Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Onderstepoort Journal of Veterinary Research
versão On-line ISSN 2219-0635
versão impressa ISSN 0030-2465
Onderstepoort j. vet. res. vol.78 no.1 Pretoria Jan. 2011
ORIGINAL RESEARCH
The diagnosis and prevalence of persistent infection with bovine viral diarrhoea virus in South African feedlot cattle
Thelma MeiringI,II; Leon ProzeskyII; Eben R. du PreezIII; Dirk J. VerwoerdIV
IIDEXX Laboratories, Onderstepoort, South Africa
IIDepartment of Paraclinical Sciences, University of Pretoria, South Africa
IIISD Morris Consulting Services, Johannesburg, South Africa
IVKaran Beef Feedlot, Heidelberg, South Africa
ABSTRACT
Bovine viral diarrhoea virus (BVDV) infection is an important viral infection affecting the cattle industry today. The prevalence of this infection in South African feedlots is unknown. Ear notch biopsies were collected from chronic poor doers and animals that appeared unthrifty upon entering feedlots, as well as animals entering the hospital pen with respiratory disease for the first time. A total of 1690 samples were collected: 1074 from the former category and 616 from the latter. A routine immunohistochemistry staining protocol showed that 49 animals tested positive, of which 43 (4%) came from the feedlot entry group and six (1%) from the hospitalised group. The prevalence of persistently infected cattle from this selected, nonrandom sample entering six large South African feedlots was found to be 2.9%, which is higher than the international rule of thumb that 0.5% of all cattle entering feedlots are persistently infected. There was no clear correlation between persistent infection and respiratory disease. Serum samples were also collected when possible and 10 positive cases were found. Results from enzyme-linked immunosorbent assays for antigen and antibody performed on these sera correlated well with those from the immunohistochemistry staining method in six cases, but in four cases the animals tested falsely positive owing to nonspecific staining. Immunohistochemistry staining on ear notch biopsies is thus a reliable diagnostic method to identify persistently infected animals with BVDV, but the pathologist should be aware of nonspecific positive staining.
Introduction
Bovine viral diarrhoea virus (BVDV) is one of the most economically important pathogens in the cattle industry today (Firat et al. 2002; Hilbe et al. 2007; Houe, Lindberg & Moennig 2006; Radostits et al. 2000; Van Vuuren 2005). It causes a multitude of different diseases including subclinical benign diarrhoea (bovine virus diarrhoea), peracute highly fatal diarrhoea, haemorrhagic and thrombocytopaenic disease, reproductive failure, fatal mucosal disease of persistently infected (PI) animals infected early in utero, and abortions and malformations (Firat et al. 2002; Radostits et al. 2000). In addition, BVDV causes a general immunosuppression (Peterhans, Jungi & Schweizer 2003) that is strongly associated with chronic nonresponsive respiratory disease in feedlots (Haines et al. 2001). BVDV is a member of the genus Pestivirus (family Flaviviridae). There are two biotypes, designated as noncytopathic (NCP) and cytopathic (CP), depending on their effect on tissue culture cells. The NCP type is the most common and the most important and is also the one causing persistent infection in animals. There is considerable antigenic diversity and cross-reactivity amongst isolates of BVDV. The virus has been divided into genotypes I and II, depending on antigenic and genetic differences (Kampa et al. 2007). Both NCP and CP isolates exist within each genotype. Genotype I has recently been subdivided into two subgenotypes, BVDV1a and BVDV1b (Potgieter 2004; Radostits et al. 2000). Additional subgenotypes have been proposed for strains that are unique to southern Africa (Potgieter 2004).
The NCP type of BVDV is predominant in nature (Firat et al. 2002; Radostits et al. 2000). When the NCP virus infects the foetus between day 42 and day 125, the immune system develops a tolerance towards the virus and an immune response to that specific BVDV strain does not develop. (Hilbe et al. 2007; Kampa et al. 2007; Luzzago et al. 2006; McClurkin et al. 1984). The calves are born persistently viraemic and continue to shed virus every day for the rest of their lives. The neonatal mortality of PI calves is high and they are often born weak (Moczygemba 2003). Others are normal and healthy at birth. Fatal mucosal disease may develop within 6-24 months of age if the animal is super-infected with a homologous strain of a CP virus or if the NCP virus in the body mutates to a homologous CP virus (Potgieter 2004; Radostits et al. 2000).
The BVDV is of economic importance because of associated abortion, congenital defects, still births, increased neonatal mortality, prenatal and postnatal growth retardation, suboptimal reproductive performance, death from mucosal disease and premature disposal of PI animals (Cornish et al. 2005; Firat et al. 2002; Hilbe et al. 2007; Radostits et al. 2000). Economic impact specifically associated with feedlots includes expenses incurred for treatment, additional labour (care and movement of animals), premature culling because of chronic disease (repeat treatments) and profit reduction because of reduced growth and performance during and after illness (Hessman et al. 2009). Pens containing a single PI animal experience a pull rate (animals removed to hospital pen because of apparent illness) that is 33% higher than pens without PI animals. Pens adjacent to those with PI animals also experience a similar increase in morbidity rate or pull rate. Loneragan et al. (2005) found a pull rate incidence as high as 43%. The rule of thumb in the United States feedlot industry is that 1% of calves born per year in an infected herd are PI animals. About half of these are expected to die before weaning, which sets the industry's average intake rate of PI animals at 0.5% (Ishmael 2003).
Feedlots are particularly at risk of receiving PI animals. Although PI calves can present as stunted animals with an unthrifty coat, not all PI animals are in poor condition and it is therefore not possible to assess infection status exclusively from the physical appearance of an animal (Potgieter 2004). The real danger associated with PI animals is that they are persistently viraemic and immunosuppressed and shed virus either constantly or intermittently, thus being the main source of infection for other animals (Cornish et al. 2005; Kampa et al. 2007; Loneragan et al. 2005; Luzzago et al. 2006; Van Vuuren 2005). Both Brock et al. (1998) and Moczygemba (2003) suggest that the levels of viraemia in PI calves are cyclic and that higher levels of virus and shedding may occur under stressful situations, for example, transport and entry into a feedlot. Niskanen, Lindberg & Traven (2002) infected 10 calves with BVDV and bovine coronavirus (BCV) and put them with noninfected calves. None of the latter calves contracted BVDV, but all became BCV positive. The finding suggests that acutely infected animals do not secrete BVDV effectively and appear not to be a major source of infection for other animals; rather, PI animals appear to be the major source of BVDV infection (Potgieter 2004).
BVDV infects the lymphoid system and is immuno-suppressive (Potgieter 2004; Ridpath et al. 2002) as it infects the dendritic or stromal macrophages that support and nurse lymphoid cells during their development. Damage to these cells destroys the lymphoid population, which leads to lymphopaenia and immunosuppression (Wren 2001).
Immunohistochemistry (IHC) staining of skin biopsies is a reliable, fast diagnostic tool to identify the presence of BVDV antigen (Hilbe et al. 2007; Houe et al. 2006; Luzzago et al. 2006; Thur, Zlinszky & Ehrensperger 1996). Researchers have sampled skin from various locations on PI animals and found that results were identical, irrespective of the location from which the sample was collected (Thur et al. 1996). The presence of BVDV antigen in keratinocytes in the stratum basale and stratum spinosum has been documented in clinically normal PI cattle (Bielefeldt Ohmann 1988; DuBois et al. 2000; Sandvik 1999; Thur et al. 1996). According to Njaa et al. (2000), BVDV has a tropism for lymphocytes, mononuclear phagocytes and epithelial cells. In their study of formalin-fixed skin biopsies, positive IHC staining for BVDV was found in the cytoplasm of keratinocytes, sebaceous epithelial cells, mononuclear cells in the dermis and vascular smooth muscle cells. The epidermal staining occurred diffusely and predominantly in the stratum basale and stratum spinosum. The most prominent staining was found in the isthmus and infundibulum of hair follicles. Previous studies showed IHC staining to be as reliable as virus isolation (VI) to detect PI cattle (Broderson, White & Smith 1998; Thur et al. 1996). The advantages of IHC staining are that tissues are fixed in formalin and the procedure is more rapid and economical than VI.
There is little chance of identifying an acutely infected animal as falsely positive according to skin biopsy. Njaa et al. (2000) found positive IHC staining for BVDV on four skin samples in animals acutely infected with a high dose of BVDV. The staining was, however, confined to a few small, discrete foci in the stratum spinosum, with little extension into follicular ostia, and was distinguishable from the more extensive staining in PI animals. Libler-Tenorio, Ridpath and Neill (2003) found that BVDV antigen in inoculated calves was present only in lymphoid tissue and the intestinal mucosa but not the skin. Ridpath et al. (2002) infected animals with six different isolates and could not detect virus in any of the skin biopsies. Hilbe et al. (2007) detected positive staining only in the skin of PI animals and no positive stains in acutely infected animals. Based on these results PI animals are rarely expected to be confused with acutely infected animals when skin biopsies are used, which suggests that the technique can be used as a reliable, fast diagnostic tool to diagnose PI animals (Luzzago et al. 2006).
Antigen-capture enzyme-linked immunosorbent assay (ELISA) can also be used to test for the presence of BVDV antigen and several different ELISAs are commercially available (Brinkhof, Zimmer & Westenbrink 1996; Brock 1995; Hilbe et al. 2007; Sandvik 1999). ELISA is a versatile diagnostic method to detect almost any immunoreactive molecule and can also be used to detect serum antibodies (Hilbe et al. 2007). Clinically healthy PI calves older than 2 months are nearly always ELISA-negative for antibodies (Sandvik 1999). Exceptions occur when PI calves ingest high titres of maternal antibodies to a different strain and some of these persist for longer than 3 months, or when exposed to different field or vaccine strains, the PI calf mounts a (poor) humoural response.
The objectives of this study were to:
• determine, by means of IHC evaluation of skin biopsies, the prevalence of PI animals entering feedlots
• determine whether PI animals are at higher risk of contracting respiratory disease in feedlots than noninfected animals
• determine the reliability of IHC by investigating nonspecific staining or staining of few mononuclear cells with re-evaluation and/or restaining with another colour
• determine the reliability of IHC staining for identifying PI animals in comparison with the antibody and antigen capture ELISAs.
Materials and methods
Collection of specimens
Ear notch biopsies were collected from calves at the point of entry into feedlots, from calves presented to the hospital pen for the first time and from chronic poor doers from the following feedlots: Karan Beef, Mollevel, Taaiboschbult, Gysbertshoek, Hurland and Sparta Beef. The calves selected at point of entry were poor looking with a dull coat, unthrifty, thin, and potbellied and were suspected to be persistently BVDV infected. The chronic poor doers were animals that had received more than three treatments in the hospital pen and were placed on pasture because of poor performance in the feedlot. Dead animals in this group were also sampled. A total of 1074 specimens were collected from first-time entrants and chronic poor doers between March 2005 and October 2005, and 616 from animals entering the hospital pen for the first time because of respiratory disease between May 2006 and August 2006.
Ear notch samples measuring 10 mm x 5 mm collected with an ear tag applicator were fixed in 10% buffered formalin and presented to the laboratory for examination, within 3-4 weeks after collection. Ear notch samples were taken because of the ease of collection, but biopsy specimens could have been taken from any skin area, as BVDV antigen is evenly distributed in skin samples (Thur et al. 1996).
Immunohistochemistry staining
Sections were cut and embedded in wax blocks by routine standard operating procedures at the IHC laboratory of the Pathology section, Faculty of Veterinary Science, Onderstepoort. Seven specimens were placed in a single wax block as demonstrated in Figure 1. Each animal could thus be identified individually and costs were minimised.
The ABC Vector Elite immunoperoxidase staining procedure was used (Vectastain Universal Elite ABC kit from Merck, Vector Laboratories, UK), shown in Figure 2.
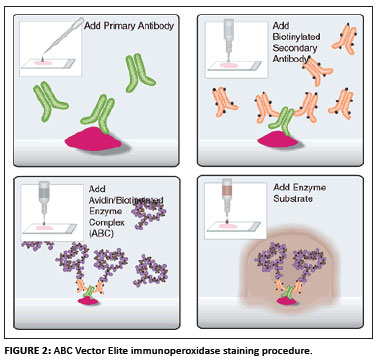
Sections of 3-4 µm were cut from the wax blocks. The tissues were then mounted on pretreated Superfrost Plus glass slides for immunoperoxidase staining. The slides were dried overnight in an oven at 58 ºC to enhance tissue section adhesion. Routine dewaxing and rehydration was performed (10 min in xylol and 3 min each in 100%, 96% and 70% alcohol according to the modified version of the method suggested by Bancroft and Stevens [1982]). The ABC Vector Elite immunoperoxidase staining procedure, as described earlier, was used (Figure 2). After dewaxing and rehydration antigen was demasked according to the proteolytic enzyme digestion (Pronase) method.
A 3% hydrogen peroxide solution (in distilled water) was used for 5 min to block endogenous peroxidases. Sections were rinsed twice: once in distilled water and once in phosphate buffered saline - bovine serum albumin (PBS-BSA) buffer for 5 min per rinse. The pronase (SIGMA Protease Type XIV) solution was prepared by adding 50 mg of the pronase powder to 100 mL of PBS-BSA buffer that had been heated to 37 ºC. The sections were incubated in the pronase-buffer solution in the oven at 37 ºC for 30 min. Sections were then rinsed in distilled water and then in PBS-BSA buffer for 5 min per rinse.
A drop of refrigerated normal rabbit serum was added to 10 drops PBS-BSA buffer, placed directly on sections and left for 20 min. The excess normal serum was gently shaken off the slides and slides were gently wiped clean around tissue sections. The primary monoclonal BVD antibody (Cornell University, New York) was applied to all the appropriate sections at a dilution of 1:1000 and left to stain for 1.5 h on the bench at room temperature. Negative controls were prepared using only PBS-BSA buffer or an irrelevant monoclonal antibody. The sections were rinsed twice; once in distilled water and once in PBS-BSA buffer for 5 min each.
The secondary biotinylated antiserum (rabbit anti-mouse antibody, diluted at 1:500) was applied on sections for 30 min and left at room temperature. The sections were then rinsed twice; with distilled water followed by PBS-BSA buffer for 5 min each. Peroxidase-conjugated avidin (Vector Laboratories, UK) was then applied (prepared according to the manufacturer's instructions) for 30 min on the bench at room temperature. The slides were rinsed twice as described before. The sections were placed in a diaminobenzidine (DAB) or NovaRED substrate (using a droplet method) for approximately 1 min. As soon as clear positive staining was observed macroscopically on the positive control slide, all other slides were rinsed in distilled water. The sections were then counterstained with haematoxylin for 3-4 min.
The sections were then rinsed under running tap water for 10 min to remove excess DAB or NovaRED substrate followed by routine dehydration through increased alcohol concentrations (70%, 96% and 100%) and xylol (Vector Laboratories, UK). Sections were then mounted and cover with a cover slip.
Enzyme-linked immunosorbent assays
Both BVDV antibody ELISAs and BVDV antigen capture ELISAs (IDEXX Laboratories BVDV Antibody Test Kit, Zul.No.BGVV-B233 version 06-44000-02 and IDEXX Laboratories BVDV Antigen Test Kit/Serum Plus, Zul.No.BGVV-B230 version 06-43860-02, respectively) were performed according to standard operating procedures by Golden VetLab (now IDEXX South Africa), Woodmead, South Africa.
Results
Immunohistochemistry staining
In total, 1690 ear notch biopsies were received for processing and IHC staining (1074 from calves entering feedlots and chronic poor doers; 616 from first-time admissions to the hospital because of respiratory disease). Of the total sample, 49 (2.9%) stained positive for BVDV antigen; 43 positive stains were found amongst the feedlot entrants and six positive stains were found amongst the samples from the hospitalised calves. Cases that showed granular DAB or NovaRED-coloured staining in the keratinocytes of the epidermis, epithelial cells in hair follicles, smooth muscle cells and fibroblasts were regarded as positive (Njaa et al. 2000). Figures 3-6 demonstrate positive staining observed with DAB or NovaRED.
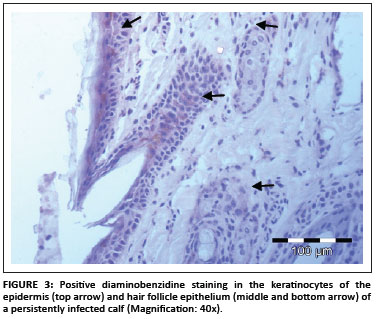
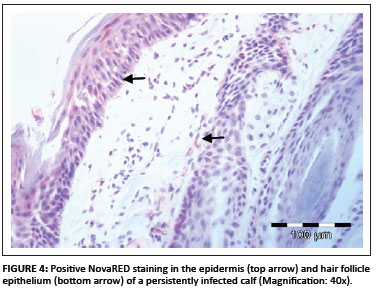
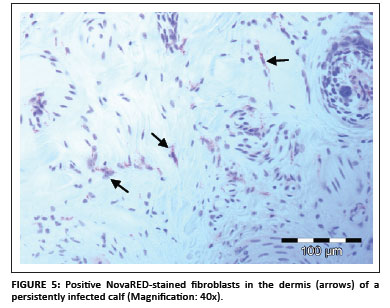
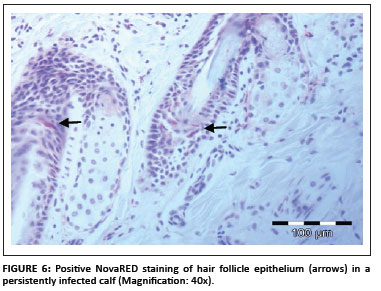
All samples were evaluated twice, because an abnormally high number of cases appeared to stain positively in one group at the first evaluation. In this group (from Taaiboschbult) 75 of 154 cases apparently stained positive. This raised concern and the staining patterns were investigated. It was concluded that a high number of cases showed nonspecific positive staining (vide infra). After re-evaluation, as described below, only 12 positive cases were identified.
Nonspecific staining was defined as nongranular DAB or NovaRED staining visible mainly in spindle and round cells in the dermis (Figure 7). In the samples investigatde during this study only spindle-shaped cells and round cells stained positive and no positive staining was evident in keratinocytes, hair follicle epithelial cells or smooth muscle cells.
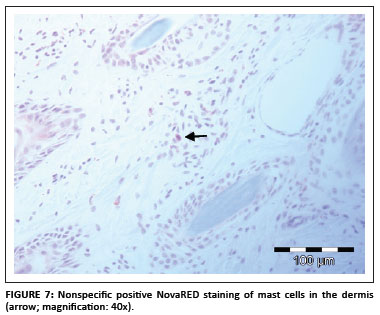
Positive staining of mast cell granules was suspected (S. Clift, pers. comm.), which was confirmed after staining the sections with Ziehl-Neelsen (ZN) stain (Figure 8). Most of the bovine mast cells in the skin were spindle shaped, which was confirmed with ZN staining. Mast cell granules stain blue (basophilic) with the ZN stain (University of Rochester 2007).
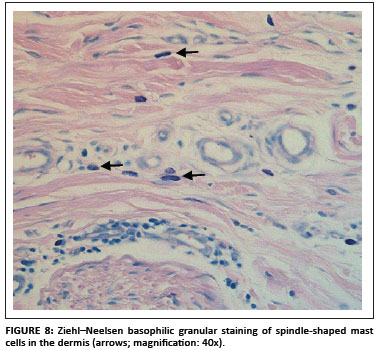
Mast cells stained positive more often with the DAB colour marker than with NovaRED. The majority of the nonspecific cases were stained with DAB. Another problem with using DAB as colour marker was that the colour pigment was sometimes difficult to distinguish from melanin pigment in the skin (yellow to golden colour). NovaRED was preferred as colour marker in this study as its unique brick-red colour precludes confusion with any other.
Enzyme-linked immunosorbent assays
Serum samples to test for BVDV antibodies and antigens were available from only 10 of the animals that originally tested positive during IHC staining. The results are presented in Table 1.
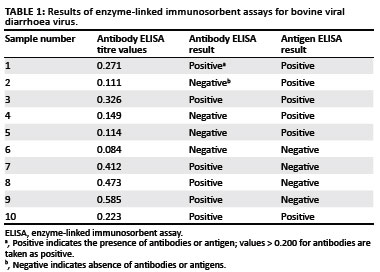
Of the 10 animals that tested positive during IHC staining, only six tested positive for viral antigen with the ELISA. PI animals will always be antigen positive, as they are consistently viraemic. This result indicated that animals 6, 7, 8 and 9 were not PI. These cases were re-evaluated, and as discussed later, they showed nonspecific positive staining in mast cells, leading to the false positive diagnosis.
A PI animal will not develop antibodies against the strain of BVDV that infected it in utero (McClurkin et al. 1984). Of the six animals that tested positive for viral antigen, three did not have antibodies against BVDV, which confirms their PI status. However, if a PI animal is infected with a significantly different strain of BVDV after birth, it could develop antibodies to that strain (McClurkin et al. 1984). This explains why three animals that tested positive for viral antigen during ELISA and IHC staining, also tested positive for antibodies against BVDV.
Discussion
IHC staining on ear notch biopsies is considered a fast and accurate diagnostic tool to identify animals that are persistently BVDV infected (Hilbe et al. 2007; Houe et al. 2006; Luzzago et al. 2006; Thur et al. 1996). The overall prevalence of PI calves entering feedlots in this study was 2.9%, which is much higher than the 0.5% estimate of Ishmael (2003). The estimate was based on the assumption that 1% PI animals are born into infected herds per year and that half of the animals are expected to die before weaning. Less than 0.5% of such animals will thus be available to enter feedlots owing to the early deaths of PI animals. Unpublished data from a South African study in July 2004 indicate a prevalence of PI animals of 0.56% in a single feedlot based on 2994 samples randomly collected from 20 000 calves entering the specific feedlot (W. Schultheiss, pers. comm., 01 September 2010). The samples in the present study were, however, taken from a selected population of calves, which were visually suspect animals and chronic poor doers, and therefore results cannot be directly compared to those of other studies. Feedlots receive large numbers of animals from a potentially large number of infected herds, increasing the possible number of PI animals that enter their feedlot population. Loneragan et al. (2005) state that when one PI animal is identified in a herd, the herd likely contains others as well and it is thus likely that PI animals entering a feedlot are clustered by herd of origin. In addition, many truckloads of calves entering South African feedlots are sourced from auctions and speculators, thus representing a collection of comingled animals from a variety of herds. Many of these high-risk calves are culled from breeding herds because of poor growth performance at weaning. A higher percentage of positive animals may thus be present in a feedlot compared to a single infected herd where, based on data from North America and Europe, approximately 1-2% of calves born are infected persistently.
The samples were collected mainly by feedlot personnel and distinctions were not made between animals entering the feedlot for the first time and chronic poor doers. It is thus not possible to determine how many suspected PI first-time entrants and how many chronic poor doers were sampled. It was, however, clear from the samples received that feedlot personnel preferred sampling chronic poor doers compared to first-time entrants. Selection of first-time entrants was based on their physical appearance, including appearing pot-bellied, unthrifty and thin. The feedlots were specifically interested in the possible causes of chronic poor doers, as it is an important problem in the feedlot industry that results in financial losses. This suggests that the likelihood of a PI animal to become a chronic poor doer in a feedlot is increased, which may also have played a role in the higher prevalence (2.9%) observed in this study. Loneragan et al. (2005) found that PI animals were more likely to become chronically ill or die. In the group of animals entering the hospital pen for the first time (collected separately at the end of the study), only 1% were infected persistently according to IHC staining.
The hypothesis that persistent infection with BVDV would increase the likelihood of contracting a respiratory illness soon after admission owing to the immunosuppressive nature of the disease was not substantiated during the study. However, the seasonal nature of bovine respiratory disease, the varying profile of calf ages and different purchasing strategies by the participating feedlots, coupled with the relatively small sample of hospital cattle in this study, preclude any firm conclusions on the contributory role of BVDV to bovine respiratory disease in South African feedlots. In addition, a significant number of calves that die following persistent infection with BVDV show severe complicated respiratory pathology with or without salmonellosis (D.J. Verwoerd, pers. comm., 03 February 2011). Further studies on the role of BVDV in the health of South Africa feedlot cattle are clearly indicated. Other factors causing respiratory disease in cattle in feedlots include the time of year (more prevalent in winter), extremely dusty conditions, stressors such as over-stocking, social structure in the pen, rain and mud, wind, poor adaptation, nonvaccinated (not pretreated) animals and respiratory disease following acidosis. In the unpublished study by Schultheiss mentioned earlier, no specific evidence of an increase in pneumonia in PI calves was found, but the presence of a PI animal in a pen increased the risk of the other calves in the pen contracting pneumonia threefold (W. Schultheiss, pers. comm.). This also corresponds to other studies such as that by Loneragan et al. (2005), who found an increase in respiratory disease in PI calves. The authors found the incidence of respiratory tract disease to be 43% greater in cattle with an opportunity for direct contact with a PI animal. These cattle also required more treatments for respiratory disease compared with cattle not exposed to a PI animal, thus incurring substantially higher medical costs.
The reliability of IHC staining to diagnose PI animals was also tested in this study. One of the most important findings was that nonspecific staining may lead to diagnosing an animal incorrectly as being infected persistently with BVDV. This problem was investigated after an inappropriate number of positive cases were diagnosed. Nonspecific positive staining was observed in round as well as spindle-shaped mast cells. As the pathologist becomes more proficient in evaluating these sections, it becomes clear when mast cells are staining positive because the granules have a distinctly different shape from the granular virus staining. All sections diagnosed as positive were re-evaluated to exclude any false positives from the final results. Although nonspecific staining was observed with both stains, it was far more frequently seen with DAB staining. It is not certain why mast cell granules stain positively with the BVDV antibody. This phenomenon is not seen in every negative case and mast cells granules do not always take up stain. The possibility of over-staining should also be considered, as many of the affected sections showed colour changes in the connective tissue and cartilage, which is indicative of over-staining.
All positively diagnosed animals must have positive staining in keratinocytes of the epidermis (stratum basale and stratum spinosum), hair follicle epithelium, smooth muscle cells of blood vessels and spindle-shaped cells in the connective tissue of the dermis (fibroblasts) (Njaa et al. 2000; also findings fromt his study). If these parameters are considered and cases are carefully examined, IHC staining can be considered to be a fast, reliable and cost-effective tool to identify PI animals. Cornish et al. (2005) found that the IHC stain detected 100% of PI calves. The monoclonal antibody used in the IHC staining method received from Cornell University was specifically developed for research purposes and is considered to be of the highest quality. All samples in this research project were stained with it.
One of the limitations of this study was the inability to collect serum samples from all animals sampled. Serum samples for testing were available for only 10 PI animals as diagnosed according to IHC staining. Of these, six calves were positive for BVDV antigen (consistently viraemic) and three had antibodies to BVDV (Table 1). The IDEXX HerdCheck BVDV Antibody Test Kit has high specificity (> 99.7%) and sensitivity (nearly 100%) and detects BVDV types I and II antibodies (IDEXX Laboratories n.d.). However, it cannot be used on its own to identify a PI animal, for the following reasons. A PI animal will not have antibodies against the BVDV strain that infected it in utero because the immune system recognises the virus as self. Animals infected in utero after the immune system is competent can develop an antibody response, as can PI animals infected after birth with a different strain (McClurkin et al. 1984). They will, however, be antibody negative towards the strain that caused the initial infection. In this study three of the six PI animals showed the presence of antibody, indicating infection by a different strain of virus after birth.
A PI animal will have a positive result with the BVDV antigen capture ELISA because it is persistently viraemic. An acutely infected animal will, however, also test positive, and this test is therefore not able to confirm PI status. The test is based on the robust Ems (gp48) antigen, which is consistently present in large quantities in both serum and tissue, making it easy to detect with the test kit. This antigen is highly stable, yielding reliable results even after long storage. Of the sampled animals, four were negative for antigen but positive for antibody, indicating that they were not infected persistently. Re-evaluation of the immunoperoxidase sections indicated that all four of these were incorrectly diagnosed originally as positive owing to the presence of nonspecific staining. Based on the information obtained in this study, IHC staining is a good and reliable tool to diagnose PI animals. The finding corroborates those of Cornish et al. (2005), Hilbe et al. (2007), Houe et al. (2006), Luzzago et al. (2006) and Thur et al. (1996). However, the pathologist evaluating these sections should be able to differentiate clearly positive from nonspecific staining according to the advised parameters.
Too few skin samples were accompanied by serum samples to compare determinations by IHC staining and ELISA antibody and antigen statistically. In general, IHC staining is more cost effective, as only one test needs to be performed compared with both ELISAs. Currently the cost for one animal's IHC stain test is approximately equal to the cost of one of the ELISAs.
Conclusion
The incidence of PI calves entering feedlots in South Africa is estimated to be approximately 0.56%. In this study the incidence was 2.9% because the samples were collected from a selected population of calves that appeared visually suspect or were recognised as poor doers. It was concluded that the possibility of a PI animal to become a chronic poor doer increases in a feedlot situation compared to calves raised extensively. This study confirmed the reliability of the IHC staining method to identify PI animals, despite the possibility of wrong diagnosis owing to nonspecific staining.
Acknowledgements
We would like to express our appreciation to the following people: Dr Sarah Clift for her support and help with interpretation of the IHC stains, and troubleshooting; Ms Vanessa Prinsloo for cutting of sections; Mrs Marie Smit for performing the IHC stains; Prof. J.A. Lawrence for his valuable inputs.
References
Bancroft, A.S. & Stevens, A., 1982, Theory and Practice of Histological Techniques, 2nd edn., Chruchill Livingstone, Edinburgh. [ Links ]
Bielefeldt Ohmann, H., 1988, 'BVD virus antigens in tissues of persistently viraemic, clinically normal cattle: Implications for the pathogenesis of clinically fatal disease', Acta Veterinaria Scandinavica 29, 77-84. PMid:2849295 [ Links ]
Brinkhof, J., Zimmer, G. & Westenbrink, F., 1996, 'Comparative study of four enzyme-linked immunosorbent assays and a co-cultivation assay for the detection of antigens associated with bovine viral diarrhoea virus in persistently infected cattle', Veterinary Microbiology 50, 1-6. doi:10.1016/0378-1135(95)00201-4 [ Links ]
Brock, K.V., 1995, 'Diagnosis of bovine viral diarrhoea virus infections', Veterinary Clinics of North America: Food Animal Practice 11, 549-561. PMid:8581862 [ Links ]
Brock, K.V., Grooms, D.L., Ridpath, J. & Bolin, S.R., 1998, 'Changes in levels of viraemia in cattle persistently infected with bovine viral diarrhoea virus', Journal of Veterinary Diagnostic Investigation 10, 22-26. PMid:9526856 [ Links ]
Broderson, B.W., White, A.K. & Smith, D.R., 1998, 'Immunohistochemical test on skin biopsies as a method for detection of cattle persistently infected with bovine viral diarrhoea virus', Proceedings of American Association of Bovine Practitioners 31, 246-252. [ Links ]
Cornish, T.E., Van Olphen, A.L., Cavender, J.L., Edwards, J.M., Jaeger, P.T., Vieyra, L.L. et al., 2005, 'Comparison of ear notch immunohistochemistry, ear notch antigen-capture ELISA, and buffy coat virus isolation for detection of calves persistently infected with bovine viral diarrhoea virus', Journal of Veterinary Diagnostic Investigation 17, 110-117. PMid:15825490 [ Links ]
DuBois, W.R., Cooper, V.L., Duffy, J.C., Dean, D.D., Ball, R.L. & Starr Jr, B.D., 2000, 'A preliminary evaluation of the effect of vaccination with modified live bovine viral diarrhoea virus (BVDV) on detection of BVDV antigen in skin biopsies using immunohistochemical methods', The Bovine Practitioner 34, 98-100. [ Links ]
Firat, I., Ak, S., Bozkurt, H.H., Ak, K., Turan, N. & Bagcigil, F., 2002, 'Distribution of bovine viral diarrhoea virus (BVDV) in the genital system tissues of cattle', Veterinarski Arhiv 72, 35-248. [ Links ]
Haines, D.M., Martin, K.M., Clark, E.G., Jim, G.K. & Janzen, E.D., 2001, 'The immunohistochemical detection of Mycoplasma bovis and bovine viral diarrhea virus in tissues of feedlot cattle with chronic, unresponsive respiratory disease and/or arthritis', Canadian Veterinary Journal 42(11),857-860. PMid:11708203, PMCid:1476660 [ Links ]
Hessman, B.E., Fulton, R.W., Sjeklocha, D.B., Murphy, T.A., Ridpath, J.F. & Payton, M.E., 2009, 'Evaluation of economic effects and the health and performance of the general cattle population after exposure to cattle persistently infected with bovine viral diarrhoea virus in a starter feedlot', American Journal of Veterinary Research 70, 73-85. doi:10.2460/ajvr.70.1.73, PMid:19119951 [ Links ]
Hilbe, M., Stalder, H., Peterhans, E., Haessig, M., Nussbaumer, M., Egli, C. et al., 2007, 'Comparison of five diagnostic methods for detecting bovine viral diarrhea virus infection in calves', Journal of Veterinary Diagnostic Investigation 19, 28-34. PMid:17459829 [ Links ]
Houe, H., Lindberg, A. & Moennig, V., 2006, 'Test strategies in bovine viral diarrhea virus control and eradication campaigns in Europe', Journal of Veterinary Diagnostic Investigation 18, 427-436. PMid:17037609 [ Links ]
IDEXX Laboratories, n.d., Bovine Viral Diarrhoea Virus (BVDV) Antibody Test Kit, Zul. Nr.BGVV-B233 version 06-44000-02, brochure, Liebefeld-Bern, Switzerland. [ Links ]
Ishmael, W., 2003, 'BVD's big take', viewed 03 April 2005, from http://www.beef-mag.com. [ Links ]
Kampa, J., Stahl, K., Renstrom, L.H.M. & Alenius, S., 2007, 'Evaluation of a commercial Erns-capture ELISA for detection of BVDV in routine diagnostic cattle serum samples', Acta Veterinaria Scandinavica 49, 7-15. doi:10.1186/1751-0147-49-7, PMid:17352830, PMCid:1828736 [ Links ]
Libler-Tenorio, E.M., Ridpath, J.F. & Neill, J.D., 2003, 'Distribution of viral antigen and development of lesions after experimental infection of calves with a BVDV 2 strain of low virulence', Journal of Veterinary Diagnostic Investigation 15, 221-232. [ Links ]
Loneragan, G.H., Thomson, D.U., Montgomery, D.L., Mason, G.L. & Larson, R.L., 2005, 'Prevalence, outcome, and health consequences associated with persistent infection with bovine viral diarrhea virus in feedlot cattle', Journal of the American Veterinary Medical Association 226, 595-601. doi:10.2460/javma.2005.226.595, PMid:15742703 [ Links ]
Luzzago, C., Frigerio, M., Tolari, F., Mazzei, M., Salvadori, C., DEL Piero, F. & Arispici, M., 2006, 'Indirect immunohistochemistry on skin biopsy for the detection of persistently infected cattle with bovine viral diarrhoea virus in Italian dairy herds', New Microbiologica 29, 127-131. PMid:16841553 [ Links ]
McClurkin, A.W., Littledike, E.T., Cutlip, R.C., Frank, G.H., Coria, M.F. & Bolin, S.R., 1984, 'Production of cattle immunotolerant to bovine viral diarrhoea virus', Canadian Journal of Comparative Medicine 48, 156-161. PMid:6326980, PMCid:1236029 [ Links ]
Moczygemba, L., 2003, 'A review of the relationship between persistent infection of cattle with bovine viral diarrhoea virus and feedlot morbidity and gain', The Bovine Practitioner 37, 155-161. [ Links ]
Niskanen, R., Lindberg, A. & Traven, M., 2002, 'Failure to spread bovine virus diarrhoea virus infection from primarily infected calves despite concurrent infections with bovine Coronavirus', The Veterinary Journal 163, 251-259. doi:10.1053/tvjl.2001.0657, PMid:12090767 [ Links ]
Njaa, B.L., Clark, E.G., Janzen, E., Ellis, J.A. & Haines, D.M., 2000, 'Diagnosis of persistent bovine viral diarrhoea virus infection by immunohistochemical staining of formalin-fixed skin biopsy specimens', Journal of veterinary Diagnostic Investigation 12, 393-399. PMid:11021424 [ Links ]
Peterhans, E., Jungi, T.W. & Schweizer, M., 2003, 'BVDV and innate immunity', Biologicals 31(2),107-112. doi:10.1016/S1045-1056(03)00024-1 [ Links ]
Potgieter, L.N.D., 2004, 'Bovine viral diarrhoea and mucosal disease', in J.A.W. Coetzer & R.C. Tustin (eds.), Infectious Diseases of Livestock, 2nd edn., pp. 946-969, Oxford University Press, Cape Town. [ Links ]
Radostits, O.M., Gay, C.C., Blood, D.C. & Hinchcliff, K.W., 2000, Veterinary Medicine, 9th edn., W.B. Saunders, New York. [ Links ]
Ridpath, J.F., Hietala, S.K., Sorden, S. & Neill, J.D., 2002, 'Evaluation of the reverse transcription-polymerase chain reaction/probe test of serum samples and immunohistochemistry of skin sections for detection of acute bovine viral diarrhoea infections', Journal of Veterinary Diagnostic Investigation 14, 303-307. PMid:12152809 [ Links ]
Sandvik, T., 1999, 'Laboratory diagnostic investigations for bovine viral diarrhoea infections in cattle', Veterinary Microbiology 64, 123-134. doi:10.1016/S0378-1135(98)00264-8 [ Links ]
Thur, B., Zlinszky, K. & Ehrensperger, F., 1996, 'Immunohistochemical detection of bovine viral diarrhoea virus in skin biopsies: a reliable and fast diagnostic tool', Journal of Veterinary Medicine Series B 43, 163-166. doi:10.1111/j.1439-0450.1996.tb00301.x [ Links ]
University of Rochester, 2007, Microwave Ziehl-Neelsen method for acid-fast bacteria, viewed 29 May 2010, from http://www.urm.rochester.edu/Path/zqu/StainsManual/index.html. [ Links ]
Van Vuuren, M., 2005, 'Bovine viral diarrhoea virus infection in livestock in southern Africa', CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources No. 009, 1-5Wren, G., 2001, 'BVDV vaccination', Bovine Veterinarian, November-December, 4-12. [ Links ]
Wren, G., 2001, 'BVDV vaccination', Bovine Veterinarian, November-December, 4-12. [ Links ]
 Correspondence to:
Correspondence to:
Thelma Meiring
Postal address: PO Box 12731
Onderstepoort 0110, South Africa
Email: thelma@idexxsa.co.za
Received: 17 Feb. 2011
Accepted: 17 Mar. 2011
Published: 25 Aug. 2011
© 2011. The Authors. Licensee: AOSIS OpenJournals. This work is licensed under the Creative Commons Attribution License.














