Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Onderstepoort Journal of Veterinary Research
On-line version ISSN 2219-0635
Print version ISSN 0030-2465
Onderstepoort j. vet. res. vol.80 n.1 Pretoria Jan. 2013
RESEARCH COMMUNICATION
Descriptions of diplostomid metacercariae (Digenea: Diplostomidae) from freshwater fishes in the Tshwane area
Esmey B.E. MoemaI; Pieter H. KingI; Johnny N. RakgoleII; Chantélle BakerIII
IDepartment of Biology, University of Limpopo, Medunsa campus, South Africa
IIDepartment of Virology, University of Limpopo, Medunsa campus, South Africa
IIIElectron Microscope Unit, University of Limpopo, Medunsa campus, South Africa
ABSTRACT
The metacercarial (larval) stages of diplostomid digeneans are known to inhabit freshwater fish, causing tissue damage in the process. Due to their widespread diversity, little is known about their life cycle. The classification of these parasitic stages to the species level using only the morphology is very challenging due to the lack of genitalia; they are regarded to be the most important structures in the identification of these organisms. In this study, additional morphological information through light and scanning electron microscopy is given for two different diplostomids found in the cranial cavity of Clarias gariepinus and the vitreous chambers of Tilapia sparrmanii and Pseudocrenilabrus philander. The diplostomid metacercaria inhabiting the cranial cavity of Clarias gariepinus was morphologically identified as Diplostomulum (Tylodelphys) mashonense and an unknown metacercaria of the genus Diplostomum was found in the vitreous chambers of Pseudocrenilabrus philander and Tilapia sparrmanii. Both parasitic species' 28S recombinant deoxyribonucleic acid genomic regions were successfully amplified using Dig 125/1500R primer pairs. The assay yielded a product of approximately 1300 base pairs as seen on the gel images. There were 14 nucleotide differences over the entire analysed sequences resulting in a 1.1% (14/1273) nucleotide difference. In line with the morphological characteristics of these parasites, there seemed to be a slight difference in their genetic makeup. The application of molecular techniques on digenetic trematodes seems very promising and may yield great potential in future descriptions of morphologically similar parasitic species.
Introduction
According to Gibson, Jones and Bray (2001), adult parasites of the family Diplostomidae are parasitic in many birds and mammals. Larval forms of this family use a variety of freshwater snails as first intermediate hosts and various fish species as second intermediate hosts.
Beverley-Burton (1963) from Zimbabwe and Niewiadomska (1984, 1986) from Poland pioneered early studies on the adults of the genus Diplostomum. Larval forms belonging to the genus Diplostomulum were also previously studied from the eyes and brain of freshwater fish from various parts of the world. These included the work done by: Pandey (1973) from India; Prudhoe and Hussey (1977), Chibwana et al. (2013) from Tanzania and Nigeria; Mashego and Saayman (1989) and Madanire-Moyo, Luus-Powell and Olivier (2010) from South Africa; Hendrickson (1978a, 1978b) and Hoglund and Thulin (1992) from the Baltic Sea.
The aim of this research was to study the morphology and phylogeny of two diplostomid metacercariae; one from the eyes and the other from the cranial cavity of different freshwater fish species from two localities in the proximity of Tshwane, Gauteng Province, South Africa.
Materials and methods
Diplostomid metacercariae were collected through dissections of three freshwater fish species, namely Pseudocrenilabrus philander Weber, 1897, Tilapia sparrmanii A. Smith, 1840 and Clarias gariepinus Burchell, 1822 (C. gariepinus). They were collected from the Supersand Dam (25°35'02.42"S, 28°10'38.98"E, altitude 1198 m above sea level [a.s.l.]) situated ± 2 km north of the Bon Accord Dam in the Onderstepoort area, Gauteng and the Boekenhoutskloof farm Dam (25°32'45.18"S, 28°26'12.47"E, altitude 1214 m, a.s.l.), approximately 15 km north of the Roodeplaat Dam Nature Reserve in the proximity of Tshwane, Gauteng Province, South Africa. Live forms of the metacercariae were studied using a compound microscope with the aid of vital stains. Specimens were fixed in 2.5% phosphate buffered glutaraldehyde (pH = 7.2-7.4), 5% saline buffered solution and 70% ethanol respectively and stored until required. Standard techniques for light and scanning electron microscopy (SEM) were employed to study the internal and external ultrastructures of these parasites. Polymerase chain reaction (PCR) techniques were also performed pertaining to molecular structures on the same species.
Light microscopy
Specimens fixed in 70% ethanol were stained in haematoxylin or eosin and Van Cleave's haematoxylin staining solutions. Stained specimens were mounted on slides using DPX mounting medium. Projection drawings of mounted specimens were made by means of a drawing tube attached to a Nikon compound microscope (Nikon, Tokyo, Japan). For each description, a maximum of 20 specimens were drawn and measured, with all measurements given in micrometres (um). Morphological measurements are presented in the text as follows: minimum to maximum values, followed by the average value and standard deviation in parentheses. All light micrographs were taken using a Nikon Coolpix®990 digital camera fitted to the compound microscope.
Scanning electron microscopy
Specimens fixed in 2.5% gluteraldehyde were washed in Millonig's phosphate buffer (pH = 7.2) and dehydrated through an graded series of ethanol (from 70% ethanol to 100% ethanol) for 30 seconds to one minute in each concentration. They were processed as follows: overnight critical point dried (Polaron, Watford, UK), mounted on aluminium stubs, sputter coated with gold (Emscope; Quorum Technologies, Ashford, UK) and examined using a Leica Stereoscan 420 SEM (Leica Electron Optics, UK).
Polymerase Chain Reaction
Specimens for PCR were suspended in 5% saline buffered solution (200 μL) and frozen in a -70 C freezer until required. During the extraction process, specimens were thawed and excess buffered saline solution (BSS: 800 mL distilled water, 8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4 and 0.24 g KH2PO4; pH = 7.4) was removed. Deoxyribonucleic acids (DNA) were extracted using a QIAamp DNA mini kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. Two parasite samples obtained from different locations, the brain and eyes of two fishes, were extracted in duplicates. In brief, specimens were transferred to 220 μL lysis solution (200 μL ATL Buffer and 20 μL Proteinase K) in microcentrifuge tubes. Samples were incubated at 56 °C for four hours followed by the addition of 200 μL of absolute ethanol. The DNA in the lysate was allowed to bind to the spin columns and washed twice with the supplied buffers before being eluted in 200 μL of elution buffer. The extracted DNA of parasites was stored at -20 °C until analysis. The 28S rDNA was amplified using the primers DIG12 (5-AAGCATATCACTAAGCGG-3) and 1500R (5-GCTATCCTGAGGGAAACTTCG-3) (Tkach et al. 1999; Tkach, Pawloski & Mariaux 2000). The PCR reaction was performed in a final volume of 25 μL. The reagents at a final concentration of 0.2 μM for dNTPs, 1.5 mM MgCl2, 0.2 μM each of primers and 1 U per reaction of Taq polymerase (Bioline, BioTaq, London, UK) were used. The PCR products were sequenced on the ABI 3100XL sequencing platform (Life Technologies, Foster City, CA, USA). The sequences were edited using ChromasPro v1.5 (Technelysium Pty, Ltd, Queensland, Australia). Additional sequences were downloaded from GenBank (accession numbers are shown on the phylogenetic tree in the results section) and aligned using ClustalW within BioEdit (Hall 1999). The phylogenetic trees were constructed using MEGA v5.22 (Tamura et al. 2011).
This research project met the requirements of the Medunsa Research Ethics Committee (BP 05/2005) and the Animal Ethics Committee (AEC 02/05) of the University of Limpopo, South Africa. Fish species that were collected were kept under controlled laboratory conditions with sufficient light, air and food according to acceptable standards. They were anaesthetised using clove oil prior to dissection.
Results
Metacercaria: Diplostomid metacercaria A [Diplostomulum (Tylodelphys) mashonense] (Figure 1a).

The host is Clarias gariepinus sampled from the Boekenhouts-kloof farm Dam in the Gauteng Province and its preferred site in the host is the brain cavity.
The metacercariae were found unencysted in the cranial cavity of the sharptooth catfish, C. gariepinus. A few were collected around the frontal lobe, however, the majority of these larval stages had migrated posteriorly into the cavity. The numbers varied from a minimum of eight to over a hundred specimens per fish host. The metacercaria appeared to be off-white and elongated (Figure 1a; Figures 2a and 2b). The foliate body measured 946 um-1917 µm (1409 µm ± 302 µm) long x 240 µm-485 µm (375 µm ± 75 µm) wide.
The body was comprised of a long and distinct forebody, followed by a small hindbody. It had an oral sucker (Figure 2c), which was situated at the anterior end of the body. It measured 36 µm-90 µm (70 µm ± 17 µm) x 42 µm-96 µm (72 µm ± 14 µm). The tegument around the oral sucker was surrounded by non-ciliated serrated receptors (Figure 2c). Bordering the oral sucker were two pseudosuckers (Figure 1a), 54 µm-120 µm (73 µm ± 22 µm) x 24 µm-84 µm (48 µm ± 16 µm), which were situated bilaterally at the anterior end of the body. The oral sucker (Figure 2c) continued into a muscular pharynx measuring 24 µm-60 µm (46 µm ± 13 µm) x 30 µm-60 µm (47 µm ± 10 µm). A short oesophagus measuring 30 µm-108 µm (40 µm ± 27 µm) x 12 µm-24 µm (19 µm ± 4 µm) bifurcated into two long intestinal caeca, 772 µm-1371 µm (1111 µm ± 247 µm) x 18 µm-36 µm (28 µm ± 7 µm), within the hindbody at the posterior end of the body.
A spherical acetabulum that was similar in size to the oral sucker (Figure 2d), was situated midventrally on the body and measured 36 µm-90 µm (71 µm ± 17 µm) x 30 µm-96 µm (67 µm ± 21 µm). The tegument around the acetabulum wall (Figure 2d) had no sensory receptors, but had ridges that were equally distributed around it. An oval-shaped holdfast organ (Figure 2e) with a deep fissure measured 156 µm-198 µm (180 µm ± 15 µm) x 24 µm-150 µm (101 µm ± 48 µm) and was situated between the acetabulum and the posterior end of the body. Genital primodia, consisting of two testes, occurred anteriorly in the hindbody. The anterior testis was asymmetrical, measuring 102 µm-467 µm (199 µm ± 120 µm) x 114 µm-263 µm (179 µm ± 50 µm), and lay in line with the junction of the forebody and the hindbody. It was also placed more to the right in the hindbody. The posterior testis on the other hand was bilobed and measured 96 µm-155 µm (126 µm ± 27 µm) x 138 µm-221 µm (176 µm ± 24 µm) (Figure 1a).
The excretory system was not observed due to the copious amount of calcareous bodies found in the body. The anterior body tegument was relatively smooth in the area around the oral sucker (Figure 2f), but became unevenly ridged posteriorly (Figure 2g) with non-ciliated papillae all over the body surface (Figure 2b-h). There were no spines on the body tegument.
Metacercaria: Diplostomid metacercaria B (Figure 1b).
The hosts are Tilapia sparrmanii and Pseudocrenilabrus philander sampled from the Supersand Dam in the Gauteng Province and its preferred site in the host is the vitreous chamber.
These metacercariae were found unencysted in the vitreous chamber of small southern mouth-brooders (P. philander) and banded tilapian (T. sparrmanii) species. The number of specimens varied from a minimum of two to a maximum of 12 per fish host. The metacercaria (Figure 1b; Figures 3a and 3b) was whitish in colour with an elongated body. The spatulate body measured 832 µm-1263 µm (1019 µm ± 138 µm) long x 257 µm-407 µm (340 µm ± 50 µm) wide.
The body was comprised of a well-developed oral sucker (Figure 3c), which measured 42 µm-72 µm (55 µm ± 9 µm) x 30 µm-60 µm (51 µm ± 10 µm). This sucker was located subterminally at the anterior end of the body and the tegument around it was not armed with receptors. Well-developed pseudosuckers (Figure 1b and Figure 3a) measuring 24 µm-42 µm (34 µm ± 6 µm) x 36 µm-84 µm (59 µm ± 15 µm) flanked the oral sucker.
The mouth that was located in the oral sucker connected to a muscular pharynx measuring 30 µm-54 µm (43 µm ± 8 µm) x 30 µm-54 µm (43 µm ± 6 µm). The pharynx lead to a very short oesophagus that measured 24 µm-84 µm (39 µm ± 18 µm) x 18 µm-24 µm (19 µm ± 2 µm). The oesophagus then bifurcated into two long intestinal caeca, 557 µm-988 µm (717 µm ± 141 µm) x 18 µm-42 µm (29 µm ± 7 µm), ending at the posterior end of the holdfast organ. A spherical acetabulum (Figure 3d) measuring 30 µm-66 µm (51 µm ± 12 µm) in diameter was situated along two-thirds of the body length. It was somewhat smaller than the oral sucker.
An elliptical holdfast organ (Figure 3e) with hymen measured 96 µm-180 µm (131 µm ± 29 µm) x 42 µm-96 µm (68 µm ± 18 µm) and was situated at the posterior end of the forebody. A conspicuous V-shaped excretory bladder (Figure 1b and Figure 3a) measuring 138 µm-240 µm (172 µm ± 32 µm) x 72 µm-132 µm (98 µm ± 21 µm) was situated at the posterior end of the hindbody. No flame cells were observed due to the rod-shaped calcareous bodies in the body. The genital primodia were also not observed, most probably due to the developmental stage of the parasites that were examined. An excretory pore was present at the posterior end of the hindbody (Figure 3h).
The non-ciliated receptors were observed posterior to the oral sucker (Figure 3f) surrounding the acetabulum (Figure 3d) and in most of the body tegument (Figures 3g and 3h).
Polymerase Chain Reaction
Parasite samples were successfully amplified using the Dig 125/1500R primer pairs. Three of the extracts tested positive: two from the brain and one from the eye. The assay yielded a product of about 1300 base pairs (bp) as seen on the gel images. Brain sample sequences were 100% similar. The sequence of eye sample differed from the one obtained from the brain by having one base pair longer in the sequenced region. In addition, there were 14 nucleotide differences over the entire analysed sequences resulting in a 1.1% (14/1273) nucleotide difference. Table 1 shows the specific locations of the base differences. The sequences were submitted to Genbank under the following accession numbers: KF189071 (brain) and KF189072 (eye).
Nucleotide positions were only based on the analysed regions of the two sequences. A phylogenetic tree (Figure 4) was constructed using the two sample sequences along with the similar sequences downloaded during a Basic Local Alignment Search Tool (BLAST) analysis from the Genbank database.
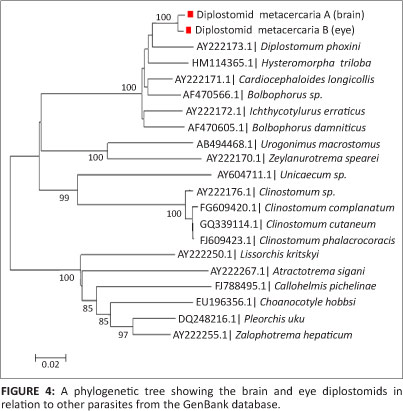
Discussion
In this study, two dissimilar diplostomids were found to infect three fish species. Diplostomid metacercaria A was only found in the cranial cavity of C. gariepinus, whereas diplostomid metacercaria B was only found in the vitreous chamber of T. sparrmanii and P. philander. From this study it is evident that the two diplostomids are host specific and site specific due to the niches where they were sampled.
Both the cranial cavity and the eye diplostomid metacercariae were found unencysted at their respective sites. They were found to be moving freely in the cerebrospinal fluid in the region of the frontal lobes and vitreous humour of the eye, respectively.
Eight catfish that were dissected and examined in the present study were infected with the larvae in their cranial cavity. However, they did not show any signs of distress caused by these parasites. Khalil (1963) described Diplostomum tregenna, Nazmi Gohar, 1932 from the cranial nerves of Clarias lazera from the River Nile. He observed three stages of this parasite; the third stage was well-developed showing genitalia. Beverley-Burton (1963) described an adult diplostomula of Diplostomulum (Tylodelphys) mashonense (D. [T.] mashonense); the metacercariae were discovered in the cranial cavity of Clarias mossambicus, which was synonymous to C. gariepinus in Southern Rhodesia (now known as Zimbabwe).
Similarly, Mashego and Saayman (1989) described D. (T.) mashonense collected from C. gariepinus from dams around the Limpopo Province (formerly known as Lebowa). The latter authors' observations were that the metacercariae concentrated more on the frontal lobes of the brain in young hosts and thereafter they were found to have moved posteriorly as the hosts aged. This phenomenon was also observed during the present research study. These larvae were found to be very similar to the one described by the abovementioned authors and further, it was also very difficult to observe the excretory system including the flame cells. The metacercariae found in the cranial cavity of C. gariepinus during our investigation can therefore be identified as the larval stage of D. (T.) mashonense.
Tilapia sparrmanii specimens were found to be more frequently infected by eye diplostomid metacercariae than P. philander. It is still unclear as to why this should be the case, but immunity and the daily activities of the fish species may possibly have an influence. Hendrickson (1978a, 1978b) made observations about the eye diplostomids Diplostomum scheuringi, Hughes, 1929 and Diplostomum spathaceum respectively that were sampled from Wyoming fish. However, in his study observations were that some fish species were less infected than others and that these parasites had little, if any, effect on the sight of the fish.
Pandey (1973) described another type of eye diplostomid, Diplostomulum opthalmi (Pandey 1970), from freshwater fish in India. The eye diplostomid metacercaria observed in the present study is very similar to the one described by Hendrickson (1978a, 1978b) and Pandey (1973). However, it differs in the body tegument and size, has suckers that are spined and there is an absence of pseudosuckers. In order to classify this diplostomid species as a new species, the adult stage of this larval parasite will have to be found or experimentally raised. Only then would the taxonomic status of this species be able to be commented on.
Although the diplostomid metacercariae in the present study seem morphologically similar to the metacercariae described by other researchers, morphological studies are simply not sufficient. The morphology of organisms may undergo micro-evolutionary adaptation over time as the environment changes due to many external factors. Factors like pH, temperature, nutrients, light, health, age, as well as angles of observation may introduce variation of the same organism and are therefore very subjective even with standardised guidelines (Huyse, Poulin & Théron 2005). Phylogenetic studies were thus conducted on these metacercarial stages.
In line with the morphological characteristics of the two parasites, there seemed to be a slight difference in their genetic make-up. From the 1273 bp of a 28S region, a 1.03% difference exists at DNA level. This is thought to be a highly conserved region for most organisms and should therefore be common to organisms of the same species. Lower homology values will be expected for non-conserved regions of these organisms, such that their full length sequences could differ by as much as 15%. The power of phylogenetic analysis is undermined by the lack of parasite sequences in the Genbank database and therefore renders a limitation into complete characterisation of these organisms. The phylogenetic tree thus constructed, clusters the parasites in the present study with those that belong to the Superfamily Diplostomoidea and can unfortunately only resolve the samples studied to the Order Strigeidida. There are mixed families within the same node when analysing this phylogenetic tree to the family level.
This is the first study to report on the larger fragment of the 28S rDNA of diplostomids from the cranial cavity of C. gariepinus and the eyes of P. philander and T. sparrmanii. Presently there are no related sequences in the GenBank database. However, there is a recent report on phylogenetic studies of diplostomids collected from the brain of C. gariepinus sampled from Nigeria and Tanzania, of which the sequences covered up to the ITS-2 region (Chibwana et al. 2013). Unfortunately the sequences of the present study cannot be compared to those generated in the previous work due to the fact that they both target exclusive DNA regions. The only sequence that links to the sequences from our study (and that of Chibwana et al. 2013) is Alaria alata (accession number JF820609). Since there are no related sequences in the database, we can only base our nomenclature on the morphology.
This was a pilot study from the Department of Biology, which aimed to assess the possibility of applying molecular biology techniques to the investigation of larval digenetic parasites. The application of molecular techniques on digenetic trematodes seems very promising and may yield great potential in future descriptions of these parasitic species.
Recommendations
We recommend a full length genome analysis of these two organisms in order to establish more definitive evidence of whether they belong to the same species. Further than that, archived specimens that have been described using biological and morphological characteristics should be genetically analysed in order to increase the information in the database of these organisms in GenBank.
Acknowledgements
SEM equipment utilised during this investigation was supported by the Department of Science and Technology in partnership with the National Research Foundation of South Africa. The Department of Virology, Medunsa campus allowed us to utilise their PCR laboratories for molecular work.
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
E.B.E.M. (University of Limpopo) was the principal investigator and this study forms part of her doctoral thesis. P.H.K. (University of Limpopo) was the postgraduate supervisor of this study. C.B. (University of Limpopo) was a co-worker involved with all aspects of micrography and technical finishing of photographic plates. J.N.R. (University of Limpopo) was involved in the phylogenetic studies of the present study.
References
Beverley-Burton, M.M., 1963, 'A new strigeid, Diplostomum (Tylodelphys) mashonense n. sp., (Trematoda: Diplostomidae), from the Grey Heron, Ardea cinerea L. in southern Rhodesia, with an experimental demonstration of part of the life cycle', Revue de Zoologie et de Botanique Africaines LXVII, 291-308. [ Links ]
Chibwana, F.D., Blasco-Costa, I., Georgiva, S., Hosea, K.M., Nkwengulila, G., Scholz, T. & Kostadinova, A., 2013, 'A first insight into the barcodes for African diplostomids (Digenea: Diplostomatidae): Brain parasites in Clarias gariepinus (Siluriformes: Clariidae)', Infection, Genetics and Evolution 17, 62-70. http://dx.doi.org/10.1016/j.meegid.2013.03.037, PMid:23542455 [ Links ]
Gibson, D.I., Jones, A. & Bray, R.A., 2001, 'Keys to the Trematoda, vol. 1', CABI Publishing, London. [ Links ]
Hall, T.A., 1999, 'BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT', Nucleic Acids Symposium Series 41, 95-98. [ Links ]
Hendrickson, G.L., 1978a, 'Observations on Strigeoid trematodes from the eyes of Southeastern Wyoming fish. I. Diplostomulum spathaceum (Rudolphi, 1819)', Proceedings of the Helminthological Society 45, 60-64. [ Links ]
Hendrickson, G.L., 1978b, 'Observations on Strigeoid trematodes from the eyes of Southeastern Wyoming fish. II. Diplostomulum scheuringi Hughes, 1929, Neascus ptychocheilus (Faust, 1917), and other types', Proceedings of the Helminthological Society 45, 64-68. [ Links ]
Höglund, J. & Thulin, J., 1992, 'Identification of Diplostomum spp. in the retina of perch Perca fluviatilis and the lens of roach Rutilus rutilus from the Baltic Sea - an experimental study', Systematic Parasitology 21, 1-19. [ Links ]
Huyse, T., Poulin, R. & Théron, A., 2005, 'Speciation in parasites: A population genetics approach', Trends in Parasitology 21(10), 469-475. http://dx.doi.org/10.1016/j.pt.2005.08.009, PMid:16112615 [ Links ]
Khalil, L.F., 1963, 'On Diplostomulum tregenna, the diplostomulum stage of Diplostomum tregenna Nazmi Gohar, 1932 with an experimental demonstration of part of the life cycle', Journal of Helminthology 37(3), 199-206. http://dx.doi.org/10.1017/S0022149X00003783 [ Links ]
Madanire-Moyo, G.N., Luus-Powell, W.J. & Olivier, P.A.S., 2010, 'Ecology of metazoan parasites of Clarias gariepinus (Osteicthyes: Clariidae) from the Nwanedi-Luphephe Dams of the Limpopo River system, South Africa' African Zoology 45(2), 233-243. http://dx.doi.org/10.3377/004.045.0202 [ Links ]
Mashego, S.N. & Saayman, J.E., 1989, 'Digenean trematodes and cestodes of Clarias gariepinus (Burchell, 1822) in Lebowa, South Africa, with taxonomic notes', South African Journal of Wildlife Research 19, 17-20. [ Links ]
Niewiadomska, K., 1984, 'Present status of Diplostomum spathaceum (Rudolphi, 1819) and differentiation of Diplostomum pseudospathaceum nom. nov (Trematoda: Diplostomidae)', Systematic Parasitology 6, 81-86. http://dx.doi.org/10.1007/BF02185515 [ Links ]
Niewiadomska, K., 1986, 'Verification of the life-cycles of Diplostomum spathaceum (Rudolphi, 1819) and D. pseudospathaceum Niewiadomska, 1984 (Trematoda: Diplostomidae)', Systematic Parasitology 8, 23-31. [ Links ]
Pandey, K.C., 1973, 'Studies on metacercariae of freshwater fishes of India. II. Description of a known and five unknown strigeid metacercariae from Lucknow', Journal of Indian Zootomy 14(3), 155-166. [ Links ]
Prudhoe, S. & Hussey, C.G., 1977, 'Some parasitic worms in fresh-water fishes and fish predators from the Transvaal, South Africa', African Zoology 12, 113-148. [ Links ]
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S., 2011, 'MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods', Molecular Biology and Evolution 28(10), 2731-2739. http://dx.doi.org/10.1093/molbev/msr121, PMid:21546353, PMCid:PMC3203626 [ Links ]
Tkach, V., Grabda-Kazubska, B., Pawlowski, J. & Swiderski, Z., 1999, 'Molecular and morphological evidences for close phylogenetic affinities of the genera Macrodera, Leptophallus, Metaleptophallus and Paralepodema (Digenea: Plagiorchidea)', Acta Parasitologica 44, 170-179. [ Links ]
Tkach, V., Pawlowski, J. & Mariaux, J., 2000, 'Phylogenetic Analysis of the suborder Plagiorchiata (Platyhelminthes: Digenea) based on partial IsrDNA sequences', International Journal of Parasitology 30, 83-93. http://dx.doi.org/10.1016/S0020-7519(99)00163-0 [ Links ]
 Correspondence:
Correspondence:
Esmey Moema
PO Box 139
Medunsa 0204, South Africa
Esmey. Moema@ul.ac.za
Received: 22 Apr. 2013
Accepted: 24 June 2013
Published: 25 Sept. 2013














