Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Onderstepoort Journal of Veterinary Research
versão On-line ISSN 2219-0635
versão impressa ISSN 0030-2465
Onderstepoort j. vet. res. vol.82 no.1 Pretoria 2015
http://dx.doi.org/10.4102/OJVR.V82I1.899
RESEARCH COMMUNICATION
Serological survey of antibodies to Toxoplasma gondii and Coxiella burnetii in rodents in north-western African islands (Canary Islands and Cape Verde)
Pilar ForondaI; Josué Plata-LuisI; Borja del Castillo-FiguerueloI; Ángela Fernández-ÁlvarezI; Aarón Martín-AlonsoI; Carlos FeliuII; Marilena D. CabralIII; Basilio ValladaresI
IUniversity Institute of Tropical Diseases and Public Health of the Canary Islands University of La Laguna, Spain
IILaboratory of Parasitology, University of Barcelona, Spain
IIIDepartment of Science and Technology, University of Cape Verde, Cape Verde
ABSTRACT
Coxiella burnetii and Toxoplasma gondii are intracellular parasites that cause important reproductive disorders in animals and humans worldwide, resulting in high economic losses. The aim of the present study was to analyse the possible role of peridomestic small mammals in the maintenance and transmission of C. burnetii and T. gondii in the north-western African archipelagos of the Canary Islands and Cape Verde, where these species are commonly found affecting humans and farm animals. Between 2009 and 2013, 108 black rats (Rattus rattus) and 77 mice (Mus musculus) were analysed for the presence of Coxiella and Toxoplasma antibodies by enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence (IFA), respectively. Our results showed a wide distribution of C. burnetii and T. gondii, except for T. gondii in Cape Verde, in both rodent species. The overall seroprevalence of C. burnetii antibodies was 12.4%; 21.1% for Cape Verde and 10.2% for the Canary Islands. With respect to T. gondii, seropositive rodents were only observed in the Canary Islands, with an overall seroprevalence of 15%. Considering the fact that both pathogens can infect a large range of hosts, including livestock and humans, the results are of public health and veterinary importance and could be used by governmental entities to manage risk factors and to prevent future cases of Q fever and toxoplasmosis.
Introduction
Coxiella burnetii and Toxoplasma gondii are intracellular pathogens with worldwide distribution (Angelakis & Raoult 2010; Dubey 2010). Q fever is caused by C. burnetii and has been found in a large range of domestic and wild animals including mammals, birds, reptiles and arthropods (Angelakis & Raoult 2010). Toxoplasmosis is caused by T. gondii and can be found in all warmblooded animals, including terrestrial and marine mammals and birds (Jenkins et al. 2013; Pan et al. 2012; Wendte, Gibson & Grigg 2011).
Both pathogens cause high economic losses in livestock as a result of reproductive disorders (Arricau-Bouvery & Rodolakis 2005; Guatteo et al. 2011; Hill, Chirukandoth & Dubey 2005). In humans, most infections remain asymptomatic; however, Q fever and toxoplasmosis can be dangerous to pregnant women and immunosuppressed patients (Angelakis & Raoult 2010; Desmonts & Couvreur 1974; Dubey & Beattie 1988).
The main source of human infection with Q fever is livestock. Pets can also transmit this disease to humans (Arricau-Bouvery & Rodolakis 2005) and oral transmission by ingesting dairy products has been also reported (Fishbein & Raoult 1992). In the case of toxoplasmosis, the main source of infection is the ingestion of raw or insufficiently cooked meat with tissue cysts, oocysts in water or raw food and via vertical transmission (Cook et al. 2000).
Rodents have been found to be a source of livestock infection with several pathogens, including T. gondii (Kijlstra et al. 2008) and C. burnetii (Reusken et al. 2011). Therefore, and considering the fact that both T. gondii and C. burnetii are endemic in the Canary Islands (Bolaños et al. 2003; Rodriguez et al. 2010; Rodríguez-Ponce, Molina & Hernandez 1995; Solá-Graffigna 1997; Velasco 2010) and the lack of information on these species for Cape Verde, the aim of the present work was to contribute to the understanding of the epidemiology of the two pathogens in these northwestern African archipelagos. To this end, a serological study to detect T. gondii and C. burnetii antibodies in peridomestic rodents from the Canary Islands and Cape Verde was carried out.
Materials and methods
Biological samples and study area
The study was carried out in five of the seven Canary Islands (Spain), namely Tenerife, El Hierro, Gran Canaria, Lanzarote and Fuerteventura, and one island belonging to the Cape Verde Republic, namely Santiago. Both archipelagos are located near the north-west coast of Africa. The Canary Islands are located 100 km off the coast of Morocco, between 27°37'N and 29°24'N and 13°23'W and 18°8'W. Cape Verde is located 550 km off the coast of Senegal, between 17°16'N and 14°44'N and 22°37'W and 25°24'W. Between 2009 and 2013, 185 rodents from the Canaries and Cape Verde were captured randomly, comprising 108 black rats (Rattus rattus) (Linnaeus, 1758) and 77 mice (Mus musculus) (Linnaeus, 1758). The sample size was determined according to the variables host species and island. The sampled areas were determined at random: Orgaos, Praia and São Domingos in Santiago, San Cristóbal de La Laguna in Tenerife, Frontera in El Hierro, Telde, Santa Brígida and San Mateo in Gran Canaria and the whole islands of Fuerteventura and Lanzarote. The animals were captured alive using Sherman traps in suburban-rural areas. The traps were set in the afternoon and the evening and checked soon after sunrise. Animals were taken to the University Institute of Tropical Diseases and Public Health of the Canary Islands or to laboratories belonging to Cape Verde University.
The animals were euthanised by cervical dislocation or by carbon dioxide inhalation and bled by cardiac puncture. Blood samples were centrifuged and serum was removed and stored in a 1:1 glycerol solution at -20 °C until analysed.
Enzyme-linked immunosorbent assay
An ELISA kit was used (Coxiella burnetii ELISA kit, Bio-X Diagnostics, Belgium); however, anti-rat antibody peroxidase conjugate and the anti-mouse antibody peroxidase conjugate (Sigma-Aldrich, USA) were used with the serum samples instead of the kit's anti-goat antibody, which was used in controls. The sera and controls were used at 1:100 dilutions. The plates were read spectrophotometrically at 450 nm with a microplate reader (Model 680, Bio-Rad Laboratories, USA). The cut-off value was determined in accordance with the manufacturer's instructions.
Indirect immunofluorescence
An IFA kit was used (Toxo-Spot IFI, Biomerieux, France) in order to detect antibodies against T. gondii. The serum samples were diluted at 1:40 and 1:80 in phosphate buffered saline (PBS) and the conjugate was made as follows: 400 μL PBS, 10 μL Evans blue and 1 μL goat anti-rat or goat anti-mouse antibodies (Thermo Scientific, USA), both labelled with fluorescein. For each slide, one negative and one positive control were included and the results were determined in accordance with these controls. A positive sample was used as positive control and PBS was used as negative control.
Statistical analysis
Data analysis was performed with statistical software SPSS 20.0 (IBM, USA). Logistic regression could not be carried out because of the limited number of outcome events. Instead, chi-square contingency tables were used to examine the relationships between rodent species and island studied and the proportion of rodents harbouring antibodies against C. burnetii or T. gondii. Fisher's exact test was used if expected cell counts were < 5. A probability value < 0.05 was considered statistically significant.
Ethical considerations
All the animal procedures were performed according to the principles of animal welfare in experimental science (Spanish Government 2007, 2013). Animal trapping and its use were approved by the environmental agencies of the relevant governmental entities, in accordance with Act 151/2001, 33/2003, 42/2007 and 4/2010.
Results
Coxiella burnetii and T. gondii antibodies were found in both species of rodents analysed, with overall prevalences of 12.4% (CI 95% 7.7-17.2) and 11.9% (CI 95% 7.2-16.6), respectively. Coxiella burnetii was present in the two archipelagos studied; T. gondii was not found in Cape Verde (Table 1).
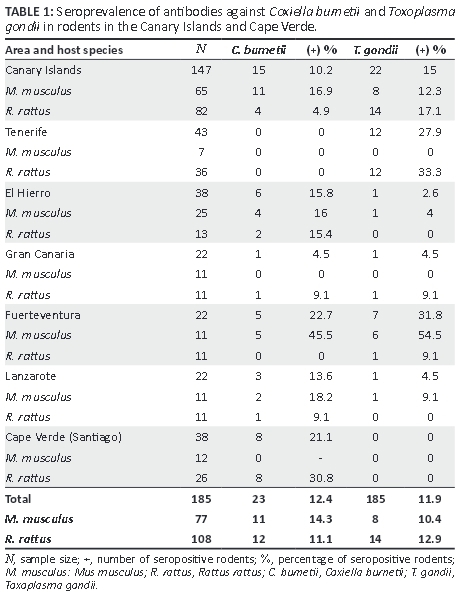
Rodents carrying antibodies against C. burnetii were found in all the islands analysed except for Tenerife, where antibodies were not found in either host. C. burnetii showed a higher seroprevalence in Cape Verde (21.1%, CI 95% 8.1-34.0) than in the Canary Islands (10.2%, CI 95% 5.3-15.1) but without significant differences. Significant differences in the proportion of rodents harbouring antibodies against both C. burnetii and T. gondii were found amongst the different islands included in the study (p < 0.01, Chi-square contingency table). When data from different hosts were compared, significant differences were observed for C. burnetii between M. musculus (16.9%, CI 95% 7.81-26.0) and R. rattus (4.9%, CI 95% 0.2-9.5) (p < 0.05) collected from the Canary Islands. However, differences between hosts were not observed when these islands were compared pairwise.
The overall prevalence for T. gondii in the Canary Islands was 15% (CI 95% 9.2-20.7); 17.1% (CI 95% 8.9-25.2) for R. rattus and 12.3% (CI 95% 4.3-20.3) for M. musculus. No significant differences between hosts were found, even when the islands were analysed separately. Comparing the seroprevalence of T. gondii between different islands, there was a significant difference for M. musculus between Fuerteventura (54.5%, CI 95% 25.1-84.0) and El Hierro (4%, CI 95% 0-11.7) islands (p < 0.001, Fisher's exact test). No positive results were found in rodents captured in Cape Verde.
Discussion
This study reveals the wide distribution of T. gondii and C. burnetii in rodents in the Canary Islands, as antibodies against both pathogens were found in all the islands analysed, except for C. burnetii in Tenerife. Furthermore, this study also indicates the role of black rats (R. rattus) as reservoirs of T. gondii in Santiago, as antibodies against this parasite were found in almost one-third of the individuals analysed.
To the authors' knowledge, there are no previous studies of C. burnetii or T. gondii in rodents from oceanic islands. When comparing the results with previous studies carried out in continental areas, the overall prevalence of T. gondii obtained in the present study (12.4%) is lower than that observed in Iran (24.41%) (Mosallanejad et al. 2012) but higher than that found in France (5%) (Gotteland et al. 2013) or Brazil (5.7%) (Siqueira et al. 2013). The overall prevalence of C. burnetii was 11.9%, similar to that obtained in the Netherlands (7.1%) (Reusken et al. 2011).
In the Canary Islands, previous studies involving small ruminant livestock (Rodriguez et al. 2010; Velasco 2010) and humans (Bolaños et al. 2003) have indicated that C. burnetii is endemic and T. gondii is widely distributed in the archipelago (Rodriguez-Ponce et al. 1995; Solá-Graffigna 1997). In the case of Cape Verde, to the authors' knowledge, there are neither available data for livestock census nor data about C. burnetii and T. gondii. However, based on personal observations during this study, the main livestock animals in Cape Verde seem to be goats, as in the Canary Islands (Gobierno de Canarias 2012). The same occurs in several African continental countries, such as Uganda, where goats are not only important to the local economy but also serve as a major source of protein (Bisson et al. 2000).
Considering the fact that C. burnetii and T. gondii can be transmitted from rodents to livestock (Kijlstra et al. 2008; Reusken et al. 2011), the presence of these pathogens in rodents from the Canary Islands and Cape Verde may have influenced the productivity of small ruminant flocks, which depends greatly on their reproductive efficiency (Arricau-Bouvery & Rodolakis 2005; Guatteo et al. 2011; Hill et al. 2005). However, the results of this study are not only important from an economic point of view; these pathogens are also relevant to public health. Both toxoplasmosis and Q fever are occupational diseases for people who work with infected animals and/or their products. In this regard, it must be remembered that the rodents captured in this study came from suburban and rural areas where people and their pets live in close contact with rodents and can acquire these pathogens (Arricau-Bouvery & Rodolakis 2005). Consequently, this study suggests that the incidence of both Q fever and toxoplasmosis, mainly in their mild forms, may have been underestimated in the Canary Islands and Cape Verde, especially in immunosuppressed patients such as pregnant women and HIV-positive people, as both pathogens can produce severe disease. For this reason, T. gondii and C. burnetii are important in sub-Saharan Africa, where HIV is a public health problem (UNAIDS 2013).
Conclusion
The present study reveals the presence and wide distribution of T. gondii and C. burnetii in peridomestic rodents from the Canary Islands and C. burnetii in Cape Verde, where there were no available data about their presence in these mammals. The two species examined, R. rattus and M. musculus, presented antibodies against both pathogens with similar prevalences.
Considering the potential health impact and the economic importance of T. gondii and C. burnetii in the archipelagos studied, the results of the study could be useful for governments to improve the management of Q fever and toxoplasmosis. However, more studies are required in order to analyse the possible role of these rodent species in the transmission of both pathogens to humans and livestock in this area.
Acknowledgements
We thank the islands councils of Tenerife, El Hierro, Gran Canaria, Lanzarote and Fuerteventura. We want to thank Leonildo Varela and all members of the PCI project for their help in taking samples. This study was supported by Rede de Investigación de Centros de Enfermedades Tropicales - RICET (RD06 0021/0005, FIS), Ministry of Health, Madrid, Spain; Agencia Espanola para la Cooperación Internacional y el Desarrollo under Programa de Cooperación Interuniversitaria (A1/035356/11); the Spanish Ministry of Foreign Affairs and Cooperation; Real Federación Espanola de Fútbol; SEMTSI; Project SEMACA (MAC73/C169) MAC 2007-2013; Programa Nacional de Biodiversidad, Ciencias de la Tierra y Cambio Global: Subprograma de Biología de Organismos y Sistemas (CGL 2006-04937 and CGL2009-07759BOS) of the Spanish Ministry of Science and Education; and the Canary Government (A.M.A., grant number TESIS20100083).
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Authors' contributions
B.V. (University of La Laguna) and C.F. (University of Barcelona) were the project leaders. P.F. (University of La Laguna), A.F.A. (University of La Laguna) and A.M.A. (University of La Laguna), C.F. and M.D.C. (University of Cape Verde) performed the field work. P.F., J.P.L. (University of La Laguna), B.d.C.F. (University of La Laguna) and A.M.A. realised the immunological and statistical analyses. P.F., J.P.L., B.C.F., A.M.A. and B.V. developed the discussion of the results. The manuscript was written by P.F., A.M.A., J.P.L. and B.C.F. and read and approved by all the authors.
References
Angelakis, E. & Raoult, D., 2010, 'Q fever', Veterinary Microbiology 140(3), 297-309. http://dx.doi.Org/10.1016/j.vetmic.2009.07.016 [ Links ]
Arricau-Bouvery, N. & Rodolakis, A., 2005, 'Is Q fever an emerging or re-emerging zoonosis?', Veterinary Research 36(3), 327-349. http://dx.doi.org/10.1051/vetres:2005010 [ Links ]
Bisson, A., Maley, S., Rubaire-Akiiki, C. & Wastling, J., 2000, 'The seroprevalence of antibodies to Toxoplasma gondii in domestic goats in Uganda', Acta Tropica 76(1), 33-38. http://dx.doi.org/10.1016/S0001-706X(00)00086-3 [ Links ]
Bolaños, M., Santana, O., Angel-Moreno, A., Pérez-Arellano, J., Liminana, J., Serra-Majem, L. et al., 2003, 'Seroprevalence of infection by Coxiella burnetii in Canary Islands (Spain)', European Journal of Epidemiology 18(3), 259-262. http://dx.doi.org/10.1023/A:1023342624475 [ Links ]
Cook, A., Gilbert, R., Buffolano, W., Zufferey, J., Petersen, E., Jenum, P. et al., 2000, 'Sources of Toxoplasma infection in pregnant women: European multicentre case-control study Commentary: Congenital toxoplasmosis - further thought for food', British Medical Journal 321(7254), 142-147. http://dx.doi.org/10.1136/bmj.321.7254.142 [ Links ]
Desmonts, G. & Couvreur, J., 1974, 'Congenital toxoplasmosis: A prospective study of 378 pregnancies', New England Journal of Medicine 290(20), 1110-1116. http://dx.doi.org/10.1056/NEJM197405162902003 [ Links ]
Dubey, J. & Beattie, C., 1988, Toxoplasmosis of animals and man, CRC Press, Boca Raton. [ Links ]
Dubey, J.P., 2010, Toxoplasmosis of animals and humans, CRC Press, Boca Raton. [ Links ]
Fishbein, D.B. & Raoult, D., 1992, 'A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products', American Journal of Tropical Medicine and Hygiene 47(1), 35-40. [ Links ]
Gobierno de Canarias, 2012, Estadísticas agrarias, viewed 06 December 2014, from http://www.gobcan.es/agricultura/otros/estadistica/ [ Links ]
Gotteland, C., Chaval, Y., Villena, I., Galan, M., Geers, R., Aubert, D. et al., 2013, 'Species or local environment, what determines the infection of rodents by Toxoplasma gondii?', Parasitology 141(2), 259-268. http://dx.doi.org/10.1017/S0031182013001522 [ Links ]
Guatteo, R., Seegers, H., Taurel, A., Joly, A. & Beaudeau, F., 2011, 'Prevalence of Coxiella burnetii infection in domestic ruminants: A critical review', Veterinary Microbiology 149(1), 1-16. http://dx.doi.org/10.1016/j.vetmic.2010.10.007 [ Links ]
Hill, D.E., Chirukandoth, S. & Dubey, J., 2005, 'Biology and epidemiology of Toxoplasma gondii in man and animals', Animal Health Research Reviews 6(1), 41-62. http://dx.doi.org/10.1079/AHR2005100 [ Links ]
Jenkins, E., Castrodale, L., de Rosemond, S., Dixon, B., Elmore, S., Gesy, K. et al., 2013, 'Tradition and transition: Parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland', Advances in Parasitology 82, 33-204. http://dx.doi.org/10.1016/B978-0-12-407706-5.00002-2 [ Links ]
Kijlstra, A., Meerburg, B., Cornelissen, J., de Craeye, S., Vereijken, P. & Jongert, E., 2008, 'The role of rodents and shrews in the transmission of Toxoplasma gondii to pigs', Veterinary Parasitology 156, 183-190. http://dx.doi.org/10.1016/j.vetpar.2008.05.030 [ Links ]
Mosallanejad, B., Avizeh, R., Jalali, R., Hossein, M. & Hamidinejat, H., 2012, 'Seroprevalence of Toxoplasma gondii among wild rats (Rattus rattus) in Ahvaz District, Southwestern Iran', Jundishapur Journal of Microbiology 5(1), 332-335. [ Links ]
Pan, S., Thompson, R.A., Grigg, M.E., Sundar, N., Smith, A. & Lymbery, A.J., 2012, 'Western Australian marsupials are multiply infected with genetically diverse strains of Toxoplasma gondii, PloS One 7(9), e45147. http://dx.doi.org/10.1371/journal.pone.0045147 [ Links ]
Reusken, C., van der Plaats, R., Opsteegh, M., de Bruin, A. & Swart, A., 2011, 'Coxiella burnetii (Q fever) in Rattus norvegicus and Rattus rattus at livestock farms and urban locations in the Netherlands: Could Rattus spp. represent reservoirs for (re) introduction?', Preventive Veterinary Medicine 101(1), 124-130. http://dx.doi.org/10.1016/j.prevetmed.2011.05.003 [ Links ]
Rodriguez, N., Carranza, C., Bolaños, M., Pérez-Arellano, J. & Gutierrez, C., 2010, 'Seroprevalence of Coxiella burnetii in domestic ruminants in Gran Canaria island, Spain', Transboundary and Emerging Diseases, 57(1-2), 66-67. http://dx.doi.org/10.1111/j.1865-1682.2010.01116.x [ Links ]
Rodriguez-Ponce, E., Molina, J.M. & Hernandez, S., 1995, 'Seroprevalence of goat toxoplasmosis on Grand Canary Island (Spain)', Preventive Veterinary Medicine 24, 229-234. http://dx.doi.org/10.1016/0167-5877(95)00491-E [ Links ]
Siqueira, D.B., Alessio, F.M., Mauffrey, J.F., Marvulo, M.F., Ribeiro, V.O., Oliveira, R.L. et al., 2013, 'Seroprevalence of Toxoplasma gondii in wild marsupials and rodents from the Atlantic forest of Pernambuco State, Northeastern Region, Brazil', Journal of Parasitology 99(6), 1140-1143. http://dx.doi.org/10.1645/GE-2855.1 [ Links ]
Solá-Graffigna, D., 1997, 'Aportación al conocimiento de la epidemiologia de la toxoplasmosis y fiebre Q en el ganado ovino y caprino del archipiélago canario', PhD Thesis, Dept. of Parasitology, Ecology and Genetics, University of La Laguna. [ Links ]
Spanish Government, 2007, Ley 32/2007, de 7 de noviembre, para el cuidado de los animales, en su explotación, transporte, experimentación y sacrificio, Boletin Oficial del Estado, 268, 45914-45920. [ Links ]
Spanish Government, 2013, Ley 6/2013, de 11 de junio, de modificación de la Ley 32/2007, de 7 de noviembre, para el cuidado de los animales, en su explotación, transporte, experimentación y sacrificio, Boletin Oficial del Estado, 140, 4428944292. [ Links ]
UNAIDS, 2013, The Gap Report, Joint United Nations Programme on HIV/AIDS (UNAIDS), Geneva, Switzerland, viewed 14 November 2014, from http://www.unaids.org/ [ Links ]
Velasco, F., 2010, 'Coxiella burnetii infections in domestic ruminants in Canary Islands (Spain)', Transboundary and Emerging Diseases 57(6), 464-464. http://dx.doi.org/10.1111/j.1865-1682.2010.01159.x [ Links ]
Wendte, J.M., Gibson, A.K. & Grigg, M.E., 2011, 'Population genetics of Toxoplasma gondii: New perspectives from parasite genotypes in wildlife', Veterinary Parasitology 182(1), 96-111. http://dx.doi.org/10.1016/j.vetpar.2011.07.018 [ Links ]
 Correspondence:
Correspondence:
Pilar Foronda
Faculty of Pharmacy
University of La Laguna
Avda. Astrofísico Fco. Sánchez, 38203 La Laguna
Canary Islands, Spain
Email: pforonda@ull.edu.es
Received: 03 Nov. 2014
Accepted: 02 Feb. 2015
Published: 29 May 2015














