Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Onderstepoort Journal of Veterinary Research
On-line version ISSN 2219-0635
Print version ISSN 0030-2465
Onderstepoort j. vet. res. vol.86 n.1 Pretoria 2019
http://dx.doi.org/10.4102/ojvr.v86i1.1673
ORIGINAL RESEARCH
Prevalence and risk factors associated with Campylobacter spp. occurrence in healthy dogs visiting four rural community veterinary clinics in South Africa
Musafiri KaramaI; Beniamino T. Cenci-GogaI, II; Alice ProsperiIII, IV; Eric EtterV, VI; Saeed El-AshramVII, VIII; Cheryl McCrindleIX; Jackson N. OmbuiX; Alan KalakeXI
IDepartment of Paraclinical Sciences, Faculty of Veterinary Science, University of Pretoria, Onderstepoort, South Africa
IIDepartment of Veterinary Medicine, Laboratorio di Ispezione degli alimenti di origine animale, University of Perugia, Perugia, Italy
IIIExperimental Zooprofilattico Institute of Lombardy and Emilia-Romagna 'Bruno Ubertini', Brescia, Italy
IVDepartment of Veterinary Medical Sciences, University of Bologna, Bologna, Italy
VDepartment of Production Animal Studies, Faculty of Veterinary Science, University of Pretoria, Onderstepoort, South Africa,
VICentre de Coopération Internationale en Recherche Agronomique pour le Développement-INRA, UMR ASTRE Baillarguet International Campus, University of Montpellier, Montpellier, France
VIICollege of Life Science and Engineering, Foshan University, Foshan, China
VIIIFaculty of Science, Kafr ElSheikh University, Kafr El Sheikh, Egypt,
IXDepartment of Agriculture and Animal Health, University of South Africa, Johannesburg, South Africa
XDepartment of Public Health, Pharmacology and Toxicology, College of Agriculture and Veterinary Sciences, University of Nairobi, Nairobi, Kenya
XIGauteng Department of Agriculture and Rural Development, Gauteng, Johannesburg, South Africa
ABSTRACT
Reports on the occurrence of Campylobacter spp. in dogs in South Africa are non-existent. This study investigated the prevalence of Campylobacter spp. in 481 dogs visiting four rural community veterinary clinics in South Africa. Dogs were screened for Campylobacter spp. by culture and polymerase chain reaction (PCR), and logistic regression analysis was performed to assess the association between sex, clinic, breed and age and the occurrence of Campylobacter spp. in dogs. The prevalence of Campylobacter spp. was 41.50% (95% confidence interval [CI], 37.39% - 46.04%). Campylobacter jejuni, C. upsaliensis and C. coli were detected in 29.31% (95% CI, 25.42% - 33.54%), 13.10% (95% CI, 10.37% - 16.42%) and 5.41% (95% CI, 3.71% - 7.82%) of dogs, respectively. Dogs carrying more than one species of Campylobacter spp. accounted for 6.23% (95% CI, 4.40% - 8.78%). Campylobacter upsaliensis and C. jejuni were detected in 3.74% (95% CI, 2.37% - 5.86%), whereas C. coli and C. jejuni were found in 2.49% (95% CI, 1.42% - 4.34%) of dogs. Age and clinic were the risk factors significantly associated with Campylobacter spp. occurrence, while age, breed and clinic were predictors of C. jejuni carriage. Furthermore, age was the only risk factor associated with a higher likelihood of carrying C. upsaliensis. The prevalence of Campylobacter spp. C. jejuni and C. upsaliensis increased significantly as dogs grew older. In addition, the odds of carrying Campylobacter spp. were higher in the Staffordshire bull terrier breed compared to crossbreed dogs. In conclusion, this study shows that dogs visiting rural community veterinary clinics in South Africa are reservoirs of Campylobacter spp. and may be potential sources of Campylobacter spp. for humans living in close proximity of the dog populations under study.
Keywords: dogs; Campylobacter spp.; C. jejuni; C. coli; C. upsaliensis; risk factors; South Africa.
Introduction
Campylobacter spp. are the leading cause of bacterial diarrhoeal diseases globally. Around 400-500 million cases of Campylobacter infections occur each year globally (Friedman et al. 2000). Although most human cases of campylobacteriosis are foodborne or waterborne (Jacobs-Reitsma, Lyhs & Wagenaar 2008), a number of studies have shown that contact with dogs is a risk factor for human campylobacteriosis (Couturier, Hale & Couturier 2012; Damborg et al. 2016; Man 2011; Mughini Gras et al. 2012; Neimann et al. 2003; Rossi et al. 2008; Tenkate & Stafford 2001). Manifestations of Campylobacter infections in humans include mild watery to severe bloody diarrhoea, nausea and vomiting and in some cases life-threatening complications such as Guillain-Barré syndrome or its variant, Miller-Fisher syndrome (Jacobs et al. 2008). In immunocompromised patients, Campylobacter jejuni is an important cause of severe bacteraemia, which may lead to death (Tee & Mijch 1998).
Dogs are considered asymptomatic carriers of Campylobacter spp. (Acke et al. 2009; Carbonero et al. 2012; Hald & Madsen 1997). A number of studies have reported Campylobacter spp. carriage rates ranging from 2.7% to 100% in dogs (Chaban, Ngeleka & Hill 2010; Hald et al. 2004; Tsai et al. 2007). Furthermore, clinical cases in dogs with Campylobacter spp. as a primary or secondary cause of diarrhoea have also been reported (Burnens, Angéloz-Wick & Nicolet 1992; McOrist & Browning 1982). Healthy and diarrhoeic dogs may harbour one or more Campylobacter species including C. jejuni, C. coli and C. upsaliensis (Carbonero et al. 2012; Chaban et al. 2010; Giacomelli et al. 2015; Holmberg et al. 2015; Parsons et al. 2011). Both C. jejuni and C. upsaliensis have been implicated in dog-associated Campylobacter spp. infections in humans (Bourke, Chan & Sherman 1998; Couturier et al. 2012; Nachamkin, Allos & Ho 1998). Zoonotic transmission of Campylobacter spp. from dogs occurs through direct contact with infected dogs or dog faeces (Damborg et al. 2016; Mughini Gras et al. 2012). There are suggestions that contact with dogs may be associated with up to 6% of human campylobacteriosis cases (Rossi et al. 2008; Tenkate & Stafford 2001).
Although dogs are considered an important reservoir of Campylobacter spp. (Hald & Madsen 1997; Hald et al. 2004), current data on the prevalence of Campylobacter spp. in dogs in South Africa and on the African continent are lacking. Therefore, the aim of this study was to investigate the prevalence and risk factors associated with Campylobacter spp. occurrence in healthy dogs visiting four rural community veterinary clinics in South Africa.
Materials and methods
Study design, area and population
This cross-sectional study was conducted at four rural community veterinary clinics located in Gauteng Province, South Africa (see Figure 1). A total of 481 dogs were screened for Campylobacter spp. including C. jejuni, C. coli and C. upsaliensis. Each dog owner had an identity card on which the following variables were recorded: name of the dog and owner, vaccination status, sex, date of birth and breed. Faecal swabs were obtained from all dogs that visited a particular clinic on the sampling day. This study was approved by the Animal Ethics Committee of the University of Pretoria (V056-15).
Sample collection and Campylobacter spp. culture methods
Sterile swabs were used to collect faecal samples from dogs during routine vaccination and deworming campaigns. Each clinic was visited once, and one sample was obtained from each animal. For culture and isolation of Campylobacter spp., swabs were spread-plated on Campy CVA agar (Brucella agar containing 5% defibrinated sheep blood supplemented with 20 mg of cefoperazone, 10 mg of vancomycin and 2 mg of amphotericin B) (Becton, Dickinson and Company, Sparks, MD, United States [US]). The inoculated plates were incubated at 37 °C for 48-72 hours in tightly sealed anaerobic system containers in which GasPakTM EZ Campy System sachets (Becton, Dickinson and Company, Sparks, MD, US) were placed to create a microaerophilic atmosphere (approximately 6% - 16% oxygen and 2% - 10% carbon dioxide). We used 37 °C to incubate Campylobacter spp. instead of the generally used 42 °C to mimic the 'natural' growth environmental temperature of Campylobacter spp. in the gastrointestinal tract of dogs. Furthermore, 37 °C was used to favour the growth of C. upsaliensis, which grows optimally at 37 °C (Lastovica & Le Roux 2001).
DNA extraction
Briefly, a sterile inoculating loop was used to harvest colony sweeps from all Campy CVA plates that showed growth after 48 h - 72 h. A loop-full of colony sweeps was suspended in a 1.5 mL Eppendorf tube containing 1 mL of FA buffer (Becton, Dickinson and Company). Bacterial suspensions were mixed and washed by vortexing, followed by centrifugation (15 000 g) for 5 minutes. After the first wash and centrifugation cycle, the supernatant was discarded and the bacterial pellet was resuspended in FA buffer (Becton, Dickinson and Company). Two additional washes and centrifugation cycles were carried out, after which the pellet was suspended in 500 µL of sterile water, vortexed and the homogeneous cell suspension was boiled to 100 °C for 15 min, then stored at -20 °C for further processing.
Campylobacter spp. screening
A Campylobacter spp. specific multiplex polymerase chain reaction (PCR) protocol (Forbes & Horne 2009) was used to screen DNA for Campylobacter spp. (C. jejuni, C. coli and C. upsaliensis). Primers lpxAC. jejuni-ACAACTTGGTGACGATGTTGTA (Klena et al. 2004), lpxAC. coli GATAGTAGACAAATAAGAGAGAATMAG (Forbes & Horne 2009) and lpxAC. upsaliensis-AAGTCGTATATTTTCYTACGCTTGTGTG (Klena et al. 2004) were used as forward primers. Primers lpxA-R1-CAATCATGTGCGATATGACAATAYGCCAT, lpxA-R2-CAATCATGA-GCAATATGACAATAAGCCAT and lpxAR KK2m CAATCATGDGCDATATGASAATAHGCCAT were used as reverse primers for C. jejuni, C. coli and C. upsaliensis (Klena et al. 2004; Forbes & Horne 2009). Each PCR reaction (25 µL) contained 2.5 µL of 10X Thermopol reaction buffer, 2.0 µL of 2.5 mM deoxyribonucleotides triphosphate (dNTPs), 0.25 µL of 100 mM MgCl2, 1.25 µL of 0.5 µM of each forward primer and 1.25 µL of 0.25 µM of each reverse primer in a 50:50 mixture, 1U of Taq DNA Polymerase (New England BioLabs® Inc., Ipswich, MA, US) and 5 µL of DNA template. Sterile water was used to top up the reaction volume to 25 µL. Campylobacter jejuni ATCC 33560, C. coli derived from ATCC 33559 (Microbiologics, St Cloud, MN, US) and an in-house dog C. upsaliensis isolate were used as positive controls, and sterile water was the negative control. All PCR reagents were supplied by New England BioLabs, except for the primers, which were supplied by Inqaba Biotec (Inqaba Biotec, Pretoria, South Africa) or Integrated DNA Technologies (IDT, San Diego, CA, US). Polymerase chain reactions were performed in a C1000 TouchTM (Bio-Rad, Hercules, CA, US) or a Veriti™ (Applied Biosystems®, Foster City, CA, US) thermal cycler. Amplified DNA was electrophoresed in 2.5% (w/v) agarose gels in tris-acetate-ethylenediaminetetraacetic acid buffer. Gels were stained with ethidium bromide (0.05 mg/µL), and amplicons were visualised under ultraviolet light in a Gel Doc system (Bio-Rad, Hercules, CA, US).
Campylobacter speciation
Colony sweeps were obtained from all Campy CVA plates that were positive for Campylobacter spp. on PCR screening, streaked on horse blood agar and incubated at 37 °C for 48 h - 72 h to obtain single colonies. Three suspect Campylobacter spp. single colonies were taken from each horse blood agar plate with a sterile plastic inoculating loop or swab, spread-plated separately on horse blood agar plates and incubated at 37 °C for 48 h - 72 h to multiply and purify the single colonies. After incubation, pure single colony bacterial sweeps were harvested using a sterile plastic inoculating loop or swab, and the bacterial cells were suspended in 1.5 mL FA buffer in an Eppendorf tube. DNA was extracted from the single colony sweeps by the boiling method and screened for C. jejuni, C. coli and C. upsaliensis using the aforementioned primers and multiplex PCR protocol (Forbes & Horne 2009; Klena et al. 2004). Single colony isolates that were confirmed as C. jejuni, C. coli or C. upsaliensis on PCR were stored at -80 °C in cryovials containing a sterile freezing mixture (70% Brucella broth and 30% glycerol).
Statistical analysis
All statistical analyses were performed using the 'base', 'epiDisplay' and 'aod' packages of the R software version 3.3.3 (R Foundation for Statistical Computing 2017, Vienna, Austria, http://www.R-project.org/). The prevalence of Campylobacter spp., C. jejuni, C. coli and C. upsaliensis, were computed on 481 dogs using a general linear model considering the error distribution as binomial and the link function 'logit' (logistic regression). The following risk factors were tested: sex, breed, clinic, age and number of vaccinations. As a categorical variable, 'breed' included crossbreed, molosser, Staffordshire bull terrier, toy and other breeds.
To prepare for the logistic regression model, the potential relationship or association between the number of vaccinations and the age of the dog was tested. Because of non-normality in the distribution of ages in relation to time and number of vaccinations and heterogeneity of variance, an analysis of variance (ANOVA) could not be applied. Instead, a general linear model using count data (family = poisson) was used to align the ages of dogs with the number of vaccinations. Because dog ages were statistically linked with the number of vaccinations, the number of vaccinations as a risk factor was removed from the logistic regression model and only sex, breed, clinic and age were kept for risk factor analysis.
All the records for which sex and breed were not determined or missing (n = 100) were removed from the database, and only 381 dog samples were used in the model. A general linear model (family = binomial) with a full model encompassing all risk factors and all possible interactions was initially performed. Using a backward stepwise model selection based on the Akaike information criterion (AIC), the best model was kept (with the smallest AIC). A likelihood ratio test was performed to test the overall significance of each risk factor (multilevel comparison). Because age was analysed as a continuous variable, odds ratio (OR) and prevalence predictions were not calculated for this risk factor. In terms of levels within each categorical risk factor, for comparison purposes, the risk factor associated with the lowest Campylobacter spp. prevalence was used as a reference. Adjusted OR that took into account all cofounder variables were calculated, and confidence intervals (CIs). Odds ratio significance was computed using the Wald's test. To predict the prevalence of Campylobacter spp. within different categories for each risk factor, the 'predict' function was applied on logistic regression results. Finally, risk factors for C. jejuni, C. upsaliensis and C. coli were tested using the same approach that was applied for Campylobacter spp. risk factor analysis. For all analyses, p-values < 0.05 were considered significant.
Results
Descriptive analysis
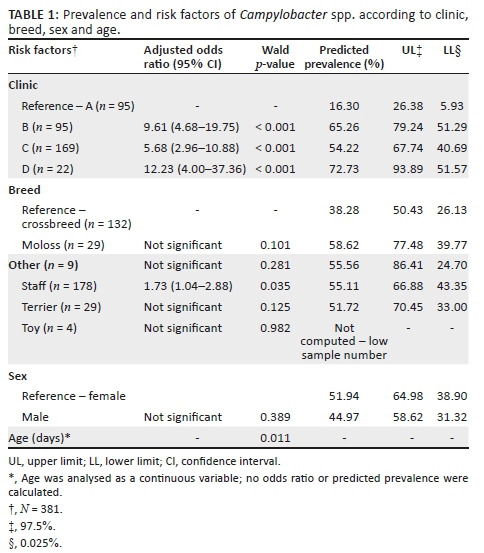
Of the 481 dogs that were tested for Campylobacter spp. (see Table 1), 41.58% (95% CI, 37.39% - 46.04%) were positive for Campylobacter spp. The distribution of individual Campylobacter species was as follows: C. jejuni (29.31%; 95% CI, 25.42% - 33.54%) was the most frequent species, followed by C. upsaliensis (13.10%; 95% CI, 10.37% - 16.42%) and C. coli (5.41%; 95% CI, 3.71% - 7.82%) (see Table 1). Similarly, 6.23% (95% CI, 4.40% - 8.78%) of dogs with mixed infections were also detected: C. jejuni + C upsaliensis, 3.74% (95% CI, 2.37% - 5.86%) and C. jejuni + C. coli, 2.49% (95% CI, 1.42% - 4.34%).
Risk factors
A total of 381 dogs were included in the final model. Logistic regression showed that clinic, age and breed were significant risk factors for carrying Campylobacter spp. (see Table 1). No interactions between any of these risk factors were statistically significant in the model. Overall, visiting a particular clinic and the age of the dog were significant risk factors for carrying C. jejuni while age was the only risk factor associated with carrying C. upsaliensis. Dogs visiting clinics B, C and D were respectively 9.61, 5.68 and 12.3 times more likely to carry Campylobacter spp. in comparison to dogs visiting clinic A (reference clinic) (see Table 1). In addition, breed was a predictor of Campylobacter spp., with the odds of carrying Campylobacter spp. significantly higher in the Staffordshire bull terrier breed in comparison to crossbreed dogs, which had the lowest prevalence of Campylobacter spp. Furthermore, the prevalence of Campylobacter spp., C. jejuni and C. upsaliensis increased significantly as dogs grew older.
Discussion
To our knowledge, this is the first study reporting on the occurrence of Campylobacter spp. in dogs visiting rural community veterinary clinics and the risk factors associated with Campylobacter spp. carriage in dogs in South Africa. Our results showed that 41.5% of dogs carried Campylobacter spp., in line with similar studies, which have reported Campylobacter spp. prevalence in dogs ranging from 35% to 43% in Europe and North America (Acke et al. 2009; Holmberg et al. 2015; Parsons et al. 2010; Procter et al. 2014; Workman et al. 2005). However, higher prevalence rates of Campylobacter spp. of up to 75.5% have been reported in dogs in different countries including Sweden, Canada, the UK and USA (Chaban et al. 2010; Engvall et al. 2003; Leahy et al. 2017; Parsons et al. 2011).
Variations in Campylobacter spp. occurrence rates in dogs have been ascribed to a number of factors, including dog living conditions - whether a dog is confined in a house, a shelter or kennel, or is a stray. Higher Campylobacter spp. occurrence rates have been reported in dogs living in shelters or kennels and stray dogs (Baker, Barton & Lanser 1999; Parsons et al. 2011; Procter et al. 2014; Tsai et al. 2007; Workman et al. 2005) in comparison to in-house dogs. Additional factors such as Campylobacter spp. culture conditions, including the incubation temperature and atmosphere, as well as antimicrobial supplements that are used for selection of Campylobacter spp. in various recovery media or enrichment broths, may also influence Campylobacter detection rates (Allos & Lastovica 2008; Aspinall et al. 1996; Lastovica & Le Roux 2001).
Campylobacter jejuni was the most frequent Campylobacter species in dogs, followed by C. upsaliensis and C. coli to a lesser extent. This finding is in agreement with previous studies, which have reported C. jejuni as the most frequent species in dogs compared to other Campylobacter species (Carbonero et al. 2012; Giacomelli et al. 2015; Tsai et al. 2007). However, a number of reports have also found C. upsaliensis to be the most frequent species in dogs (Acke et al. 2009; Chaban et al. 2009; Holmberg et al. 2015; Parsons et al. 2011; Rossi et al. 2008). A number of studies have found that C. upsaliensis was more frequent in dogs confined in household compounds while C. jejuni was more common in stray dogs and shelter or kennel dogs (Carbonero et al. 2012; Leonard et al. 2011; Parsons et al. 2011; Procter et al. 2014). The majority of dog owners in this study indicated that their dogs were not housed in fenced yards and dogs were allowed to leave their living premises and freely roam in the neighbourhood, thereby living a 'semi-stray' life. The stray nature of dogs sampled in this study may have played a role in the predominance of C. jejuni over C. upsaliensis. Roaming, scavenging and hunting behaviours of dogs living a semi-stray life exposes dogs to environments, food and water sources that may favour higher environmental contamination levels with C. jejuni compared to C. upsaliensis, which has been found to be more frequent in dogs living in-house that are fed home-cooked food (Leonard et al. 2011).
While the role played by C. jejuni in human disease is well recognised globally, of particular interest in this study was the presence of dogs infected with C. upsaliensis. Campylobacter upsaliensis has emerged in the last 20 years (Bourke et al. 1998) as an important species in dogs worldwide (Chaban et al. 2010; Engvall et al. 2003; Parsons et al. 2010) and a cause of campylobacteriosis in humans (Allos & Lastovica 2008; Couturier et al. 2012; Labarca et al. 2002; Nakamura et al. 2015). This is the first time C. upsaliensis has been reported in dogs in South Africa. This finding is of public health significance as C. upsaliensis has been previously reported as the third most frequent Campylobacter species in South Africa over a period of 10 years in paediatric patients, accounting for 23% of Campylobacter spp. cases (Lastovica & Engel 2001).
Carriage of more than one Campylobacter species was observed in 6.2% of dogs. Dogs with mixed infections carried C. jejuni and C. upsaliensis or C. jejuni and C. coli. Dogs carrying multiple Campylobacter species may have been exposed to environments and sources that allow these Campylobacter species to thrive favourably. Similar findings have been reported elsewhere (Bojanić et al. 2017; Chaban et al. 2010; Engvall et al. 2003; Hald et al. 2004; Koene et al. 2004). A number of studies have recommended the use of more than one Campylobacter spp. culture medium to facilitate the isolation and increase the chance of recovering multiple Campylobacter species of public health importance from faecal samples (Endtz et al. 1991; Baker et al. 1999; Koene et al. 2004). In the aforementioned studies in which multiple Campylobacter species were detected in individual dogs, at least two media were used to isolate Campylobacter spp. While evaluation of the sensitivity of the medium used in this study to recover Campylobacter spp. is beyond the scope of this investigation, detection of dogs carrying multiple Campylobacter species indicates that Campy CVA agar was a reliable single medium for direct and simultaneous recovery of more than one Campylobacter species from dog faeces.
Concerning the different risk factors that were investigated in this study, our findings showed that the overall prevalence of Campylobacter spp. and particularly the prevalence of C. jejuni and C. upsaliensis increased as dogs grew older, with predominance of C. jejuni in dogs younger than 1 year in comparison to dogs older than 1 year. Similar studies have reported that dogs less than 1 year old were more likely to be colonised by Campylobacter spp. (Acke et al. 2009; Guest, Stephen & Price 2007; Hald et al. 2004; Leahy et al. 2017; Parsons et al. 2010; Procter et al. 2014; Sandberg et al. 2002). The high prevalence of Campylobacter spp. in younger dogs may be most probably ascribed to an immature immune system and an underdeveloped enteric microbiota that is unable to outcompete and displace Campylobacter spp. in the intestine. This finding was not surprising as the dog population under study was skewed towards a higher number of dogs younger than 1 year (88%) compared to dogs that were older than 1 year.
Consistent with previous studies, the prevalence of Campylobacter spp. was not significantly different in male and female dogs (Nair et al. 1985; Olson & Sandstedt 1987; Sandberg et al. 2002; Torre & Tello 1993). However, breed was overall a predictor for Campylobacter carriage, with the Staffordshire bull terrier breed more likely to carry Campylobacter spp. in comparison to crossbreed dogs. Although breed has never been reported as a risk factor for Campylobacter spp. occurrence in dogs, this finding may indicate that dogs belonging to the Staffy breed, which is a pure breed, may be more susceptible to disease in comparison to crossbreeds, which are generally considered more resistant to disease.
Visiting a particular clinic was identified as a risk factor for carrying C. jejuni, with dogs visiting clinics B, C and D presenting a higher risk of carrying Campylobacter spp. and particularly C. jejuni. While the reasons behind this finding are not clear, the authors postulate that there are yet-unidentified factors such as dog living conditions (in-house vs. stray dogs) that may be favouring a higher occurrence rate of C. jejuni in dogs living in the communities serviced by clinics B, C and D in comparison to clinic A, which had the lowest Campylobacter spp. prevalence.
Conclusion
This study provides useful information on the prevalence and risk factors of C. jejuni, C. upsaliensis and C. coli in dogs visiting rural community veterinary clinics in South Africa. Our results indicate that dogs visiting the veterinary rural community clinics under study are reservoirs and may be an important source of Campylobacter spp. for humans. However, a limitation of this study is that the dogs studied were not recruited randomly and the prevalence of Campylobacter presented in this study may not be a reflection of the larger dog population of South Africa. Future epidemiological and characterisation studies comparing dog and human Campylobacter spp. isolates are needed to establish the zoonotic potential of Campylobacter spp. carried by dogs in South Africa.
Acknowledgements
Funding from the National Research Foundation including the South Africa-Italy and South Africa-Kenya Joint Science and Technology Research Cooperation programmes, the Faculty of Veterinary Science, University of Pretoria, and the Gauteng Department of Agriculture and Rural Development is gratefully acknowledged.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
M.K., B.T.C.-G., A.K., J.N.O. and C.M. conceived and designed the study. A.P. and M.K. collected the samples and performed the research; E.E. and M.K. analysed the data. M.K. and S.E-A. wrote the article.
References
Acke, E., McGill, K., Golden, O., Jones, B.R., Fanning, S. & Whyte, P., 2009, 'Prevalence of thermophilic Campylobacter species in household cats and dogs in Ireland', Veterinary Record 164, 44-47. https://doi.org/10.1136/vr.164.2.44 [ Links ]
Allos, B.M. & Lastovica, A.J., 2008, 'Clinical significance of Campylobacter and related species other than Campylobacter jejuni and Campylobacter coli', in I. Nachamkin, C.M. Szymanski & M.J. Blaser (eds.), Campylobacter, 3rd edn., pp. 123-149, American Society for Microbiology Press, Washington, DC, viewed 28 June 2018, from http://www.asmscience.org/content/book/10.1128/9781555815554.ch07. [ Links ]
Aspinall, S.T., Wareing, D.R.A., Hayward, P.G. & Hutchinson, D.N., 1996, 'A comparison of a new Campylobacter selective medium (CAT) with membrane filtration for the isolation of thermophilic campylobacters including Campylobacter upsaliensis', Journal of Applied Bacteriology 80, 645-650. https://doi.org/10.1111/j.1365-2672.1996.tb03269.x [ Links ]
Baker, J., Barton, M.D. & Lanser, J., 1999, 'Campylobacter species in cats and dogs in South Australia', Australian Veterinary Journal 77, 662-666, viewed 15 July 2018, from http://doi.wiley.com/10.1111/j.1751-0813.1999.tb13159.x [ Links ]
Bojanić, K., Midwinter, A.C., Marshall, J.C., Rogers, L.E., Biggs, P.J. & Acke, E., 2017, 'Isolation of Campylobacter spp. from client-owned dogs and cats, and retail raw meat pet food in the Manawatu, New Zealand', Zoonoses and Public Health 64, 438-449, viewed 28 June 2018, from http://doi.wiley.com/10.1111/zph.12323 [ Links ]
Bourke, B., Chan, V.L. & Sherman, P., 1998, 'Campylobacter upsaliensis: Waiting in the wings', Clinical Microbiology Reviews 11, 440-449, viewed 06 July 2018, from http://www.ncbi.nlm.nih.gov/pubmed/9665977. [ Links ]
Burnens, A.P., Angéloz-Wick, B. & Nicolet, J., 1992, 'Comparison of Campylobacter carriage rates in diarrheic and healthy pet animals', Journal of Veterinary Medicine, Series B 39, 175-180. https://doi.org/10.1111/j.1439-0450.1992.tb01155.x [ Links ]
Carbonero, A., Torralbo, A., Borge, C., García-Bocanegra, I., Arenas, A. & Perea, A., 2012, 'Campylobacter spp., C. jejuni and C. upsaliensis infection-associated factors in healthy and ill dogs from clinics in Cordoba, Spain. Screening tests for antimicrobial susceptibility', Comparative Immunology, Microbiology and Infectious Diseases 35, 505-512, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/22640550. [ Links ]
Chaban, B., Musil, K.M., Himsworth, C.G. & Hill, J.E., 2009, 'Development of cpn60-based real-time quantitative PCR assays for the detection of 14 Campylobacter species and application to screening of canine fecal samples', Applied and Environmental Microbiology 75, 3055-3061. https://doi.org/10.1128/AEM.00101-09. [ Links ]
Chaban, B., Ngeleka, M. & Hill, J.E., 2010, 'Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals', BMC Microbiology 10, 73, viewed 06 July 2018, from http://www.ncbi.nlm.nih.gov/pubmed/20219122. [ Links ]
Couturier, B.A., Hale, D.V.C. & Couturier, M.R., 2012, 'Association of Campylobacter upsaliensis with persistent bloody diarrhea', Journal of Clinical Microbiology 50, 3792-3794. https://doi.org/10.1128/JCM.01807-12 [ Links ]
Damborg, P., Broens, E.M., Chomel, B.B., Guenther, S., Pasmans, F., Wagenaar, J.A., et al., 2016, 'Bacterial zoonoses transmitted by household pets: State-of-the-art and future perspectives for targeted research and policy actions', Journal of Comparative Pathology 155, S27-S40. https://doi.org/10.1016/j.jcpa.2015.03.004 [ Links ]
Endtz, H.P., Ruijs, G.J., Zwinderman, A.H., Van der Reijden, T., Biever, M. & Mouton, R.P., 1991, 'Comparison of six media, including a semisolid agar, for the isolation of various Campylobacter species from stool specimens', Journal of Clinical Microbiology 29, 1007-1010, viewed 16 July 2018, from http://www.ncbi.nlm.nih.gov/pubmed/2056033. [ Links ]
Engvall, E.O., Brändstrom, B., Andersson, L., Båverud, V., Trowald-Wigh, G. & Englund, L., 2003, 'Isolation and identification of thermophilic Campylobacter species in faecal samples from Swedish dogs', Scandinavian Journal of Infectious Diseases 35, 713-718, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/14606609. [ Links ]
Forbes, K.J. & Horne, J., 2009, 'The molecular epidemiology of Scottish Campylobacter isolates from human cases of infection using Multilocus Sequence Typing (MLST). CaMPS - Campylobacter MLST project in Scotland. Food Standards Agency Scotland Contract S14006', viewed 15 July 2018, from https://www.food.gov.uk/sites/default/files/media/document/339-1-595_CaMPS_S14006_Final_Report.pdf. [ Links ]
Friedman, C.R., Neimann, J., Wegener, H.C. & Tauxe, R.V., 2000, 'Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations', in I. Nachamkin & M.J. Blaser (eds.), Campylobacter, 2nd. edn., vol. II(6), pp. 121-138, American Society for Microbiology, Washington, DC. [ Links ]
Giacomelli, M., Follador, N., Coppola, L.M., Martini, M. & Piccirillo, A., 2015, 'Survey of Campylobacter spp. in owned and unowned dogs and cats in Northern Italy', Veterinary Journal 204, 333-337. https://doi.org/10.1016/j.tvjl.2015.03.017 [ Links ]
Guest, C.M., Stephen, J.M. & Price, C.J., 2007, 'Prevalence of Campylobacter and four endoparasites in dog populations associated with hearing dogs', Journal of Small Animal Practice 48, 632-637, viewed 28 June 2018, from http://doi.wiley.com/10.1111/j.1748-5827.2007.00367.x [ Links ]
Hald, B. & Madsen, M., 1997, 'Healthy puppies and kittens as carriers of Campylobacter spp., with special reference to Campylobacter upsaliensis', Journal of Clinical Microbiology 35, 3351-3352, viewed 24 April 2018, from http://www.ncbi.nlm.nih.gov/pubmed/9399557. [ Links ]
Hald, B., Pedersen, K., Wainø, M., Jørgensen, J.C. & Madsen, M., 2004, 'Longitudinal study of the excretion patterns of thermophilic Campylobacter spp. in young pet dogs in Denmark', Journal of Clinical Microbiology 42, 2003-2012, viewed 29 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/15131162. [ Links ]
Holmberg, M., Rosendal, T., Engvall, E.O., Ohlson, A. & Lindberg, A., 2015, 'Prevalence of thermophilic Campylobacter species in Swedish dogs and characterization of C. Jejuni isolates', Acta Veterinaria Scandinavica 57, 1-8. [ Links ]
Jacobs, B., Van Belkum, A. Endtz, H.P., 2008, 'Guillain-Barré syndrome and Campylobacter infection', in I. Nachamkin, C.M. Szymanski & M.J. Blaser (eds.), Campylobacter, 3rd edn., pp. 245-265, American Society for Microbiology Press, Washington, DC, viewed 15 July 2018, from http://www.asmscience.org/content/book/10.1128/9781555815554.ch13. [ Links ]
Jacobs-Reitsma, W., Lyhs, U. & Wagenaar, J., 2008, 'Campylobacter in the food supply', in I. Nachamkin, C.M. Szymanski & M.J. Blaser (eds.), Campylobacter, 3rd edn., pp. 627-644, American Society for Microbiology, Washington, DC. [ Links ]
Klena, J.D., Parker, C.T., Knibb, K., Claire Ibbitt, J., Devane, P.M.L., Horn, S.T., et al., 2004, 'Differentiation of Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis by a multiplex PCR developed from the nucleotide sequence of the lipid A gene lpxA', Journal of Clinical Microbiology 42, 5549-5557. https://doi.org/10.1128/JCM.42.12.5549-5557.2004 [ Links ]
Koene, M.G.J., Houwers, D.J., Dijkstra, J.R., Duim, B. & Wagenaar, J.A., 2004, 'Simultaneous presence of multiple Campylobacter species in dogs', Journal of Clinical Microbiology 42, 819-821, viewed 15 July 2018, from http://www.ncbi.nlm.nih.gov/pubmed/14766860. [ Links ]
Labarca, J.A., Sturgeon, J., Borenstein, L., Salem, N., Harvey, S.M., Lehnkering, E., et al., 2002, 'Campylobacter upsaliensis: Another pathogen for consideration in the United States', Clinical Infectious Diseases 34, e59-e60, viewed 28 June 2018, from https://academic.oup.com/cid/article-lookup/doi/10.1086/340266. [ Links ]
Lastovica, A.J. & Engel, M.E., 2001, 'Epidemiology of other Campylobacter species', in World Health Organization, The increasing incidence of human campylobacteriosis. Report and proceedings of a WHO consultation of experts, Copenhagen, Denmark, 21-25 November 2000, pp. 61-64, World Health Organization, Geneva. http://www.who.int/iris/handle/10665/67767. [ Links ]
Lastovica, A.J. & Le Roux, E., 2001, 'Efficient isolation of Campylobacter upsaliensis from stools', Journal of Clinical Microbiology 39, 4222-4223, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/11712518. [ Links ]
Leahy, A.M., Cummings, K.J., Rodriguez-Rivera, L.D., Hamer, S.A. & Lawhon, S.D., 2017, 'Faecal Campylobacter shedding among dogs in animal shelters across Texas', Zoonoses and Public Health 64, 623-627, viewed 28 June 2018, from http://doi.wiley.com/10.1111/zph.12356 [ Links ]
Leonard, E.K., Pearl, D.L., Janecko, N., Weese, J.S., Reid-Smith, R.J., Peregrine, A.S., et al., 2011, 'Factors related to Campylobacter spp. carriage in client-owned dogs visiting veterinary clinics in a region of Ontario, Canada', Epidemiology and Infection 139, 1531-1541. https://doi.org/10.1017/S0950268810002906 [ Links ]
Man, S.M., 2011, 'The clinical importance of emerging Campylobacter species', Nature Reviews Gastroenterology and Hepatology 8, 669-685, https://doi.org/10.1038/nrgastro.2011.191 [ Links ]
McOrist, S. & Browning, J.W., 1982, 'Carriage of Campylobacter jejuni in healthy and diarrhoeic dogs and cats', Australian Veterinary Journal 58, 33-34, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/7082228. [ Links ]
Mughini Gras, L., Smid, J.H., Wagenaar, J.A., De Boer, A.G., Havelaar, A.H., Friesema, I.H.M., et al., 2012, 'Risk factors for campylobacteriosis of chicken, ruminant, and environmental origin: A combined case-control and source attribution analysis', PLoS One 7(8), e42599. https://doi.org/10.1371/journal.pone.0042599 [ Links ]
Nachamkin, I., Allos, B.M. & Ho, T., 1998, 'Campylobacter species and Guillain-Barré syndrome', Clinical Microbiology Reviews 11, 555-567, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/9665983. [ Links ]
Nair, G.B., Sarkar, R.K., Chowdhury, S. & Pal, S.C., 1985, 'Campylobacter infection in domestic dogs', The Veterinary Record 116, 237-238, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/4002595. [ Links ]
Nakamura, I., Omori, N., Umeda, A., Ohkusu, K. & Matsumoto, T., 2015, 'First case report of fatal sepsis due to Campylobacter upsaliensis', Journal of Clinical Microbiology 53, 713-715, viewed 29 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/25411172. [ Links ]
Neimann, J., Engberg, J., Mølbak, K. & Wegener, H.C., 2003, 'A case-control study of risk factors for sporadic Campylobacter infections in Denmark', Epidemiology and Infection 130, 353-366, viewed 15 July 2018, from http://www.journals.cambridge.org/abstract_S0950268803008355. [ Links ]
Olson, P. & Sandstedt, K., 1987, 'Campylobacter in the dog: A clinical and experimental study', The Veterinary Record 121, 99-101, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/2821668. [ Links ]
Parsons, B.N., Porter, C.J., Ryvar, R., Stavisky, J., Williams, N.J., Pinchbeck, G.L., et al., 2010, 'Prevalence of Campylobacter spp. in a cross-sectional study of dogs attending veterinary practices in the UK and risk indicators associated with shedding', The Veterinary Journal 184, 66-70, viewed 28 June 2018, from https://www.sciencedirect.com/science/article/pii/S1090023309000124. [ Links ]
Parsons, B.N., Williams, N.J., Pinchbeck, G.L., Christley, R.M., Hart, C.A., Gaskell, R.M., et al., 2011, 'Prevalence and shedding patterns of Campylobacter spp. in longitudinal studies of kennelled dogs', The Veterinary Journal 190, 249-254, viewed 28 June 2018, from https://www.sciencedirect.com/science/article/pii/S1090023310003527. [ Links ]
Procter, T.D., Pearl, D.L., Finley, R.L., Leonard, E.K., Janecko, N., Reid-Smith, R.J., et al., 2014, 'A cross-sectional study examining Campylobacter and other zoonotic enteric pathogens in dogs that frequent dog parks in three cities in South-Western Ontario and risk factors for shedding of Campylobacter spp.', Zoonoses and Public Health 61, 208-218. https://doi.org/10.1111/zph.12062 [ Links ]
Rossi, M., Hänninen, M.L., Revez, J., Hannula, M. & Zanoni, R.G., 2008, 'Occurrence and species level diagnostics of Campylobacter spp., enteric Helicobacter spp. and Anaerobiospirillum spp. in healthy and diarrheic dogs and cats', Veterinary Microbiology 129, 304-314. https://doi.org/10.1016/j.vetmic.2007.11.014 [ Links ]
Sandberg, M., Bergsjø, B., Hofshagen, M., Skjerve, E. & Kruse, H., 2002, 'Risk factors for Campylobacter infection in Norwegian cats and dogs', Preventive Veterinary Medicine 55, 241-253, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/12392875. [ Links ]
Tee, W. & Mijch, A., 1998, 'Campylobacter jejuni bacteremia in Human Immunodeficiency Virus (HIV)-infected and non-HIV-infected patients: Comparison of clinical features and review', Clinical Infectious Diseases 26, 91-96, viewed 28 June 2018, from https://academic.oup.com/cid/article-lookup/doi/10.1086/516263. [ Links ]
Tenkate, T.D. & Stafford, R.J., 2001, 'Risk factors for Campylobacter infection in infants and young children: A matched case-control study', Epidemiology and Infection 127, 399-404, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/11811871. [ Links ]
Torre, E. & Tello, M., 1993, 'Factors influencing fecal shedding of Campylobacter jejuni in dogs without diarrhea', American Journal of Veterinary Research 54, 260-262, viewed 28 June 2018, from http://www.ncbi.nlm.nih.gov/pubmed/8430936. [ Links ]
Tsai, H.J., Huang, H.C., Lin, C.M., Lien, Y.Y. & Chou, C.H., 2007, 'Salmonellae and campylobacters in household and stray dogs in Northern Taiwan', Veterinary Research Communications 31, 931-939. https://doi.org/10.1007/s11259-007-0009-4 [ Links ]
Workman, S.N., Mathison, G.E., Marc, C. & Lavoie, M.C., 2005, 'Pet dogs and chicken meat as reservoirs of Campylobacter spp. in Barbados', Journal of Clinical Microbiology 43, 2642-2650. https://doi.org/10.1128/JCM.43.6.2642-2650.2005 [ Links ]
 Correspondence:
Correspondence:
Musafiri Karama
musafiri.karama@up.ac.za
Received: 23 July 2018
Accepted: 17 Jan. 2019
Published: 28 May 2019














