Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Onderstepoort Journal of Veterinary Research
versión On-line ISSN 2219-0635
versión impresa ISSN 0030-2465
Onderstepoort j. vet. res. vol.83 no.1 Pretoria 2016
http://dx.doi.org/10.4102/ojvr.v83i1.1170
ORIGINAL RESEARCH
Cellular immune responses induced in vitro by Ehrlichia ruminantium secreted proteins and identification of vaccine candidate peptides
Nontobeko ThemaI, II; Alri PretoriusI, II; Selaelo I. TshilwaneI; Junita LiebenbergI; Helena SteynI; Mirinda van KleefI, II
INew Generation Vaccines Programme, Agricultural Research Council- Onderstepoort Veterinary Institute, South Africa
IIDepartment of Veterinary Tropical Diseases, University of Pretoria, South Africa
ABSTRACT
Secreted proteins are reported to induce cell-mediated immunity characterised by the production of interferon-gamma (IFN)-γ. In this study three open reading frames (ORFs) (Erum8060, Erum7760, Erum5000) encoding secreted proteins were selected from the Ehrlichia ruminantium (Welgevonden) genome sequence using bioinformatics tools to determine whether they induce a cellular immune response in vitro with mononuclear cells from needle and tick infected animals. The whole recombinant protein of the three ORFs as well as four adjacent fragments of the Erum5000 protein (Erum5000A, Erum5000B, Erum5000C, Erum5000D) were successfully expressed in a bacterial expression system which was confirmed by immunoblots using anti-His antibodies and sheep sera. These recombinant proteins were assayed with immune sheep and cattle peripheral blood mononuclear cells (PBMCs), spleen and lymph node (LN) cells to determine whether they induce recall cellular immune responses in vitro. Significant proliferative responses and IFN-γ production were evident for all recombinant proteins, especially Erum5000A, in both ruminant species tested. Thus overlapping peptides spanning Erum5000A were synthesised and peptides that induce proliferation of memory CD4+ and CD8+ T cells and production of IFN-γ were identified. These results illustrate that a Th1 type immune response was elicited and these recombinant proteins and peptides may therefore be promising candidates for development of a heartwater vaccine.
Introduction
Heartwater, a disease of cattle, sheep, goats and wild ruminants is caused by an intracellular rickettsiales, Ehrlichia (Cowdria) ruminantium (Dumler et al. 2001; Moshkovski 1947). Heartwater affects sub-Saharan Africa and the Caribbean where it is transmitted by ticks of the genus Amblyomma. The only commercial vaccine against heartwater is a live blood vaccine that requires antibiotic treatment to prevent a serious course of the disease (Van der Merwe 1987). It is, however, risky to use in non-endemic areas where the vector resides because it contains viable organisms. It also requires a cold chain for storage and distribution and does not protect against all isolates (Collins et al. 2003). Attempts to develop an alternative to the blood vaccine have involved inactivated (Martinez et al. 1994), attenuated (Zweygarth et al. 2005) and recombinant DNA vaccines that included genes coding for the major antigenic protein 1 (MAP1) (Nyika et al. 2002), low molecular weight proteins of E. ruminantium (Sebatjane et al. 2010), or a cocktail of four 1H12 E. ruminantium open reading frames (ORFs) (Pretorius et al. 2007, 2008). When a cocktail of the four 1H12 E. ruminantium ORFs was tested in sheep as a DNA vaccine as well as a DNA vaccine prime or recombinant protein boost, it elicited complete protection after needle challenge. However, only limited protection was achieved with these vaccines when the animals were challenged by tick infestation. Therefore, it is necessary that additional ORFs need to be identified in order to improve the efficacy of a recombinant heartwater vaccine.
Approaches used to identify vaccine candidates for recombinant vaccine development are, in general, guided by the type of immune responses that are likely to mediate protection. It is known that a cellular T helper 1 (Th1) immune response is fundamental in destruction of intracellular pathogens like E. ruminantium (Totté et al. 1999). Th1 responses are mediated by the cytokine interferon-gamma (IFN-γ), which is expressed primarily by CD4+ helper T cells and cytotoxic CD8+ T cells. Thus, identification of antigens that induce similar responses is needed for evaluation as immunogenic agents. Several E. ruminantium proteins have already been investigated for their ability to induce cellular immune responses in vitro. Five ORFs were selected from the E. ruminantium genome and their ability to induce proliferative responses and IFN-γ production was evaluated in vitro (Sebatjane et al. 2010). All five recombinant proteins induced proliferation of immune peripheral blood mononuclear cells (PBMCs) and IFN-γ production. The corresponding five genes were each individually incorporated into pCMViUBs, mammalian expression vector and tested as a potential vaccine in sheep using a DNA prime-protein boost immunisation regimen. A cocktail of these DNA constructs protected one out of five sheep against a virulent E. ruminantium (Welgevonden) needle challenge. Similarly, using a reverse vaccinology strategy, recombinant proteins that induced a cellular immune response in immune bovine and ovine PBMCs characterised by the induction of Th1 cytokines that includes, IFN-γ, iNOS, GM-CSF and TNF-α were identified (Liebenberg et al. 2012). These studies all used needle infected animals to screen for protective antigens. However, indications are that antigens that induce protection against natural and not artificial challenge should be identified for inclusion in a DNA vaccine. A successful DNA vaccine may therefore need to contain a combination of recombinant proteins which induce cell-mediated immunity to ensure protection against heartwater.
One of the complicating factors is that whole proteins from pathogens may contain epitopes, discrete sites recognised by lymphocytes presented in combination with major histocompatibility complex (MHC), which inhibit protective immune responses or induce immunopathology (Wang et al. 2005). Hence, these negative effects can be avoided if T cell epitopes that specifically stimulate immune responses are identified. Including only the protective epitopes from multiple antigens and discarding the unnecessary sequence will also allow efficient use of limited space needed to package numerous antigens in a subunit vaccine. Research directed at elucidating the epitopes of selected E. ruminantium proteins will provide a better understanding of which fragment of the protein is immunogenic for incorporation into a multivalent vaccine. Furthermore, to ensure that the vaccine protects under field conditions, several immunogenic epitopes that are associated with cell-mediated immunity need to be identified and characterised using mononuclear cells from tick immune animals.
In addition to several E. ruminantium promising vaccine targets which function extremely well as DNA vaccines under experimental conditions, secreted proteins have been shown to be of particular relevance as protective antigens against several pathogens including Chlamydia muridarum (Murthy et al. 2007), Ehrlichia canis and Ehrlichia chaffeensis (Doyle et al. 2006), Mycobacterium tuberculosis (Langermans et al. 2005) and Anaplasma marginale (Leal et al. 2000). These antigens have been reported to induce cell-mediated and humoral immunity characterised by the production of IFN-γ and antibodies. This indicates that secreted proteins may be potential vaccine candidates and warrants further investigation. In this study we therefore investigated whether secreted E. ruminantium recombinant proteins and peptides would induce similar immune responses in vitro by PBMC from needle infected and challenged (NI) and tick infected and challenged (TI) animals.
Materials and methods
Expression of recombinant proteins
Ehrlichia ruminantium genes of putatively secreted proteins were identified in silico using bioinformatics algorithms as described previously (Liebenberg et al. 2012). Protein expression was performed using the pET102/TOPO® expression system (Invitrogen) according to the instructions of the manufacturer. Briefly, the whole E. ruminantium ORFs Erum8060 (0.6 kb), Erum7760 (0.75 kb) and Erum5000 (1.47 kb) as well as the equally divided four 348 bp adjacent fragments (Erum5000A, Erum5000B, Erum5000C, Erum5000D) were PCR amplified using specifically designed primers (Table 1-A1). Plasmids of the expected size were sequenced to confirm the presence of inserts and that the ORFs were in-frame. Recombinant (His6-tagged) proteins were purified from soluble supernatant or the inclusion bodies using the Protino® Ni 150 prepacked columns kit (Macherey-Nagel) according to the instructions of the manufacturer. The purified proteins were assayed using SDS-PAGE analysis, Western blot analysis using anti-His6 antibodies (Roche) and heartwater immune sheep sera (Figure 1-A1, Supporting information). The recombinant proteins were acetone precipitated for use in immune assays as previously described (Van Kleef et al. 2002). The concentration of the recombinant proteins was determined using the Pierce™ BCA Protein Assay Kit (Thermo Scientific).
Synthesis of peptides
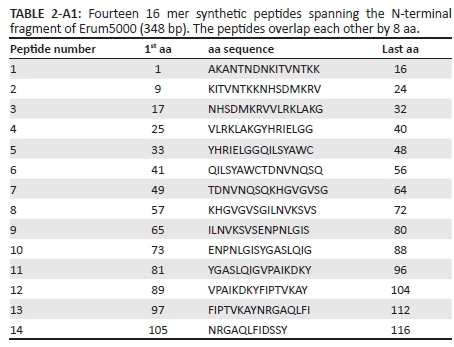
Sixteen mer peptides overlapping by eight amino acids spanning the N-terminal fragment of Erum5000 (Erum5000A) (~348bp) (Table 2-A1) were synthesised by Genscript (USA) and the purity of the peptides were > 98% as analysed by high-performance liquid chromatography. The peptides were dissolved in water or 100% dimethyl sulfoxide to 1 mg/mL and stored at -20 °C. Peptides were further diluted to 100 µg/mL in complete medium prior to use in immunological assays which include lymphocyte proliferation, IFN-γ Enzyme-Linked ImmunoSpot (ELISPOT) assay and flow cytometry.
Immunological assays
Inoculation of animals as source of immune mononuclear cells
Six- to eight-month-old merino sheep (s147, s6010) and three heartwater naïve Nguni cattle (b8460, b8347 and b8404) were immunised by the needle infection and treatment method and needle challenged as described previously (Liebenberg et al. 2012). In addition to this, to mimic field or natural immunisation, four sheep (s6355, s6821, s6822, s6823) were tick infected and treated with ticks infected with the Welgevonden strain in the laboratory as described previously (Mahan et al. 1998) with some modifications. Briefly, uninfected Amblyomma hebraeum nymph ticks were infected by feeding on a sheep that had been infected intravenously with E. ruminantium Welgevonden stock. Engorged nymphs were allowed to moult to adults in the laboratory. A sheep was then infected by feeding 10 adults (5 males and 5 females) heartwater infected ticks on it. The sheep was monitored daily for the onset of clinical signs and treated on the third day of febrile reaction with Terramycin®100 (Pfizer). The sheep were tick challenged with the Welgevonden infected ticks. Heartwater infection of ticks and sheep were confirmed by pCS20 real-time PCR (Steyn et al. 2008). All animal research was performed in accordance with the stipulations of the animal ethics committee at the ARC Onderstepoort Veterinary Institute and the University of Pretoria animal use and care committee and Section 20 approval from Department of Agriculture, Forestry and Fisheries.
Purification of peripheral blood mononuclear cells
PBMCs were purified from whole blood under sterile conditions. Briefly, blood was collected in BD (Becton, Dickinson) Vacutainer®- Ethylenediaminetetraacetic acid (EDTA) tubes (Becton, Dickinson) and PBMCs were isolated by density gradient centrifugation (Histopaque®-1077; Sigma-Aldrich®) as described by Liebenberg et al. (2012). The cells were washed three times and counted using TC10™ Automated cell counter (BioRad) and the cells resuspended (4 × 106 cells/mL) in complete medium, RPMI-1640 (GIBCO® RPMI + GlutaMAXTM-I) (Invitrogen) supplemented with 10% foetal bovine serum (FBS), 55 mM 2-mercaptoethanol and 1% GIBCO® Pen Strep (Invitrogen).
Lymphocyte proliferation assay
Lymphocyte proliferation assays (LPA) were carried out in triplicate wells as described by van Kleef et al. (2000). Responder cells at a final concentration of 2 x 106 PBMC/mL were added to respective test wells together with one of the following: E. ruminantium crude antigen (1 µg/mL, positive control), ConA (positive control), E. ruminantium recombinant proteins (10 µg/mL), synthetic peptides (10 µg/mL), Erum5000A (positive control for peptides), E. ruminantium recombinant protein that tested negative previously (rErum4930, rNegative, negative control) or medium only (unstimulated PBMCs). Proliferation was determined by measuring the incorporation of 0.5 µCi of [methyl-3H] thymidine added during the final 18 h of the assay using a scintillation counter. Results are presented as a stimulation index (SI) ± standard deviation (s.d.), where SI is the mean counts per min (cpm) of cells stimulated with antigen divided by mean cpm of unstimulated cells. Only SI two times higher than the negative recombinant protein and p ≤ 0.05 was considered to be significant antigen-specific proliferation.
IFN-γ ELISPOT assay
The ELISPOT assay was performed as described by (Sebatjane et al. 2010). Briefly, PBMCs (2 x 106 PBMC/mL) were seeded in ELISPOT 96 well plates (Millipore MAIPS 4510) precoated with mouse anti-bovine IFN-γ mAb CC302 coating antibody (1 μg/mL). PBMCs were stimulated with recombinant protein, peptides and controls as described for LPA. The plates were developed after 48 h incubation at 37 ºC in a humidified 5% CO2 incubator. The number of spots per million cells (spmc) of the antigen was compared to the number of spmc of the corresponding negative control. Only the number of spmc that were two times higher than the negative control and with a significant p value (p ≤ 0.05) as determined by Student's t-test were regarded as positive. Additionally, for peptide responses only samples with at least 10 spmc were considered positive.
Cell surface staining
Immune PBMCs (2 x 106 cells/mL) were stimulated with antigens for 48 h at 37 °C. The cells were stained with the following commercial monoclonal antibodies: CD4 (IgM, cell line GC50A), CD8 (IgG1, cell line CACT80C) and CD45RO (IgG3, cell line ILA116A) (Washington State University Monoclonal Antibody Centre, Pullman, WA) at a 1:100 dilution in PN buffer (PBS, 0.5% FBS containing 0.2% sodium azide). Following washing secondary antibodies goat anti-mouse IgM-APC (Invitrogen), goat anti-mouse IgG1-PE (Serotec) and goat anti-mouse IgG3-FITC (Serotec) were added at dilutions of 1:10, 1:40 and 1:10 respectively. All incubations were for 15 min at room temperature and washing was done twice with PN buffer. Cells were fixed with 0.2% formaldehyde in PBS. Samples were assayed on a FC 500 Beckman Coulter flow cytometer and data analysed using the Kaluza software version 1.2 (Beckman Coulter).
Intracellular IFN-γ staining
Immune PBMCs were purified from s6821 and s6823 for intracellular IFN-γ staining using the BD Cytofix/Cytoperm™ Kit (BD Biosciences) and protocol. Briefly cells (2 x 106 cells/mL) were incubated for 72 h at 37 °C in the presence or absence of 10 µg/mL peptide or recombinant protein. Golgi stop solution was added 4 h prior to harvesting. Cells were first surface stained as described above and subsequently, intracellular IFN-γ staining was performed with fluorochrome-conjugated anti-cytokine antibody (Alexa fluor®488, Serotec) at a dilution of 1:20. The cells were incubated at 4 °C for 30 min in the dark, followed by washing with the supplied buffers. The cells were analysed with an FC 500 Beckman Coulter flow cytometer and data analysed using the Kaluza software version 1.2 (Beckman Coulter).
Major histocompatibility complex typing of experimental animals
Samples of genomic DNA of animals used in this study were obtained from whole blood collected in BD Vacutainer® K2E tubes containing EDTA. Genomic DNA purification was done using the Generation®Capture Column Kit (Gentra systems) according to the instructions of the manufacturer. Typing for Ovine MHC Ovar-DRB1 and BoLA-DRB3 was performed using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) as described by Konnai et al. (2003) and Van Eijk, Steward-Haynes and Lewin (1992), respectively. Briefly, the second exon of Ovar-DBR1 and BoLA-DRB3 was amplified by nested PCR using different primers. The resulting DNA (nested PCR product) was digested overnight at 37 °C with 5 U of either RsaI, HaeIII, PsuI, SacI, SacII, DdeI, NciI, Hin1I or EcoRI restriction enzymes (Roche), or at 60 °C with 5 U of BstNI. However, DRB RFLP patterns could not be distinguished and thus the nested PCR products were cloned to pGEM®-T Easy vector (Promega) and sequenced. The restriction patterns obtained were compared with published restriction maps (Konnai et al. 2003; Van Eijk et al. 1992).
Statistical analysis
The significance of differences between immunological assay results was determined by means of the Student's t-test. Differences with p ≤ 0.05 were considered significant.
Results
Expression and purification of recombinant proteins
Three ORFs (rErum5000, rErum7760, rErum8060) were chosen randomly out of a total of 24 exported proteins previously predicted by bioinformatics (Table 1, Liebenberg et al. 2012). They were successfully expressed in an Escherichia coli BL21Star™ (DE3) host strain in conjunction with the pET102/D-TOPO® vector. Recombinant rErum8060 and Erum7760 were purified from both the soluble (s) and insoluble (i) fractions while rErum5000 was found to be insoluble and showed distinct bands at ~36 kDa, ~42 kDa and ~70 kDa respectively in SDS-PAGE and in Western blot assays that corresponded to their predicted molecular weights. In addition, heartwater immune sheep sera could detect rErum7760 and -8060 in both the soluble and insoluble fractions while rErum5000 could only be recognised in the insoluble fraction (Figure 1-A1, Supporting information).
Proliferation induced by recombinant proteins
No proliferation was induced in PBMC from the naïve s5408. Each recombinant protein induced significant proliferation in PBMC from a different sheep (Erum8060, TI s6355; Erum7760, NI s6010; Erum5000, NI s147; Table 2). Varying but significant proliferation was induced by all rproteins with PBMC from NI bovine (Table 3). When spleen cells from NI and TI sheep and NI bovine were used, Erum5000 performed the best out of the three rproteins with a SI of 21 with lymph node cells from TI s6355. All three rproteins induced the highest significant proliferation with spleen and lymph node cells from TI s6355 compared to NI s6010. Similarly, ovine spleen and lymph node cells responded with higher SI than PBMC from the same animal.
IFN-γ responses induced by recombinant proteins
No IFN-γ was induced in PBMC from the naïve s5408 (Table 4). The highest significant response was induced by Erum5000 with PBMC from TI s6822 (200 spmc) and lymph node cells from TI s6355 (272 spmc) (Table 4). This was followed by Erum8060 with PBMC from NI s147 (101 spmc) and spleen cells from TI s6355 (88 spmc). Ovine spleen and lymph node cells responded with higher spmc than PBMC from the same animal. Varying but significant proliferation was induced by Erum5000 rprotein with PBMC from NI bovine (Table 4). Erum8060 and Erum7760 only induced IFN-γ by PBMC from two bovine.
Expression and purification of four recombinant protein fragments of Erum5000
Because rErum5000 induced significant and reproducible results, it was divided into four to provide a better understanding of which fragment of the protein is immunogenic for epitope mapping. Ehrlichia ruminantium ORF Erum5000 (~1.39 kb) was PCR amplified into four consecutive ~348 bp fragments (Erum5000A, Erum5000B, Erum5000C, Erum5000D) using specifically design primers (Table 1-A1). The corresponding recombinant proteins were produced in an E. coli Topo tools expression system resulting in Thioredoxin or His-tag fusion proteins with distinct bands at 34 kDa (Figure 1-A1).
Proliferation and IFN-γ secretion of peripheral blood mononuclear cells s in response to four fragments of rErum5000
PBMCs from NI bovine (b8374, b8404, b8460), sheep (s147, s6010), TI sheep (s6355, s6821, s6822, s6823) and a naïve sheep (s5408) were stimulated with the four fragment proteins, Erum5000A, Erum5000B, Erum5000C and Erum5000D. All four rproteins induced immune bovine PBMCs to proliferate with SI ranging from ~2 to 18 (Table 3). In correlation with the proliferation results, PBMCs from the naïve sheep (s5408) and one NI sheep (s6010) did not produce IFN-γ after they had been incubated with proteins (Table 4). All proteins tested with immune bovine PBMCs induced significant production of IFN-γ (Table 3). The highest magnitude of IFN-γ responses was obtained when tested with TI sheep PBMCs (s6821, s6822 and s6823). Erum5000A induced the highest IFN-γ responses in all animals tested. Erum5000D and Erum5000C induced highest IFN-γ production in sheep as compared to Erum5000B. However, Erum5000B induced reproducible immune responses in both cattle and sheep. Hence Erum5000A and Erum5000B fragments were further tested for their ability to induce immune responses in spleen and lymph nodes of TI and NI sheep. Both fragments specifically induced immune cells from spleen and lymph node to secrete IFN-γ. Once again ovine spleen and lymph node cells responded with higher spmc than PBMC from the same animal.
Peptide-specific lymphocyte proliferation and IFN-γ production
Significant proliferation and IFN-γ secretion after Erum5000A stimulation provided the rationale for mapping Erum5000A peptides for vaccine development. Thus fourteen 16-mer peptides (P1-P14) overlapping by eight amino acids were synthesised to determine the exact minimal epitope sequence that can induce recall cellular immune responses. All peptides induced significant IFN-γ with spmc ranging from ~20 to 140 when tested with PBMC from TI sheep (Figure 1) and ranged from 2 spmc to 160 spmc for cattle (Table 5). The peptides that induced the best recall responses were P9 and P14 in TI sheep and P7 and P10 in cattle.
Determination of T cell subsets responsive to peptides of Erum5000A
In an attempt to assess phenotype of PBMCs responsive to 14 Erum5000A peptides PBMCs from three sheep (s6821 s6822 and s6823) were used. CD4+ and CD8+ T cells expressing memory markers (CD45RO+) were measured by flow cytometry after 48 h stimulation. The percentages of CD4+ T cells expressing CD45RO+ was significantly induced by all peptides and varied between s6821 and s6822 with P2, P6, P8 and P14 showing best results (Figure 2). Whereas for PBMCs from s6823, only peptides P9 and P13 induced significant CD4+ T cells expressing CD45RO+. Similarly, the percentage of CD8+ T cells expressing memory markers (CD45RO+) was remarkably high and varied between sheep. Peptide P8 (aa 57-72) induced a high percentage of CD8+ T cells expressing memory markers (CD45RO+) in all three sheep followed by P14 (aa 105-116).
Intracellular IFN-γ staining analyses revealed that both CD4+ and CD8+ T cells from sheep 6821 produced high amounts of IFN-γ in response to P6, P8 and P11. Similarly, CD4+ and CD8+ T cells from sheep 6823 produced high amounts of IFN-γ in response to P8 (Table 6). In the case of other peptides lower IFN-γ production by both subsets was evident. These results also indicate that CD8+ T cells are the main producer of IFN-γ in the presence of P8.
PCR-RFLP typing of the Ovar-DRB1 and BoLA-DRB3 genes from sheep
The polymorphism of the ovine MHC class II DRB1 second exon (Ovar-DRB1, Konnai et al. 2003) was studied in four sheep, (s147, s6821 s6822 and s6823) by PCR-RFLP and BoLA-DRB3 for three bovine (b8347, b8460 and b8404). When each amplified DNA product was cleaved by the restriction enzymes, different patterns were observed. Patterns, Ovar-DRB1 and BoLA-DRB3 alleles based on restriction endonuclease enzyme digestion are listed (Tables 7 and 8). Three different alleles (*0332 and *0323, *0333) were obtained for sheep s147. Sheep s6821 and s6823 has two different sets of alleles (*0109 and *0201) and (*0201 and *0801) respectively. Whereas s6822 has homologous alleles (*0201, *0201). The alleles identified for s6821, s6822 and s6823 were also reported by Konnai et al. (2003). For bovine, only one allele was similar to alleles reported by van Eijk et al. (1992) and the remaining alleles were not present in any of the published work. Therefore, new and unknown alleles were found in this study.
Discussion
T cell responses characterised by the expression of IFN-γ are essential in protection against E. ruminantium infection (Totté et al. 1999). A DNA vaccine with four 1H12 ORFs induced IFN-γ and protection in sheep against a needle challenge with E. ruminantium Welgevonden but not against a tick challenge (Pretorius et al. 2008). Subsequently genome-based high through put technologies were then exploited to study E. ruminantium immunogenic antigens in an effort to improve this vaccine. Many E. ruminantium proteins of unknown function, and some of the membrane-associated proteins, secreted, transporters, particularly the ABC transport system and proteases were identified and immune characterised. Expressed recombinant proteins were assayed using NI immune sheep or bovine PBMC and they induced recall T cell responses characterised by elevation of IFN-γ in vitro and in vivo (Sebatjane et al. 2010), IFN-γ, TNF-α, GM-CSF, iNOS and TLR4 expression (Liebenberg et al. 2012).
These studies all used NI animals to screen for protective antigens. However, indications are that antigens that induce protection against natural and not artificial challenge should be identified for inclusion in a DNA vaccine. In this study we therefore investigated whether secreted E. ruminantium recombinant proteins would induce similar immune responses in vitro by PBMC from NI and TI animals. From a total of 27 secreted proteins (Liebenberg et al. 2012) three were selected for this study. Secreted proteins are regarded as promising vaccine candidates based on their ability to induce cell-mediated immunity characterised by production of IFN-γ (Carlisle et al. 2007). These proteins were successfully expressed in E. coli. They induced varied but significant proliferative responses and production of IFN-γ in PMBC from most animals tested. Recombinant proteins Erum5000 and Erum7760 induced the best response in PBMC from TI sheep whereas Erum8060 induced the best results in PBMC from NI sheep. The responses induced by all three proteins were also greater in cells from spleen and lymph nodes than PBMC from TI sheep. This is expected because these compartments have larger numbers of lymphocytes than PBMC improving the chance for antigen encounter with specific T cells (Blum & Pabst 2007).
The difference in immune responses obtained also suggests that the lymph node and spleen T cells may have distinct subsets of T lymphocytes (Langeveld, Gamadia & Ten Berge 2006). In addition, the spleen and LN cells of the tick infected animal produced the highest levels of IFN-γ protein, whereas the LN cells isolated from the needle infected animal remained unresponsive. This may be linked to the two different routes of infection that led to different homing patterns for the respective memory cells. This highlights the necessity to use TI animals to search for vaccine candidate antigens.
In addition to the cellular immune response, serum of NI immune animal detected the recombinant proteins (Figure 1-A1, supporting information) confirming that all proteins were secreted and exposed to the humoral immune system of the host during infection. However, when measuring IL-4 (associated with Th2 type responses) IL-4 production was not detected (results not shown) thus the antibodies detected could be opsonising antibodies (IgG2) associated with a Th1 response. Alternatively, the proteins may contain cross-reacting epitopes of a protein that induces a humoral immune response. It has been indicated that antibody-mediated immunity for intracellular parasites is not unusual (Doyle et al. 2006). Because the recombinant protein Erum5000 induced the best overall response it was equally divided and four recombinant fragment proteins Erum5000A, Erum5000B, Erum5000C and Erum5000D were expressed. Similar proliferative responses and production of IFN-γ were induced by the fragments in PBMCs isolated from the animals. However, the N-terminal fragment of Erum5000 rprotein Erum5000A induced the highest recall responses and was selected for epitope mapping.
Epitope mapping for this protein was done to identify vaccine candidates for incorporation into a multivalent vaccine. Epitope mapping of Erum5000 was determined using 14 overlapping peptides (P1-P14) spanning the fragment and PBMCs from NI cattle and TI sheep. The peptides that induced the best response in both cattle and sheep were P3, P5, P8 and P14. These peptides induced memory CD4+ and in particular CD8+ to proliferate and secrete IFN-γ.
Each recombinant protein induced immune responses and it varied between animals. Similarly, peptides were recognised to various extents by immune animals. This can be expected because out bred animals were used for these assays. It has been indicated previously that MHC class II molecules are highly polymorphic and different alleles vary in their peptide binding specificity (Groothuis et al. 2005; Sommer 2006). The results obtained from MHC typing using ovine MHC II DRB1 and bovine BoLA II-DRB3 and PCR-RFLP highlighted the diversity between the animals because different alleles were found for each animal. This may also explain the variation of responses between sheep and bovine PBMCs. Nevertheless, the data confirm that heartwater immune sheep and cattle were exposed to the proteins and peptides during infection and that recall immune responses developed in the hosts.
Both CD4+ and CD8+ T cells are IFN-γ-producing lymphocytes that are required in the development of protective immunity against heartwater. The CD4+ T cells play a major role to both cell-mediated and humoral immunity and act through the production of IFN-γ, as helper cells for immunoglobulin secretion and as effector cells to activate macrophages. Cytotoxic CD8+ T cells limit bacteria replication by killing infected cells or by releasing cytokines that mediate intracellular killing of the pathogen through the production of iNOS (Yewdell & Haeryfar 2005). Previously, T cell growth factors (Mahan, Smith & Byrom 1994) and IFN-γ (Totté et al. 1996) have been shown to inhibit E. ruminantium growth in vitro. The effect of IFN-γ may be because of upregulation of MHC class I and II expression on monocytes leading to increased antigen presentation to immune cells, or by increased phagocytosis, reactive oxygen intermediates, nitric oxide and lysosomal enzyme production. Hence the role of both CD4+ and CD8+ T cells, and IFN-γ production in the development of protective immunity against heartwater should be a high priority.
In conclusion, we have demonstrated that predicted secreted E. ruminantium proteins induced a more robust Th1 type response by TI than NI animals, as indicated by proliferation of mononuclear cells (blood, spleen and lymph node) and higher production of IFN-γ. Furthermore, epitope mapping of Erum5000A identified four peptides that were recognised by immune PBMCs from both NI cattle and TI sheep. These epitopes require further investigation as components for inclusion in the multiepitope DNA vaccine against heartwater.
Acknowledgements
We are very grateful to Dr Erich Zweygarth and Antoinette Josemans for providing culture material for the purification of E. ruminantium elementary bodies and extraction of DNA and Ms E. Faber for DNA sequencing. This study was supported by Joy Liebenberg Trust Fund and South African Department of Science and Technology.
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Authors' contributions
N.T. planned the study and did most of the experimental lab work, the data analyses as well as the preparation of manuscript. A.P. was the study supervisor, contributed to the planning of the study, the data analyses and the editing of the manuscript. S.I.T. contributed to the experimental lab work and the editing of manuscript; J.L. was responsible for the selection of vaccine candidates using bioinformatics tools and contributed to the editing of the manuscript; H.S. was responsible for the immunisation of the animals and contributed to the editing of the manuscript; M.v.K. is a programme manager and contributed to the editing of the manuscript.
References
Blum, K.S. & Pabst, R., 2007, 'Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs?', Immunology Letters 108(1), 45-51. http://dx.doi.org/10.1016/j.imlet.2006.10.009 [ Links ]
Carlisle, J., Evans, W., Hajizadeh, R., Nadaf, M., Shepherd, B., Ott, R.D. et al., 2007, 'Multiple Mycobacterium antigens induce interferon-γ production from sarcidosis peripheral blood mononuclear cells', Clinical and Experimental Immunology 150(3), 460-468. http://dx.doi.org/10.1111/j.1365-2249.2007.03510.x [ Links ]
Collins, N.E., Pretorius, A., Van Kleef, M., Brayton, K.A., Allsopp, M.T., Zweygarth, E. et al., 2003, 'Development of improved attenuated and nucleic acid vaccines for heartwater', Developments in Biologicals 114, 121-36. [ Links ]
Doyle, C.K., Nethery, K.A., Popov, V.L. & Mcbride, J.W., 2006, 'Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats', Infection and Immunity 74(1), 711-720. http://dx.doi.org/10.1128/IAI.74.1.711-720.2006 [ Links ]
Dumler, J.S., Barbet, A.F., Bekker, C.P., Dasch, G.A., Palmer, G.H., Rikihisa, S.C. et al., 2001, 'Reorganisation of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia, and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia, equi and "HGE agent" as subjective synonyms of Ehrlichia, phagocytophila', International Journal of Systematic and Evolutionary Microbiology 51, 2145-2165. http://dx.doi.org/10.1099/00207713-51-6-2145 [ Links ]
Groothuis, T.A.M., Griekspoor, A.C., Neijssen, J.J., Herberts, C.A. & Neefjes, J.J., 2005, 'MHC class I alleles and their exploration of the antigen-processing machinery', Immunological Reviews 207(1), 60-76. http://dx.doi.org/10.1111/j.0105-2896.2005.00305.x [ Links ]
Konnai, S., Nagaoka, Y., Takesima, S., Onuma, M. & Aida, Y., 2003, 'Technical note: DNA typing for Ovine MHC DRB1 using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP)', Journal of Dairy Science 86(10), 3362-3365. http://dx.doi.org/10.3168/jds.S0022-0302(03)73939-3 [ Links ]
Langermans, J.A., Doherty, T.M., Vervenne, R.A., Van Der Laan, T., Lyashchenko, K., Greenwald, R. et al., 2005, 'Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6', Vaccine 23(21), 2740-2750. http://dx.doi.org/10.1016/j.vaccine.2004.11.051 [ Links ]
Langeveld, M., Gamadia, L.E. & Ten Berge, I.J.M., 2006, 'T-lymphocyte subset distribution in human spleen', European Journal of Clinical Investigation 36(4), 250-256. http://dx.doi.org/10.1111/j.1365-2362.2006.01626.x [ Links ]
Leal, M., Noda, A., Reyna-Bello, A., Casas, B., Précigout, E., Aso, P.M. et al., 2000, 'Identification and characterization of corpuscular, soluble and secreted antigens of a Venezuelan isolate of Anaplasma marginale', Veterinary Parasitology 94(1-2), 1-15. http://dx.doi.org/10.1016/S0304-4017(00)00371-X [ Links ]
Liebenberg, J., Pretorius, A., Faber, F.E., Collins, N.E., Allsopp, B.A. & Van Kleef, M., 2012, 'Identification of Ehrlichia ruminantium proteins that activate cellular immune responses using a reverse vaccinology strategy', Veterinary Immunology and Immunopathology 145(1-2), 340-349. http://dx.doi.org/10.1016/j.vetimm.2011.12.003 [ Links ]
Mahan, S.M., Smith, G.E. & Byrom, B., 1994, 'Concanavalin a stimulated bovine T-cell supernatants inhibit growth of Cowdria ruminantium in bovine endothelial cells in vitro', Infection and Immunity 62(2), 747-750. [ Links ]
Mahan, S.M., Kumbula, D., Burridge, M.J. & Barbet, A.F., 1998, 'The inactivated Cowdria ruminantium vaccine for heartwater protects against heterologous strains and against laboratory and field tick challenge', Vaccine 16(11-12), 1203-1211. http://dx.doi.org/10.1016/S0264-410X(98)80120-5 [ Links ]
Martinez, D., Maillard, J.C., Coisue, S., Sheikboudou, C. & Bensaid, A., 1994, 'Protection of goats against heartwater acquired by immunisation with inactivated elementary bodies of Cowdria ruminantium', Veterinary Immunology and Immunopathology 41(1-2), 153-163. http://dx.doi.org/10.1016/0165-2427(94)90064-7 [ Links ]
Moshkovski, S.D., 1947, 'Comments by readers', Science 106, 62. http://dx.doi.org/10.1126/science.106.2742.62 [ Links ]
Murthy, A.K., Chambers, J.P., Meier, P.A., Zhong, G. & Arulanandam, B.P., 2007, 'Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production', Infection and Immunity 75(2), 666-676. http://dx.doi.org/10.1128/IAI.01280-06 [ Links ]
Nyika, A., Barbet, A.F., Burridge, M.J. & Mahan, S.M., 2002, 'DNA vaccination with map 1 gene followed by protein boost augments protection against challenge with Cowdria ruminantium, the agent of heartwater', Vaccine 20(7-8), 1215-1225. http://dx.doi.org/10.1016/S0264-410X(01)00430-3 [ Links ]
Pretorius, A., Collins, N.E., Steyn, H.C., Van Strijp, F., Van Kleef, M. & Allsopp, B.A., 2007, 'Protection against heartwater by DNA immunization with four Ehrlichia ruminantium open reading frames', Vaccine 25(12), 2316-2324. http://dx.doi.org/10.1016/j.vaccine.2006.11.061 [ Links ]
Pretorius, A., Van Kleef, M., Collins, N.E., Tshikhudo, N., Louw, E., Faber, F.E. et al., 2008, 'A heterologous prime/boost immunisation strategy protects against Ehrlichia ruminantium Welgevonden needle challenge but not against tick challenge', Vaccine 26(34), 4363-4371. http://dx.doi.org/10.1016/j.vaccine.2008.06.006 [ Links ]
Sebatjane, S.I., Pretorius, A., Liebenberg, J., Steyn, H. & Van Kleef, M., 2010, 'In vitro and in vivo evaluation of five low molecular weight proteins of Ehrlichia ruminantium as potential vaccine components', Veterinary Immunology and Immunopathology 137(3-4), 217-225. http://dx.doi.org/10.1016/j.vetimm.2010.05.011 [ Links ]
Sommer, S., 2006, 'The importance of immune gene variability (MHC) in evolutionary ecology and conservation', Frontiers in Zoology 2, 16. http://dx.doi.org/10.1186/1742-9994-2-16 [ Links ]
Steyn, H.C., Pretorius, A., Mccrindle, C.M.E., Steinmann, C.M.L. & Van Kleef, M., 2008, 'A quantitative real-time PCR assay for Ehrlichia ruminantium using pCS20', Veterinary Microbiology 131(3-4), 258-265. http://dx.doi.org/10.1016/j.vetmic.2008.04.002 [ Links ]
Totté, P., Bensaid, A., Mahan, S.M., Martinez, D. & Mackeever, D., 1999, 'Immune responses to Cowdria ruminantium infections', Parasitology Today 15(7), 286-290. http://dx.doi.org/10.1016/S0169-4758(99)01467-2 [ Links ]
Totté, P., Vachiéry, N., Martinez, D., Trap, I., Ballingall, K.T., Machugh, N.D. et al., 1996, 'Recombinant bovine interferon gamma inhibits the growth of Cowdria ruminantium but fails to induce major histocompatibility complex class II following infection of endothelial cells', Veterinary Immunology and Immunopathology 53(1-2), 61-71. http://dx.doi.org/10.1016/0165-2427(96)05603-6 [ Links ]
Van der Merwe, L., 1987, 'The infection and treatment method of vaccination against heartwater', Onderstepoort Journal of Veterinary Research 54(3), 489-491. [ Links ]
Van Eijk, M.J.T., Steward-Haynes, J.A. & Lewin, H.A., 1992, 'Extensive polymorphism of the BoLA-DRB3 gene distinguished by PCR-RFLP', Animal Genetics 23(6), 483-496. http://dx.doi.org/10.1111/j.1365-2052.1992.tb00168.x [ Links ]
Van Kleef, M., Gunter, N.J., Macmillan, H., Allsopp, B.A., Shkap, V. & Brown, W.C., 2000, 'Identification of Cowdria ruminantium antigens that stimulate proliferation of lymphocytes from cattle immunized by infection and treatment or with inactivated organisms', Infection and Immunity 68(2), 603-614. http://dx.doi.org/10.1128/IAI.68.2.603-614.2000 [ Links ]
Van Kleef, M., Macmillan, H., Gunter, N.J., Zweygarth, E., Allsopp, B.A., Shkap, V. et al., 2002, 'Low molecular weight proteins of Cowdria ruminanitum (Welgevonden isolate) induce bovine CD4+ -enriched T-cells to proliferate and produce interferon-γ', Veterinary Microbiology 85(3), 259-273. http://dx.doi.org/10.1016/S0378-1135(01)00516-8 [ Links ]
Wang, Y., Whittall, T., Mcgowan, E., Younson, J., Kelly, C., Bergmeier, L.A. et al., 2005, 'Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells', The Journal of Immunology 174(6), 3306-3316. http://dx.doi.org/10.4049/jimmunol.174.6.3306 [ Links ]
Yewdell, J.W. & Haeryfar, S.M.M., 2005, 'Understanding presentation of viral antigens to CD8+ T cells in vivo: The key to rational vaccine design', Annual Review of Immunology 23, 651-682. http://dx.doi.org/10.1146/annurev.immunol.23.021704.115702 [ Links ]
Zweygarth, E., Josemans, A.I., Van Strijp, M.F., Lopez-Rebollar, L., Van Kleef, M. & Allsopp, B., 2005, 'An attenuated Ehrlichia ruminantium (Welgevonden stock) vaccine protects small ruminants against virulent heartwater challenge', Vaccine 23(14), 1695-1702. http://dx.doi.org/10.1016/j.vaccine.2004.09.030 [ Links ]
 Correspondence:
Correspondence:
Nontobeko Thema
theman@arc.agric.za
Received: 03 Feb. 2016
Accepted: 14 Apr. 2016
Published: 30 Aug. 2016
Research Project no.: 9/27/c243 and 30/01/V011
Appendix 1
Results