Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Onderstepoort Journal of Veterinary Research
On-line version ISSN 2219-0635
Print version ISSN 0030-2465
Onderstepoort j. vet. res. vol.77 n.1 Pretoria Jan. 2010
http://dx.doi.org/10.4102/ojvr.v77i1.1
ORIGINAL RESEARCH
Seroprevalence survey of Chlamydophila abortus infection in breeding goats on commercial farms in the Otavi Veterinary District, northern Namibia
Alaster SamkangeI; Tendai C. KatsandeII; Georgina Tjipura-ZaireIII; Jan E. CraffordIV
IMinistry of Agriculture, Water and Forestry, Directorate of Veterinary Services, Namibia
IIARC-Onderstepoort Veterinary Institute, South Africa
IIICentral Veterinary Laboratory, Namibia
IVDepartment of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria, South Africa
ABSTRACT
A total of 1076 sera from breeding goats were randomly collected from 24 different farms and tested with CHEKIT®-ELISA (IDEXX Laboratories B.V., 1 119 NE Schiphol-Rijk, Nederland) for antibodies against Chlamydophila abortus. The farms were divided into two categories of twelve farms each, based on their previous history of observed abortions over the previous 12 months: those with low (< 5%) levels of abortion and those with high (> 5%) levels of abortion. The farmers were also interviewed on their level of awareness about chlamydophilosis, its zoonotic importance and vaccination measures against the disease. The study detected overall seroprevalence levels of 25% for the farms and 8% for the individual animals (at 95% confidence). A total of six out of twenty-four farms (25%) had at least one positive breeding animal. Only five out of the twenty-four (20.8%) farmers interviewed were aware of chlamydophilosis and its zoonotic dangers. None of the 24 farmers interviewed practised any vaccination against chlamydophilosis. There was a significantly higher number of seropositive animals from farms with high levels of abortion, compared to those animals from farms with low levels of abortion (p = 0.0001). This study underscores the need for a higher level of farmer awareness and training on chlamydophilosis and its zoonotic dangers.
Keywords: Abortions; breeding goats; CHEKIT®-ELISA; Chlamydophila abortus; chlamydophilosis
INTRODUCTION
Chlamydophila abortus (formerly Chlamydia psittaci serotype 1) is a zoonotic bacterium that commonly causes abortions in ruminants. The disease is generally referred to as ovine enzootic abortion (OEA), or simply enzootic abortion, and is one of the most important infectious agents causing abortion and major economic losses in sheep and goats worldwide (Longbottom et al. 2002; Smith & Sherman 1994; Aitken 2000, cited in Szeredi & Bacsadi 2002). In the United Kingdom, it is estimated that chlamydophilial abortion accounts for about 50% of all diagnosed abortions, resulting in losses estimated to be in excess of £20 million annually (Longbottom et al. 2002). In the United States of America, C. abortus is the most common cause of infectious abortion in goats (Aiello & Mays 1998). Apart from causing abortion and foetal loss in sheep, cattle, goats and pigs (Woollen et al. 1990, cited in Longbottom et al. 2002), C. abortus can also cause abortion in humans who come into contact with aborting livestock (Ward 2006). The disease, therefore, has important zoonotic implications to pregnant female farmers who come in contact with aborted foetuses and foetal fluids.
In Namibia, goats are very important both economically and socially. Apart from being a status symbol in some indigenous communities, they are a ready source of income and meat. In the Otavi Veterinary District (OVD), the area in which this study was conducted, there are about 75 000 cattle, 35 000 goats and 26 000 sheep, meaning that goats are second only to cattle in terms of numbers. However, Namibia has about 2.6 million sheep, 2.3 million cattle and 2 million goats, which means that goats are ranked third in numbers for the whole country (Directorate of Veterinary Services 2004). Goats are therefore among the three most important domestic animals in Namibia, as well as in the OVD.
The last survey of C. abortus in goats in Namibia was carried out about 20 years ago (Apel, Huebschle & Krauss 1989) and it is likely that the epidemiological pattern of this disease has changed since then. With the advent of independence in 1990, Namibia has undergone major changes in human and livestock population dynamics (Agrivet International 2006). There has been movement of previously disadvantaged groups of people, together with controlled movement of their small stock, from communal areas in the extreme north of the country, where no C. abortus seroprevalence studies have ever been done, to the commercial farming areas in the south of the country, where the disease has been reported to be endemic (Apel et al. 1989). In light of this, an updated study was therefore needed to determine the current C. abortus seroprevalence and to determine the level of farmer awareness of this disease and its zoonotic importance. A study of this nature would also facilitate the implementation of targeted control strategies that include raising awareness of the economic and zoonotic importance for this disease among farmers.
MATERIALS AND METHODS
Study area
This study was conducted in the OVD, which is approximately located between 18.6º - 21.4º North and 16.7º - 17.7º East, in the commercial farming area in northern Namibia (Figure 1). It is surrounded by four other veterinary districts namely, Grootfontein to the east, Oshikoto to the north, Outjo to the west, and Otjiwarongo to the south.
There are a total of 313 farms with goats (among other livestock) in the OVD.
Study animals
The Boer goat is one of the two main goat breeds found in Namibia (Directorate of Veterinary Services 2006) and was therefore selected for this study. The study targeted only breeding animals.
Sampling methods and sample size
Twenty-four farms were randomly selected from a total of 313 farms. Goat flocks that were vaccinated against chlamydophilosis were excluded from the study in order to avoid interference with the serology results (Gerber et al. 2007).
A sample size of 1076 goat sera out of a total of 1970 breeding goats (does and bucks) was used. The total number of goats, including non-breeding goats, from the 24 farms that were selected for the study was 3245. Sample size was determined at 95% confidence with an estimated prevalence of 10% (Martin, Meek & Willeberg 1987). An average of 45 goats was sampled per farm and the average breeding flock size was 82 animals.
The selected farms were divided into two categories, (1) farms with a history of high levels of abortion (> 5%) during the previous 12 months and (2) farms with a low level of abortion (< 5%) during the same period. The levels of abortion were calculated using the total number of breeding animals per farm using data acquired from a questionnaire.
The goats were bled from the jugular vein using 20-gauge needles and sterile vacutainer tubes (BD Vacutainer Systems, Plymouth, Devon PL6 7BP, United Kingdom). The blood samples were allowed to clot before the sera were separated and transported to the Central Veterinary Laboratory in Windhoek, where they were stored at -20 ºC until testing.
Laboratory testing
The commercially available group-specific indirect ELISA kit, CHEKIT®-CHLAMYDIA (IDEXX Laboratories B.V., 1119 NE Schiphol-Rijk, Nederland), was used to detect the presence of chlamydophilial antibodies.
Briefly, test serum and control samples were diluted at 1:400 in the wells of microtitre plates that were pre-coated with C. psittaci antigen (this is the official name of the antigen although the name of the bacterium was changed to C. abortus) and then incubated for 90 min at room temperature in a humid chamber to allow for the binding of any C. psittaci-reactive antibody. After incubation, each microtitre plate was washed twice with CHEKIT washing and dilution solution and 100 µL of diluted (1:200) monoclonal anti-ruminant-IgG conjugated with horseradish peroxidase, was added to each well and incubated as above. After a further two washing steps, 100 µL of substrate containing CHEKIT-Chromogen, pre-warmed to 25 ºC, was added to each well. The reaction was stopped with CHEKIT-Stopping Solution within 10 min - 30 min after addition of the chromogen, as soon as the difference in optical density (OD) between the negative and positive controls was at least 0.3 Absorbance Units.
The OD was measured at 405 nm. The results were normalised using the positive and negative control sera and expressed as percentage positivity (PP) according to the following formula:
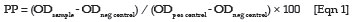
In line with the guidelines given by the manufacturer, sera with a PP values below 30% were interpreted as negative, while sera with PP values between 30% and 40% were regarded as ambiguous or weak positives and were therefore considered as negative for the purposes of this study. Sera with PP values equal to, or above 40% were considered positive for antibodies to C. abortus.
Questionnaire
A questionnaire was used to collect information on goat flocks included in this study. Specific questions included farm location, farm name, owner's name, telephone number, date, flock size, the number of breeding does and bucks, the goats' vaccination history and the farmer's awareness of chlamydophilosis, and the number of observed abortions, stillbirths, kids that were born weak, retained placentas, eye problems, arthritis and coughing, as well as cases of orchitis or epididymitis in bucks. These clinical conditions were recorded using a scoring system where the presence of any one of the eight above-listed clinical conditions was indicated by a score of '1' and the absence thereof was indicated by a score of '0'. The scores for the two categories of farms were separately averaged and compared in order to determine which category of farm had the higher average score.
Statistical analysis
Descriptive statistics were used to explain both the study population and the seroprevalence of C. abortus at farm level. The Chi-square test was used to compare the seroprevalence of C. abortus on farms with a high rate of abortion (> 5%) to farms with a low abortion rate (< 5%).
Odds ratios (OR) were calculated to estimate the comparative risk between the two categories of farms that were studied. The OR compared the likelihood of farms having high levels of abortion being seropositive to the likelihood of farms having low levels of abortion being seropositive. All statistical analyses were performed using Intercooled Stata version 7.0 computer software.
RESULTS
Disease prevalence results
Table 1 summarises the disease prevalence results for the breeding goat population on each study farm. Of the samples, 86 of the 1076 animals tested positive, giving an individual prevalence level of 8% with a range between 0% and 54%. Six out of the twenty-four farms (25%) had at least one positive breeding animal, while the seroprevalence on these farms ranged from 2.4% to 54%.
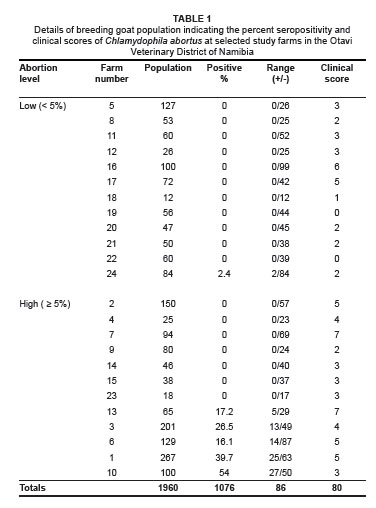
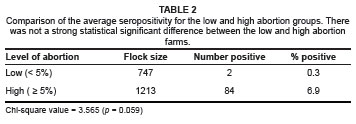
At individual animal level, there was a very significant difference (p < 0.0001) in prevalence between farms with high abortion levels (6.9%) and those with low abortion levels (0.3%). The odds of an animal being seropositive on a farm with high levels of abortion were calculated to be 113.7 times greater than that of an animal on a farm with low levels of abortion. Chi-square statistical analysis indicated that this was a highly statistically significant association (p < 0.0001).
There were a higher number of seropositive farms (41.7%) in the category with high abortion levels compared to the category with low abortion levels (8.3%). The odds of a farm with high levels of abortion being seropositive were calculated to be 7.86 times greater than for a farm with low levels of abortion. Chi-square analysis indicated that there was only a slight statistical significant difference between these two categories of farms (p = 0.059).
A total of five (83.3%) out of the six seropositive farms indicated high levels (> 5%) of abortion. The seroprevalence levels on these five farms ranged from 17.2% to 54%. The remaining seropositive farm had a low level of abortion (< 5%) and the lowest seroprevalence level of only 2.4%.
Questionnaire results
Demographic information of the goat population
The 24 farms that were included in this study had an average number of 6 bucks and 75 does per farm. The number of bucks ranged from 1 to 24 (mean 6.8) and from 1 to 13 (mean 5.7) for the farm categories with high and low abortion levels, respectively. The does ranged from 13 to 262 (mean 94.3) for high abortion level farms and from 10 to 117 (mean 56.6) for those farms with low levels of abortion. The mean breeding goat population (does and bucks only) per farm was 101 for the high abortion level farms and 62 for low abortion level farms. The total average goat population per farm (inclusive of non-breeding animals) was 159.6 for the high abortion level category and 110.8 for low abortion level category. The buck-to-doe ratio averaged 1:14 for the farms with a high abortion level and 1:10 for those with a low abortion level.
Farms with a high level of abortion therefore had significantly higher (p = 0.03) mean goat population, mean number of bucks and does and buck-to-doe ratio.
Clinical conditions present on the farms
A total of 22 farms (92%) reported at least one of the eight listed clinical conditions that are suggestive of chlamydophilosis. All 12 farms that had high levels of abortion reported at least three of these clinical conditions and they had a significantly higher average score of 4.25, compared to an average score of only 2.33 (p < 0.05) for the 12 remaining farms with low levels of abortion.
The results of the scores with respect to the clinical conditions in the two farm categories are shown in Figure 2.
Abortions were the most common problem reported by the interviewed farmers (88%), followed by the birth of weak kids (67%), retained placentas (50%), eye problems (42%), stillbirths and coughing (both 29%), arthritis (5%) and orchitis, which was reported by only 2% of the farmers.
Disease awareness and vaccination
Out of a total of 24 farmers that were interviewed, only five (20.8%) of these were aware of chlamydophilosis and its zoonotic dangers. Similarly, only two out of the twelve farmers who had experienced high levels of abortion in their does were aware of chlamydophilosis and its zoonotic dangers. None of the selected farms practised any form of vaccination against chlamydophilosis.
DISCUSSION
Various literature sources have stated that it is normal for up to 5% of ewes and does in a flock to abort (Edey 1969; Kelly 1984; Kelly 1986; Quinlivan et al. 1966; Robinson 1951; Schoenian 2000). It is on this basis that the 5% level of abortion was used as a cut-off point in determining whether or not the levels of abortion observed in a given flock were regarded as clinically significant or not.
Previous work in Namibia by Apel et al. (1989) indicated that C. abortus infections were prevalent in all the geographical regions that were tested. They detected seroprevalence levels in goats ranging from 12% (Otjiwarongo) to as high as 50% (Otavi). In their study, which covered three State Veterinary Districts (Otavi, Otjiwarongo and Windhoek), 299 out of 1185 (25.2%) caprine sera that were tested were positive for chlamydophilial antibodies. However, in the current study the prevalence of chlamydophilial antibodies for farms and individual goats was 25% and 8%, respectively. These figures were significantly lower than the serological results obtained by Apel et al. (1989) for the Otavi Veterinary District, who found the apparent prevalence levels at farms and for individual goats to be 86% and 50%, respectively. However, such differences were not altogether unexpected, especially when one considers that there was a 16-year period between the two studies and that Namibia's independence, which came less than a year after the results of the first study were published, had some impact on the livestock population structure.
Many changes in the livestock population dynamics were implemented in Namibia after independence (Agrivet International 2006). These included controlled movements of small stock, especially goats, from communal areas in the extreme north of the country, where no C. abortus seroprevalence studies had ever been done, into the commercial areas where chlamydophilosis has been shown to be endemic.
In serological surveys conducted for five consecutive years (1996-2000) in Slovakia by Tràvnicek et al. (2001), the seroprevalences for chlamydophilosis in goats were 3.94%, 10.02%, 2.96%, 3.96% and 6.08%, for each of the years respectively. It is therefore not uncommon in cross-sectional studies to discover different prevalence rates, because, unlike cohort studies that provide incidence data, prevalence studies only provide data for a specific point in time. This makes the results of prevalence studies more prone to variation. Other factors that can contribute to variations in prevalence rates between different studies done at different time periods include sampling methods, samples size and interference by vaccine-induced antibodies. These factors should therefore be carefully considered in cross-sectional studies.
This study indicated that there was not a highly statistically significant difference (p = 0.059) in C. abortus seroprevalence levels between the two categories of farms studied. The 12 farms with high levels of abortion (> 5%) in breeding does had a higher farm prevalence level of 41.7%, compared to 8.3% for the other 12 farms with low levels of abortion (< 5%).
The farms with high abortion levels consistently scored higher on all clinical conditions suggestive of caprine chlamydophilosis. These included abortions, birth of weak kids, retained placentas and eye problems. The farms with high abortion levels had a significantly higher average score of 4.25, compared to 2.33 (p < 0.05) for farms with low abortion levels. These findings are in agreement with those of a previous study by Apel et al. (1989), who found that high Chlamydophila seroprevalences correlated well with clinical signs suggestive of Chlamydophila infections, especially abortions and keratoconjunctivitis. Other workers also found similar trends in cattle (Cavirani et al. 2001).
Only five out of the twenty-four farmers (21%) interviewed were aware of chlamydophilosis and its zoonotic nature. No previous studies have addressed the aspect of farmer awareness of chlamydophilosis. The low level of awareness detected in this study demonstrates the need to educate farmers on goat husbandry practices, especially among new and inexperienced farmers who are engaged in commercial farming.
None of the 24 farmers interviewed practised any vaccination against chlamydophilosis. There is therefore a need for farmers to vaccinate against chlamydophilosis, considering that seroprevalence levels detected on farms with high levels of 'abortion ranged from 17.2% to 54%.
The relatively small farmer sample size of 24 farmers means that caution is needed when extrapolating these results to the whole study region and to the rest of the country. A countrywide study involving farms in all the districts is needed to determine the true prevalence, true awareness level and the use of vaccination in the control of chlamydophilosis in the country as a whole. This study should be conducted as a broad study that will include other important diseases of goats in order to produce wider recommendations on improved goat production systems and disease control practices in Namibia.
CONCLUSION
In spite of the above limitations, this study has clearly identified a need for more farmer education and awareness about chlamydophilosis. Effective control of this disease is important not only because of its zoonotic importance, but also because of its adverse impact on animal production. Intervention by the government, the private sector and other stakeholders through farmer training and awareness campaigns is therefore recommended. The government can become involved through its field workers in the Ministry of Agriculture, Water and Forestry. The private veterinarians and farmers' unions can also become involved in sensitising farmers.
ACKNOWLEDGEMENTS
We thank the late Dr O.J.B. Huebschle, the technical staff in the serology section of the Central Veterinary Laboratory in Windhoek and the Government of the Republic of Namibia, for providing all the logistical, material and human resources needed for the project, without which this study would not have been possible.
REFERENCES
Agrivet International, 2006, Report on the study to identify the optimal geographical sites for selected state veterinary offices, Ministry of Agriculture, Water and Rural Development, Windhoek. [ Links ]
Aiello, S.E. & Mays, A. (eds.), 1998, The Merck veterinary manual, Merck & Co., Inc., Whitehouse Station. [ Links ]
Apel, J., Huebschle, O.J. & Krauss, H., 1989, 'Seroprevalence of Chlamydia psittaci-specific antibodies in small stock in Namibia - Epidemiological study with an enzyme-linked immunosorbent assay (ELISA)', Journal of Veterinary Medicine Series B - Infectious Diseases Immunology Food Hygiene Veterinary Public Health 36(6), 447-458, viewed on 22 September 2005, from http://www.ncbi.nlm.nih.gov/pubmed/2800787. [ Links ]
Cavirani, S., Cabassi, C.S., Donofrio, G., De Iaco, B., Taddei, S. & Flammini, C.F., 2001, 'Association between Chlamydia psittaci seropositivity and abortion in Italian dairy cows', Preventive Veterinary Medicine 50, 145-151. [ Links ]
Directorate of Veterinary Services, 2006, Annual report 2006, Ministry of Agriculture, Water and Rural Development, Windhoek. [ Links ]
Directorate of Veterinary Services, 2004, Namibia stock census December 2004, Ministry of Agriculture, Water and Rural Development, Windhoek. [ Links ]
Edey, T.N., 1969, 'Prenatal mortality in sheep. A review', Animal Breeding Abstracts 7, 173-190. [ Links ]
Gerber, A., Thoma, R., Vretou, E., Psarrou, E., Kaiser, C., Doherr, M.G. et al., 2007, 'Ovine Enzootic Abortion (OEA): A comparison of antibody responses in vaccinated and naturally infected Swiss sheep over a two-year period', BMC Veterinary Research 3, 24. [ Links ]
Intercooled Stata Version 7.0, 2001, computer software, StataCorp LP, College Station, Texas 77845. [ Links ]
Kelly, R.W., 1984, 'Fertilization failure and embryonic wastage', in D.R. Lindsay & D.T. Pearce (eds.), Reproduction in Sheep, Butterworth, London. [ Links ]
Kelly, R.W., 1986, 'Reproductive wastage in sheep', Journal of Agriculture 27, 22-26. [ Links ]
Longbottom, D., Fairley, S., Chapman, S., Psarrou, E., Vretou, E. & Livingtsone, M., 2002, 'Serological diagnosis of ovine enzootic abortion by enzyme-linked immunosorbent assay with a recombinant protein fragment of the polymorphic outer membrane protein POMP90 of Chlamydophila abortus', Journal of Clinical Microbiology 40, 4235-4243. [ Links ]
Martin, S.W., Meek, A.H. & Willeberg, P., 1987, Veterinary epidemiology, principles and methods, Iowa State University Press, Ames, Iowa. [ Links ]
Quinlivan, T.D., Martin C.A., Taylor W.B. & Cairney, I.M., 1966, 'Estimates of pre- and perinatal mortality in the New Zealand Romney Marsh ewe. I. Pre- and perinatal mortality in those ewes that conceived to one service', Journal of Reproduction and Fertility 11, 379-390. [ Links ]
Robinson, T.J., 1951, 'The control of fertility in sheep. II. The augmentation of fertility by gonadotropin treatment of the ewe in the normal breeding season', Journal of Agricultural Science, Cambridge 41, 6-63. [ Links ]
Schoenian, S., 2000, Infectious causes of abortion in ewes, Western Maryland Research & Education Centre, Maryland Cooperative Extension, University of Maryland, viewed on 11 September 2006, from http://www.sheepandgoat.com/articles/abortion.html. [ Links ]
Szeredi, L. & Bacsadi, À., 2002, 'Detection of Chlamydophila (Chlamydia) abortus and Toxoplasma gondii in smears from cases of ovine and caprine abortion by the streptavidin-biotin method', Journal of Comparative Pathology 127, 257-263. [ Links ]
Tràvnicek, M., Kovacova, D., Zubricky, P. & Cislakova, L., 2001, 'Serosurvey of sheep and goats to Chlamydia psittaci in Slovakia during the years 1996-2000', Veterinarni Medicina 46(11/12), 281-285. [ Links ]
Vretou, E., Radouani, F., Psarrou, E., Kritikos, I., Xylouri, E. & Mangana, O., 2007, 'Evaluation of two commercial assays for the detection of Chlamydophila abortus antibodies', Veterinary Microbiology 123, 153-161. [ Links ]
Ward, M. (Webmaster), 2006, 'Chlamydophila abortus: Human infection', in Chlamydial infections; chlamydial infections in animals, viewed on 11 September 2006, from http://www.chlamydiae.com/ [ Links ]
 Correspondence to:
Correspondence to:
Alaster Samkange
Postal address: PO Box 788
Grootfontein, 9000, Namibia
Received: 27 Apr. 2010
Accepted: 21 July 2010
Published: 27 Aug. 2010
This article is available at: http://www.ojvr.org
© 2010. The Authors. Licensee: OpenJournals Publishing. This work is licensed under the Creative Commons Attribution License.














