Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Onderstepoort Journal of Veterinary Research
On-line version ISSN 2219-0635
Print version ISSN 0030-2465
Onderstepoort j. vet. res. vol.80 n.1 Pretoria Jan. 2013
Histomorphometrical and ultrastructural study of the effects of carbendazim on the magnum of the Japanese quail (Coturnix coturnix japonica)
Wahabu H. KimaroI, II; Mary-Catherine MadekurozwaI; Herman B. GroenewaldI
IDepartment of Anatomy and Physiology, University of Pretoria, South Africa
IIDepartment of Veterinary Anatomy, Sokoine University of Agriculture, Tanzania
ABSTRACT
The study investigated the effect of various doses of carbendazim on the morphology of the magnum of the Japanese quail. No morphological changes were observed in the magnum in birds treated with carbendazim at doses of 25 mg/kg and 100 mg/kg bodyweight. A carbendazim dose of 400 mg/kg bodyweight was the lowest dose which caused morphological changes in the magnum. Histologically, carbendazim caused pyknosis and glandular atrophy in the magnum mucosa. Carbendazim also caused significant decreases in the height of the mucosal folds, epithelial height, glandular width and glandular luminal diameter at 400 mg/kg and 800 mg/kg (p < 0.05). At ultrastructural level, dose-dependent deciliation was observed. Pyknotic nuclei, dilated cisternae of rough endoplasmic reticulum, swollen mitochondria, numerous vacuoles and lysosomes in the luminal and glandular epithelia were identified. The observed degenerative changes could be due to cytoskeletal disruption caused by carbendazim toxicity. Degeneration of the luminal and glandular cells in the magnum pose a potential threat to the egg production and reproduction of exposed birds.
Introduction
Carbendazim (methyl-2-benzimidazole carbamate) is a derivative of the benzimidazole group of fungicides, which are N-substituted esters of carbamic acid (carbamate). Carbendazim is also a metabolite of benomyl (methyl-1-butylcarbamoyl) when dissolved in water. Both chemicals (carbendazim and benomyl) are used as fungicides on ornamental plants, vegetables, fruits and cereals (International Program on Chemical Safety [IPCS] 1986). The fungicidal effect of these chemicals relies on their ability to disrupt microtubule assembly (Davidse & Flach 1977; Burland & Gull 1984). According to the Finnish National Board of Health report (1982), the level of benomyl and carbendazim metabolites such as methyl (4-hydroxy-1H-benzimidazol-2-yl) carbamate, methyl (5-hydroxy-1H-benzimidazol-2-yl) carbamate and 4,5-dihydrodiol-MBC in food and water is increasing. Carbendazim metabolite residues are present in plants (Still & Mansager 1975), soil (International Program on Chemical Safety [IPCS] 1986), surface and ground water (IPCS 1986) and fruits (Pico et al. 2007). Due to the increasing concentrations of benomyl and carbendazim metabolites in the environment, it is likely that aquatic and terrestrial organisms are being exposed to carbendazim metabolites.
The magnum is the longest region of the avian reproductive tract, making up to 50% of the total oviductal length in domestic fowls (Wyburn et al. 1970). The magnum releases egg-white proteins such as ovalbumen, conalbumen, ovomucoid, lysozyme and avidin (Palmiter & Gutman 1972). Davidson (1986) reported the production of watery eggs associated with morphological changes in the luminal and glandular epithelia of the magnum. It is clear that morphological changes in the magnum will adversely affect the quality of eggs, as well as the egg laying process.
Carbendazim causes morphological changes in mammalian reproductive organs (Carter & Laskey 1982; Goldman et al. 1989; Hess et al. 1991; Lim & Miller 1997) and male birds (Aire 2005). Little is known on the effect of carbendazim in the reproductive tract of female birds. This study therefore reports the effect of various doses of carbendazim on the morphology of the magnum of the Japanese quail.
Materials and methods
Animal management and sample collection
A total of 35 sexually mature female Japanese quail (Coturnix coturnix japonica) were purchased from Irene Improvement Research Farm, Pretoria. The birds were housed in an avian facility and allowed to breed freely. Both males and females were kept together. After acclimatisation at the research site, the birds were divided into two groups, the control (seven birds) and treatment groups (28 birds). The treatment group was further divided into four groups of seven birds each, according to the dose administered. Carbendazim (97% Sigma Aldrich) was dissolved in sunflower oil and administered once per os to the four experimental groups at doses of 25 mg/kg, 100 mg/kg, 400 mg/kg and 800 mg/kg bodyweight respectively. The doses were selected based on a previous experiment on male Japanese quails (Aire 2005). Control birds were given only the sunflower oil base orally. During the experiment, food (grower mash, containing maize grain) and water were provided ad libitum. Light was controlled at 14 h light and 10 h darkness throughout the experiment.
Forty-eight hours post-exposure to carbendazim, the birds were sacrificed by inhalation anaesthesia using carbon dioxide (CO2). Following the death of a bird, the thoracoabdominal cavity was opened and the reproductive tract was dissected out immediately. The protocol of this research (# V031/07) was approved by the animal use and care committee (AUCC) of the University of Pretoria.
Light microscopy studies
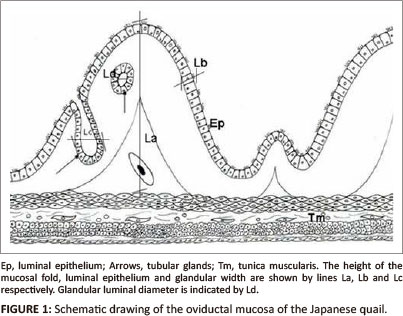
Tissue samples from the middle part of the magnum were fixed in 10% buffered formalin for 48 h. Tissue samples were then processed routinely for light microscopy (Drury & Wallington 1976) using an automated tissue processor (Shandon excelsior®, Thermo Electron Corporation, Germany). After staining with haematoxylin and eosin (H&E), sections of the tissue samples were examined under bright field light microscope (Olympus BH-2) at a magnification 40 χ and/or 100 χ for histopathological changes. In addition, tissue morphometry such as the height of primary folds, epithelial height, tubular gland width and glandular luminal diameters were evaluated at magnification 20 χ and/or 40 χ using an image analyser (AnalySIS®; Olympus BX 50, Optical Company LTD, Japan). The height of a primary fold was measured by drawing a vertical line from the base to the luminal end (Figure 1). Epithelial height was determined by measuring the height of 15 cells in five different primary folds (Berg et al. 2001). The individual glandular width was measured by drawing a perpendicular line across the widest part of the gland (Figure 1). Glandular luminal diameter was determined by measuring the perpendicular distance between two opposing gland cells. Morphometrical data were analysed using analysis of variance (ANOVA), SPSS version 17. A probability of 5% was considered significant. Photomicrographs were taken using a CC-12 digital camera mounted on the image analyser.
Scanning and transmission electron microscopy studies
Tissue samples from the middle part of the magnum were immersion fixed in 2.5% glutaraldehyde in 0.1 M Millonig's buffer (pH 7.3) for 48 h. The tissue samples were subsequently post-fixed in 2.0% osmium tetroxide for 2 h. After dehydration in graded concentrations of alcohol, tissue samples were processed for scanning electron microscopy (SEM) and transmission electron microscopy (TEM) using standard techniques. Samples for SEM were viewed and photographed with a Philips XL20 scanning electron microscope. The transmission electron microscopy samples were viewed and photographed with a Philips CM10 electron microscope.
Results
Tissue morphometry
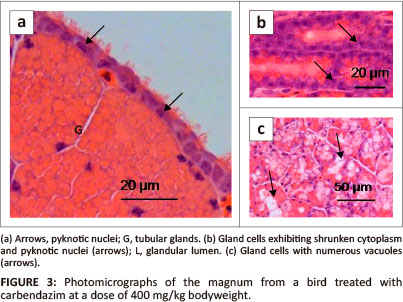
Morphometric data measured in the control and carbendazim treated birds are summarised in Table 1.
Control birds
The height of primary folds in the magnum measured between 234.84 µm and 515.88 µm (mean 378.23 ± 21.3). The height of the luminal epithelium ranged from 15.72 µm to 31.51 µm (mean 22.98 + 0.25). The width of glands measured between 22.99 µm and 49.12 µm (mean 33.96 ± 0.84). The glandular luminal diameters varied from 2.04 µm to 23.12 µm (mean 11.47 + 0.81).
Carbendazim-treated birds
There was a general decrease in the height of primary mucosal folds in carbendazim-treated groups. The decrease was significantly different at doses of 400 mg/kg and 800 mg/kg bodyweight carbendazim when compared to the control group (Table 1). A significant decrease in the height of the mucosal folds was also observed between carbendazim treatment groups, such as: 25 mg/kg and 400 mg/kg; 25 mg/kg and 800 mg/kg; 100 mg/kg and 400 mg/kg, as well as, 100 mg/kg and 800 mg/kg bodyweight carbendazim. Administration of carbendazim also decreased the height of the luminal epithelium. A significant decrease in the height of the luminal epithelium was seen at doses of 100 mg/kg, 400 mg/kg and 800 mg/kg bodyweight carbendazim when compared to the control group. In addition, a decrease in the luminal epithelial height was also observed between carbendazim treatment groups such as: between 25 mg/kg and 400 mg/kg; 25 mg/kg and 800 mg/kg; 100 mg/kg and 400 mg/kg; and between 100 mg/kg and 800 mg/kg bodyweight carbendazim.
There was a general decrease in the width of the magnal glands after exposure to carbendazim (Table 1). However, a significant decrease in glandular width was observed at doses of 100 mg/kg, 400 mg/kg and 800 mg/kg bodyweight carbendazim when compared to the control. In addition, glandular width was significantly decreased between 25 mg/kg and 100 mg/kg; 25 mg/kg and 400 mg/kg, as well as between 100 mg/kg and 800 mg/kg bodyweight carbendazim. Glandular luminal diameters were also decreased by carbendazim administration. Significant decreases in luminal diameters were detected at doses of 25 mg/kg, 100 mg/kg, 400 mg/kg and 800 mg/kg bodyweight carbendazim when compared to the control group (Table 1). In addition, a significant decrease in luminal diameter was observed between 100 mg/kg and 800 mg/kg bodyweight carbendazim treatment groups.
Histological observations
Control birds
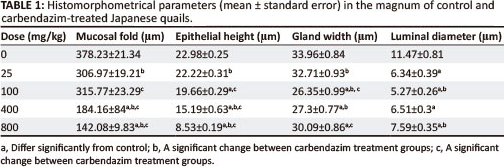
The mucosal and submucosal layers of the magnum were visible as primary and secondary folds (Figure 2a). Occasional tertiary folds were also observed. Simple columnar epithelium consisting of ciliated and non-ciliated cells lined the mucosal layer (Figure 2b).
The lamina propria-submucosa contained simple and branched tubular glands (glandulae magni). A scanty, loose connective tissue and a few blood vessels were observed interspaced between the glands. The gland cells contained round nuclei and eosinophilic granular cytoplasm. The lumena of these glands contained eosinophilic secretory material.
A thin tunica muscularis containing inner circular and outer longitudinal layers was present. Loose connective tissue, the tunica serosa, enclosed the magnum. A simple squamous epithelium lined the serosa.
Carbendazim-treated birds
No morphological changes were observed in the magnum mucosa or in the lamina propria-submucosa post-exposure to carbendazim at doses of 25 mg/kg and 100 mg/kg bodyweight.
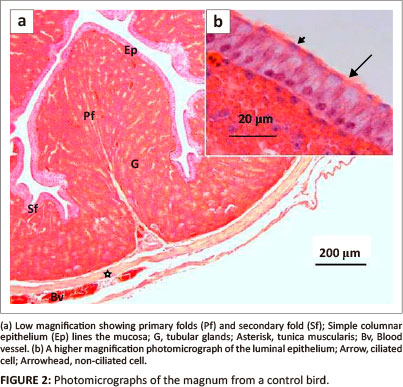
At a dose of 400 mg/kg bodyweight, a few degenerating luminal epithelial cells contained pyknotic nuclei (Figure 3a). Isolated areas of glandular atrophy were evident in the lamina propria-submucosa. The atrophic glands were characterised by cells with shrunken cytoplasm, as well as, karyorrhetic and pyknotic nuclei (Figure 3b). In a few areas, degenerating gland cells contained numerous vacuoles, pale cytoplasm, as well as, karyorrhetic and pyknotic nuclei (Figure 3c).
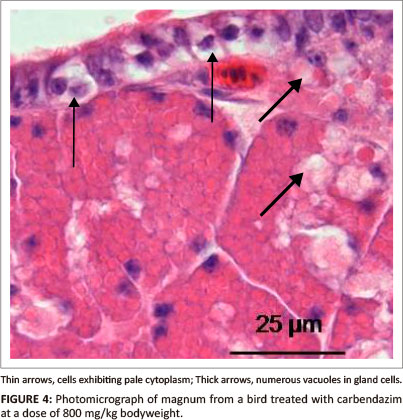
At a dose of 800 mg/kg bodyweight, vacuolation and cell swelling were observed in degenerating epithelial cells (Figure 4). Hyperaemia and leukocytic infiltrations were occasionally observed in the lamina propria-submucosa underlying the epithelium. In the glandular tissue, numerous vacuoles were observed in degenerating gland cells. No degenerative changes were identified in the tunica muscularis.
Scanning electron microscopy
Control birds
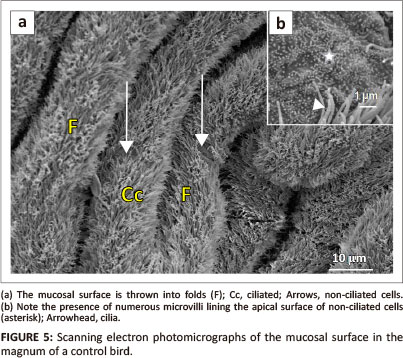
The mucosal layer of the magnum formed longitudinally oriented luminal folds (Figure 5a). Both primary and secondary folds were identified. The luminal epithelium consisted of ciliated and non-ciliated cells. The non-ciliated cells exhibited a dome-shaped apical surface. The surface of the non-ciliated cells was lined by microvilli (Figure 5b). The ciliated cells, which appeared to be predominant, displayed long cilia. In some instances, the cilia tended to obscure the non-ciliated cells. Round to oval-shaped glandular openings were identified between the epithelial cells.
Carbendazim-treated birds
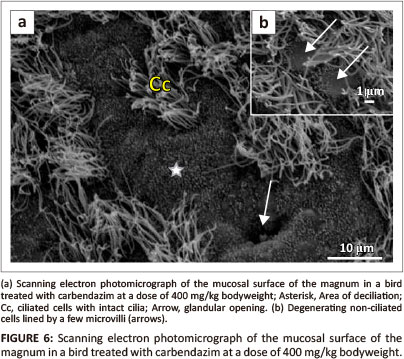
No degenerative changes were observed on the magnal mucosal surface of birds treated with carbendazim at doses of 25 mg/kg or 100 mg/kg bodyweight. Degenerative changes were observed at doses of 400 mg/kg and 800 mg/kg bodyweight. At doses of 400 mg/kg and 800 mg/kg bodyweight, isolated areas of deciliation were observed (Figure 6a). In these areas, short ciliary stems, which indicated cilia degeneration, were observed. The non-ciliated cells contained sparsely distributed microvilli (Figure 6b).
Transmission electron microscopy
Control birds
Simple columnar epithelium, composed of ciliated and non-ciliated cells, lined the magnum (Figure 7a). The ciliated cells presented electro-lucent cytoplasm. A round to oval nucleus was located in the central cytoplasmic region of the cell. Several cytoplasmic organelles which included cisternae of rough endoplasmic reticulum (RER) and mitochondria were observed adjacent to the nucleus. Golgi complexes and lysosomes were identified in the supranuclear region. A few filaments were observed perinuclearly. On the apical plasma membrane, long cilia lined the ciliated cells (Figure 7a). A few microvilli were observed between the cilia. The cilia were rooted in the cytoplasm and anchored by basal bodies. Supporting the basal bodies were basal feet and striated rootlets.
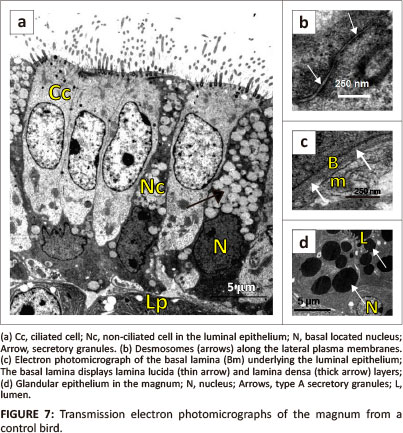
The non-ciliated cells contained round to irregular-shaped nuclei, which were located basally (Figure 7a). In these cells, the cytoplasm appeared electron dense due to presence of dense particles. Numerous membrane-bound secretory granules were observed in the central and apical cytoplasmic regions (Figure 7a). The secretory granules contained particles of intermediate electron density. Cisternae of RER and mitochondria were observed infranuclearly. The apical plasma membrane was lined by microvilli. Desmosomes linked adjacent epithelial cells along the lateral plasma membranes (Figure 7b).
The luminal epithelial cells in the magnum rested on a granular basal lamina of approximately 78 nm in thickness. The basal lamina contained a lamina lucida on the epithelial aspect and a lamina densa on the side adjacent lamina propria-submucosa (Figure 7c).
Simple, branched tubular glands were observed in the lamina propria-submucosa. The gland cells contained round euchromatic nuclei located basally (Figure 7d). The cytoplasm contained numerous cisternae of RER and free ribosomes. In addition, small to medium-sized secretory granules were observed in the apical cytoplasmic regions. Two types of secretory granules were identified: type A and type B. Type A secretory granules contained electron-dense particles (Figure 7d). Type B granules were larger than type A and contained particles of an intermediate electron density. The gland cells contained a few mitochondria which were located perinuclearly. Long and slender microvilli lined the apical plasma membranes. Desmosomes and occasional tight junctions were observed along the lateral plasma membranes. Underlying the magnal glands was a basal lamina of approximately 34 nm in thickness, which blended with the loose connective tissue of the lamina propria-submucosa. The basal lamina contained homogeneous particles of intermediate electron density. Supporting cells, associated with tubular glands, contained elongated euchromatic nuclei surrounded by scanty cytoplasm of intermediate electron density. Several cisternae of RER and a few mitochondria were observed in the cytoplasm.
Carbendazim treated birds
Carbendazim did not cause observable morphological changes at doses of 25 mg/kg and 100 mg/kg bodyweight. At a dose of 400 mg/kg bodyweight, degenerating ciliated cells contained pyknotic nuclei. Blebbing of the nuclear membrane was also a notable feature. In a few ciliated cells, crenated nuclei and those exhibiting marginalised nuclear chromatin were observed. At this dose, the ciliated cells were lined by relatively few cilia. Several lysosomes and vacuolated mitochondria were observed in the apical cytoplasmic regions (Figure 8a). Although the basal bodies were intact, the rootlets supporting them were indistinct. Degenerating non-ciliated cells were lined by a few microvilli. In addition, vacuoles and formation of myelin figures were occasionally observed. In these cells, swollen mitochondria were also identified. Desmosomes linking adjacent cells were intact. No degenerative changes were seen in the basal lamina. The lamina was approximately 83 nm in thickness.
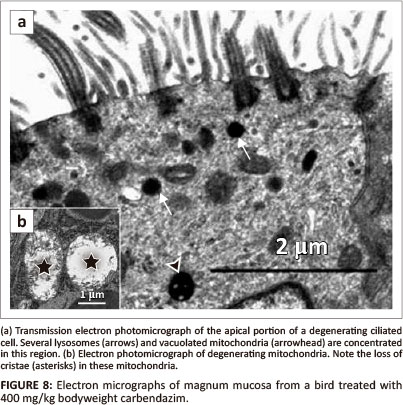
Degenerating glands were characterised by the presence of several vacuoles and swollen mitochondria (Figure 8b). Cellular junctions along the lateral plasma membranes were intact. Basal lamina which measured approximately 36 nm in thickness appeared normal. At this dose, supporting cells contained electron-dense cytoplasm. A few vacuoles and degenerating mitochondria were also identified.
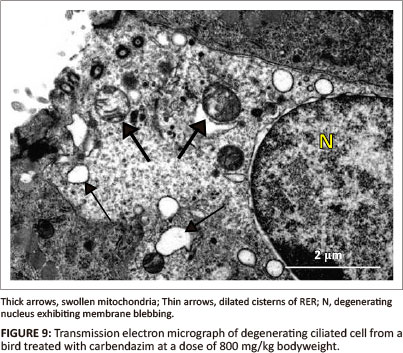
At a dose of 800 mg/kg bodyweight, deciliation and nuclear degeneration were observed in ciliated cells. The observed nuclear degeneration included: pyknosis, margination of nuclear chromatin and blebbing of the nuclear membrane (Figure 9). Although the basal bodies appeared intact, rootlet striations were indistinct. Swollen mitochondria, vacuoles and dilated RER cisternae were observed in the apical cytoplasmic region (Figure 9). A few lysosomes were also identified in this region. Degenerating non-ciliated cells contained pyknotic nuclei and numerous vacuoles. At this dose (800 mg/kg), the non-ciliated cells contained a few secretory granules which were concentrated in the apical regions of the cytoplasm. The degenerating secretory granules contained fragmented particles of an intermediate electron density. Desmosomes, which were observed along the lateral plasma membrane, were intact. The epithelial cells rested on the basal lamina of approximately 70 nm thick. The structure of the basal lamina was similar to that observed in 400 mg/kg bodyweight carbendazim-treated group.
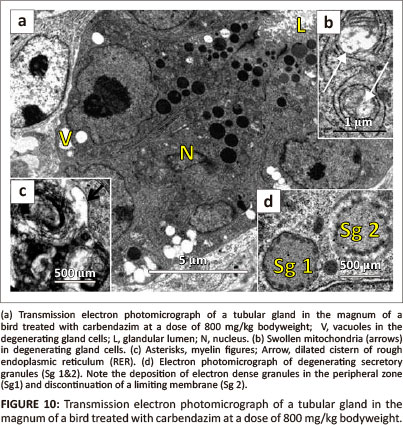
Vacuolation and pyknosis were observed in degenerating gland cells (Figure 10a). In addition, swollen mitochondria were also observed in these cells (Figure 10b). Dilated RER cisternae and myelin figures were observed in the perinuclear region of degenerating gland cells (Figure 10c). In some instances, degenerating secretory granules were observed. The degenerating secretory granules displayed condensed electron dense particles, which were displaced to the peripheral zone (Figure 10d). In addition, degeneration of the limiting membrane was observed. Relatively few microvilli lined the apical plasma membranes of the gland cells. These microvilli appeared thick when compared to the control group. Desmosomes along the lateral plasma membranes were structurally normal. Invaginations of the basal lamina lining the glandular cells were occasionally observed. At this dose, the basal lamina measured approximately 43 nm in thickness. Supporting cells contained crenated nuclei. In a few cells, blebbing of the nuclear membrane was observed. Swollen mitochondria and dilated RER cisternae were observed throughout the cytoplasm. A few lysosomes and vacuoles were occasionally identified.
Discussion
The present study has highlighted the effect of various doses of carbendazim on the morphology of the magnum in the Japanese quail. This appears to be the first report of the effect of cytoskeletal-disrupting fungicide in the female reproductive tract of birds. The results show that carbendazim caused dose dependent morphological changes in the magnum. The minimum toxic dose determined in the current study was 400 mg/kg bodyweight carbendazim, which caused identifiable morphological changes in this section. Severe morphological changes were apparent when the dose of carbendazim was increased to 800 mg/kg bodyweight. Similar findings were observed in the male Japanese quail (Aire 2005), as well as in the rat, rabbit and hamster (Mantovani et al. 1998). No morphological changes were observed in birds treated with 25 mg/kg and 100 mg/kg bodyweight carbendazim. This could be due to rapid elimination of the carbendazim from body tissues. Elimination of carbendazim from animal tissues is rapid through urine, bile and faeces after hydrolysis and conjugation in the liver (Gardiner et al. 1974). According to Ahdaya, Monroe and Guthrie (1981), no metabolite residues of carbendazim were found in the animal tissues or their products when administered at low doses.
The results of morphometrical study show that, carbendazim reduced the height of mucosal folds, luminal epithelium, as well as glandular width at a dose of 400 mg/kg bodyweight. This decrease could be due to atrophy of the oviduct as a result of carbendazim toxicity. In addition, reduced height could have also been caused by atrophy due to the androgenic effect exerted by carbendazim exposure. In the rat, carbendazim treatment caused androgenic effects which included atrophy and degeneration of the genital tract (Lu et al. 2004).
In the control birds, histological morphology of the magnum was in agreement with earlier investigations in the Japanese quail by Fitzgerald (1969), as well as Eroschenko and Wilson (1974). It was also similar to the observations made in the domestic fowl (Wyburn et al. 1970), rhea (Parizzi et al. 2008) and brood-parasitic birds (Rueda-Cediel, Kattan & Ramirez-Pinilla 2008). In contrast to the control birds, the results of carbendazim-treated birds indicated degeneration of luminal and glandular epithelial cells at high doses.
The morphology of the mucosal surface in the magnum of the control birds as revealed by scanning electron microscopy is in consonance with observations made in the domestic fowl by Bakst and Howarth (1975). Loss of cilia by ciliated cells was observed in the mucosal epithelial lining, following carbendazim exposure at doses of 400 mg/kg and 800 mg/kg bodyweight. Deciliation could have been caused by weakening of the cilia shaft due to disorganisation of the axial microtubular complex (axoneme). Carbendazim binds the β-tubulin sub-unit of the microtubule (Burland & Gull 1984) and consequently inhibits the binding of guanosine triphosphate (GTP) to the tubulin (Winder, Strandgaard & Miller2001). The interaction between carbendazim and axonemal tubulin could have resulted in the disruption of microtubules and consequently defective cilia. Deciliation is a common reaction observed in the epithelial lining of the mucosa when the female reproductive tract is exposed to toxins. For example, loss of cilia was observed in the magnum of pekin ducks fed diets containing high doses of methyl mercury for 28 days (Balachandran, Bhatnagar & Gcissinger 1985).
TEM results also showed nuclear degenerative changes, such as pyknosis and karyorrhexis in birds treated with carbendazim at doses of 400 mg/kg and 800 mg/kg bodyweight. These observations represent chromosomal damage due to carbendazim toxicity. Reports by Styles and Garner (1974), Piati, Mirabini and Chiesara (1994) and Lebailly et al. (1997) support this observation. Styles and Garner (1974) reported chromosomal damage in the rat using cultured cells and following oral exposure to carbendazim and carbendazim parent compound (benomyl). Piati, Mirabini and Chiesara (1994) reported degenerative changes in the nuclei in the hepatocytes of rats exposed to benomyl. According to these authors, the nuclear degeneration presented as increased frequency of micronuclei and chromosomal damage. By using single-cell gel electrophoresis assay, Lebailly et al. (1997) showed chromosomal damage in human lymphocytes post-exposure to carbendazim.
In addition to nuclear degeneration, degenerative changes were also observed in the cytoplasmic organelles, such as: swollen mitochondria, dilated cisternae of RER and increased number of lysosomes and vacuoles. Similar degenerative changes have been observed in the magnum of pekin ducks post-exposure to methyl mercury (Balachandran, Bhatnagar & Gcissinger 1985). Degeneration of mitochondria indicates stimulation of the apoptotic process through activation of the caspase chain. The increased number of lysosomes and vacuoles observed suggest intracellular clearance of the degenerative material within the cell. This hypothesis is supported by a research report by Chousalkar and Roberts (2008), which stated that vacuoles serve as a reservoir for unutilised secretory material during egg formation.
In conclusion, luminal and glandular epithelial cells in the magnum play a major role in the process of egg formation. Degeneration of these cells, following carbendazim treatment, poses a potential threat to the reproductive activities of exposed birds.
Acknowledgements
This work was supported by the Deutscher Akademischer Aus-tauschdienst (DAAD doctoral scholarship), the University of Pretoria and the South African Veterinary Foundation (SAVF). The authors thank staff of the University's EM unit and histopathology laboratory for their technical assistance. Mrs W. Olivier is acknowledged for assisting with the schematic drawing of Figure 1.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this article.
Author contributions
M-C.M. (University of Pretoria) and H.B.G. (University of Pretoria) designed the project and supervised the research. W.H.K. (Sokoine University of Agriculture and University of Pretoria) performed the experiments and prepared the manuscript.
References
Aire, T.A., 2005, 'Short-term effects of carbendazim on the gross and microscopic features of the testes of Japanese quails (Coturnix coturnix japonica)', Anatomy and Embryology 210, 43-49. http://dx.doi.org/10.1007/s00429-005-0001-0, PMid:16034611 [ Links ]
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E., 1981, 'Absorption and distribution of intubated insecticides in fasted mice', Pesticide Biochemistry and Physiology 16, 38-46. http://dx.doi.org/10.1016/0048-3575(81)90070-5 [ Links ]
Bakst, M. & Howarth, B. (Jr.), 1975, 'SEM preparation and observations of hen's oviduct' Anatomical Record 181, 211-226. http://dx.doi.org/10.1002/ar.1091810205 PMid:1090204 [ Links ]
Balachandran, A., Bhatnagar, M.K. & Gcissinger, H.D., 1985, 'Scanning and transmission electron microscopic studies on the oviducts of pekin ducks fed methyl mercury containing diets', Scanning Electron Microscopy 1, 311-322. [ Links ]
Berg, C., Holm, L., Brandt, I. & Brunstrom, B., 2001, 'Anatomical and histological changes in the oviducts of Japanese quail, Coturnix coturnix japonica, after embryonic exposure to ethynyloestradior, Reproduction 121, 155-165. http://dx.doi.org/10.1530/rep.0.1210155, PMid:11226039 [ Links ]
Burland, T.G. & Gull, K., 1984, 'Molecular and cellular aspects of the interaction of benzimidazole fungicides with tubulin and microtubules), in A.P.J. Trinci & J.F. Riley (eds.), Mode of action of antifungal agents, p. 299-320, Cambridge University Press, Cambridge. [ Links ]
Carter, S.D. & Laskey, J.W., 1982, 'Effect of benomyl on reproduction in the male rat) Toxicology Letters 11, 87-94. http://dx.doi.org/10.1016/0378-4274(82)90111-4 [ Links ]
Chousalkar, K.K. & Roberts, J.R., 2008, 'Ultrastructural changes in the oviduct of the laying hen during the laying cycle), Cell Tissue and Research 332, 349-358. http:// dx.doi.org/10.1007/s00441-007-0567-3, PMid:18236079 [ Links ]
Davidse, S.D. & Flach, W., 1977, 'Differential binding of methyl benzimidal-2-yl carbamate to fungal tubulin as a mechanism of resistance to this antimitotic agent in mutant strains of Aspergilus nidulans'Journal of Cell Biology 72, 174-193. http://dx.doi.org/10.1083/jcb.72.L174, PMid:12184 [ Links ]
Davidson, M.F., 1986, 'Histological studies of changes in the magnum of the domestic hen associated with the production of watery white eggs', British Poultry Science 27, 353-354. http://dx.doi.org/10.1080/00071668608416889, PMid:3742272 [ Links ]
Drury, R.A.B. & Wallington, E.A. (eds), 1976, Carleton's histological techniques, 4th edn., Oxford University Press, London. [ Links ]
Eroschenko, V.P. & Wilson, W.O., 1974, 'Histological changes in the regressing reproductive organs of sexually mature male and female Japanese quail), Biology of Reproduction 11, 168-179. http://dx.doi.org/10.1095/biolreprod11.2.168, PMid:4457133 [ Links ]
Finnish National Board of Health, 1982, 'The toxicity of the benzimidazole type fungicides: benomyl, carbendazim and thiophanate-methyr, Second report, Finnish National Board of Health, Helsinki. [ Links ]
Fitzgerald, T.C. (ed.), 1969, The coturnix quail: Anatomy and histology, Iowa State University Press, Ames. [ Links ]
Gardiner, J.A., Kirkland, J.J., Klopping, H.L. & Sherman, H., 1974, 'Fate of benomyl in animals), Journal of Agriculture and Food Chemistry 22, 419-427. http://dx.doi.org/10.1021/jf60193a046, PMid:4840504 [ Links ]
Goldman, J.M., Rehinberg, G.L., Cooper, R.L., Gray, L.E., Hein, J.F. & McElroy, W.K., 1989, 'Effects of the benomyl metabolite carbendazim on the hypothalamic-pituitary reproductive axis in the male rat), Toxicology 57, 173-182. http://dx.doi.org/10.1016/0300-483X(89)90163-7 [ Links ]
Hess, R.A., Moore, B.J., Linder, R.E. & Abuel-Atta, A.A., 1991, 'The fungicide benomyl (methyl-1-(butylcarbamate)-2-benzimidazolecarbamate) causes testicular dysfunction by inducing sloughing of germ cells and occlusion of efferent ductules), Fundamentals of Applied Toxicology 17, 733-745. http://dx.doi.org/10.1016/0272-0590(91)90181-3 [ Links ]
International Program on Chemical Safety (IPCS), 1986, 'Report No. 64: Environmental health criteria for carbamate pesticides: A general introduction), www.inchem.org [ Links ]
Lebailly, P., Vigreux, C., Godard, T., Sichel, F., Bar, E., Letalaer, J.Y., Henry-Amar, M. & Gauduchon, P., 1997, 'Assessment of DNA damage induced in vitro by etoposite and two fungicides (carbendazim and chlorothalonil) in human lymphocytes with the comet assay), Mutation Research 375, 2005-217. http://dx.doi.org/10.1016/S0027-5107(97)00015-8 [ Links ]
Lim, J. & Miller, M.G., 1997, 'The role of the benomyl metabolite carbendazim in benomyl-induced testicular toxicity', Toxicology and Applied Pharmacology 142, 401-410. http://dx.doi.org/10.1006/taap.1996.8042, PMid:9070363 [ Links ]
Lu, S-Y., Liao, J-W., Kuo, M-L., Wang, S-C., Hwang, J-S. & Ueng, T-H., 2004, 'Endocrine disrupting activity in carbendazim-induced reproductive and developmental toxicity in rats', Journal of Toxicology and Environmental Health 67, 1501-1515. http://dx.doi.org/10.1080/15287390490486833, PMid:15371226 [ Links ]
Mantovani, A., Maranghi, F., Ricciardi, C., Macri, C., Stazi, A.V., Attias, L. & Zappini, G.A., 1998, 'Developmental toxicity of carbendazim: Comparison of no-observed-adverse-effect level and benchmark dose approach', Food and Chemical Toxicology 36, 37-45. http://dx.doi.org/10.1016/S0278-6915(97)00116-6 [ Links ]
Palmiter, R.D. & Gutman, G.A., 1972, 'Fluorescent antibody localization of ovalbumin, conalbumin, ovomucoid and lysozyme in chick oviduct magnum', The Journal of Biological Chemistry,6459-6461. [ Links ]
Parizzi, R.C., Santos, J.M., Oliviera, M.F., Maia, M.O., Sousa, J.A., Miglino, M.A. & Santos, T.C., 2008, 'Macroscopic and microscopic anatomy of the oviduct in the sexually mature hea (Rhea americana)', Anatomia Histologia Embryologia 37, 169-176. http://dx.doi.org/10.1111/j.1439-0264.2007.00822.x, PMid:18070241 [ Links ]
Piati, E., Mirabini, L. & Chiesara, E., 1994, 'Increase of micronucleus frequency in cultured rat hepatocytes treated in vitro with benomyl and pirimiphos-methyl separately and in mixture', Mutation Research 324, 59-64. http://dx.doi.org/10.1016/0165-7992(94)90068-X [ Links ]
Pico, Y., La Farre, M., Soler, C. & Barcelo, D., 2007, 'Identification of unknown pesticides in fruits using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry Imazilil as a case study of quantification', Journal of Chromatography 1176, 123-134. http://dx.doi.org/10.1016/'.chroma.2007.10.071, PMid:18021786 [ Links ]
Rueda-Cediel, P., Kattan, G. & Ramirez-Pinilla, M.P., 2008, 'Ovarian and oviductal morphology of a brood-parasitic bird, Molothrus bonariensis (Passerifomes, Icteridae)', Acta Zoologica 89, 261-276. http://dx.doi.org/10.1111/j.1463-6395.2007.00315.x [ Links ]
Still, G.G. & Mansager, E.R., 1975, 'Alfalfa metabolism of propham', Pesticide Biochemical Physiology 5, 515-522. http://dx.doi.org/10.1016/00483575(75)90026-7 [ Links ]
Styles, J.A. & Garner, R., 1974, 'Benzimidazole carbamate methyl ester - Evaluation of its effects in vivo and in vitro', Mutation Research 26, 177-187. http://dx.doi.org/10.1016/S0027-5107(74)80072-2 [ Links ]
Winder, B.S., Strandgaard, C.S. & Miller, M.G., 2001, 'The role of GTP binding and microtubule-associated proteins in the inhibition of microtubule assembly by carbendazim', Toxicological Science 59, 138-146. http://dx.doi.org/10.1093/toxsci/59.1.138, PMid:11134553 [ Links ]
Wyburn, G.M., Johnston, H.S., Draper, M.H. & Davidson, M.F., 1970, 'The fine structure of the infundibulum and magnum of the oviduct of Gallus domesticus', Quarterly Journal of Experimental Physiology 55, 213-232. PMid:5201499 [ Links ]
 Correspondence:
Correspondence:
Wahabu Kimaro
Email: kim16wh@yahoo.com
Postal address: Private Bag X04, Onderstepoort 0110, South Africa
Received: 20 Feb. 2013
Accepted: 29 Apr. 2013
Published: 19 July 2013














