecenero S., Coetzee B.W.T. and Alard G.F. (2007). South African Environmental Observation Network: vision, design and status. S. Afr. J. Sci. 103, 289–294. [ Links ]
47. Ainsworth E. A. and Long S. P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytologist 165, 351–371. [ Links ]
48. Stokes C., Ash A., Tibbett M. and Holtum J. (2005). OzFACE: the Australian savanna free air CO2 enrichment facility and its relevance to carbon-cycling issues in a tropical savanna. Aust. J. Bot. 53, 677–687. [ Links ]
49. Evans K.L., van Rensburg B.J., Gaston K.J. and Chown S.L. (2006). People, species richness and human population growth. Global Ecol. Biogeog. 15, 625–636. [ Links ]
50. van Rensburg B.J., Chown S.L. and Gaston K.J. (2002). Species richness, environmental correlates, and spatial scale: a test using South African birds. Am. Nat. 159, 566–577. [ Links ]
51. Ellery W.N., Scholes R.J. and Mentis M.T. (1991). Differentiation of the grassland biome of South Africa based on climatic indices. S. Afr. J. Sci. 87, 499–503. [ Links ]
52. Hastings A. (2004). Transients: the key to long-term ecological understanding? Trends Ecol. Evol. 19, 39–45. [ Links ]
53. Nicholson S.E. (2001). Climatic and environmental change in Africa during the last two centuries. Climate Res. 17, 123–144. [ Links ]
54. Tyson P.D. and Preston-Whyte R.A. (2000). The Weather and Climate of South Africa. Oxford University Press, Oxford. [ Links ]
55. Glantz M.H. (2001). Currents of Change: Impacts of El Niño and La Niña on climate and society. Cambridge University Press, Cambridge. [ Links ]
56. Nicholls N. (1995). Long-term climate monitoring and extreme events. Climatic Change 31, 231–245. [ Links ]
57. Gaines S.D. and Denny M.W. (1993). The largest, smallest, highest, lowest, longest, and shortest: extremes in ecology. Ecology 74, 1677–1692. [ Links ]
58. Carpenter S.R. and Brock W.A. (2006). Rising variance: a leading indicator of ecological transition. Ecol. Lett. 9, 308–315. [ Links ]
59. Vasseur D.A. and Yodzis P. (2004). The color of environmental noise. Ecology 85, 1146–1152. [ Links ]
60. Parr C.L. and Chown S.L. (2003). Burning issues for conservation: a critique of faunal fire research in southern Africa. Austral Ecol. 28, 384–395. [ Links ]
61. Midgley G.F., Chapman R.A., Hewitson B., Johnston P. et al. (2005). A status quo, vulnerability and adaptation assessment of the physical and socio-economic effects of climate change in the Western Cape. CSIR Environmentek, Stellenbosch. [ Links ]
62. Gillett N.P., Weaver A.J., Zwiers F.W. and Flannigan M.D. (2004). Detecting the effect of climate change on Canadian forest fires. Geophys. Res. Lett. 31, L18211. [ Links ]
63. Stohlgren T.J., Owen A.J. and Lee M. (2000). Monitoring shifts in plant diversity in response to climate change: a method for landscapes. Biodiversity and Conservation 9, 65–86. [ Links ]
64. Botes A., McGeoch M.A., Robertson H.G., van Niekerk A., Davids H.P. and Chown S.L. (2006). Ants, altitude and change in the northern Cape Floristic Region. J. Biogeog. 33, 71–90. [ Links ]
65. Musil C.F., Schmiedel U. and Midgley G.F. (2005). Lethal effects of experimental warming approximating a future climate scenario on southern African quartz-field succulents: a pilot study. New Phytologist 165, 539–547. [ Links ]
66. van Wijk M.T., Clemmensen K.E., Shaver G.R., Williams M. et al. (2004). Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Global Change Biology 10, 105–123. [ Links ]
67. Willems J.H. and Nieuwstadt M.G.L. v. (1996). Long-term after effects of fertilization on above-ground phytomass and species diversity in calcareous grassland. J. Veg. Sci. 7, 177–184. [ Links ]
68. Hannah L., Midgley G.F., Hughes G. and Bomhard B. (2005). The view from the Cape: extinction risk, protected areas and climate change. BioScience 55, 231–242. [ Links ]
69. Lloret F., Penuelas J. and Estiarte M. (2004). Experimental evidence of reduced diversity of seedlings due to climate modification in a Mediterranean-type community. Global Change Biology 10, 248–258. [ Links ]
* Authors for correspondence. E-mails: midgley@sanbi.org; slchown@sun.ac.za














