Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.79 no.1 Johannesburg feb. 2024
http://dx.doi.org/10.17159/sadj.v79i01.16697
CASE REPORT
A gigantic unilateral neck mass in the submandibular region
RE Rikhotso
Risimati Ephraim Rikhotso, PhD, Department of Maxillofacial and Oral Surgery, Wits School of Oral Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
INTRODUCTION
Pleomorphic adenoma (PA) is the most common salivary gland neoplasm, mainly affecting the major rather than the minor salivary glands. The parotid gland is the most commonly affected site (75-80%), followed by the submandibular (10%) and minor (10%) salivary glands.1-4
Intra-orally, the palate represents the most common location of PA, followed by the lip, cheeks, tongue, floor of the mouth and oropharynx.5-6 Histogenesis of PA is characterised by simultaneous proliferation of parenchymatous glandular cells along with myoepithelial components.7 Cells of epithelial origin give rise to ductal structures and are closely intermingled with mesenchymal elements that may give rise to myxoid, hyaline, cartilaginous and osseous change. The expression of varying proportions of epithelial and mesenchymal features give rise to a wide spectrum of histological findings, hence the term "pleomorphic". It is also called a "mixed tumour", because it possesses a mixture of ductal and myoepithelial elements in one single tumour. Unlike in minor salivary glands, PA is usually encapsulated when it arises in the major salivary glands.8-9
PAs are most prevalent in the fourth to six decades, with a slight predominance in women.8,10 PA appears as a painless, slow growing round or ovoid rubbery tumour with a smooth surface, exhibiting firm consistency and variable dimensions. Treatment of choice for PA is surgical excision. Untreated or recurrent PAs may reach giant proportions, especially in major salivary glands.
We report on a case of a giant pleomorphic adenoma of the submandibular gland of 10 years' duration.
CASE REPORT
A 59-year-old black female presented to the Maxillofacial and Oral Surgery unit at Wits Oral Health Centre for evaluation of a painless mass at the right side of her neck in the right submandibular region that had been enlarging slowly for 10 years (Figures 1A, B, C). There was no history of dysphagia, dyspnea or dysphonia. According to the patient, a similar but smaller mass was excised from the same location by general surgeons in another district more than 10 years previously. The patient soon thereafter noticed that the mass started growing again. She had no knowledge of the diagnosis of the excised lesion. The swelling has been growing slowly throughout the years, but the patient noted a gradual increase in size in the past three years. The patient's medical history was significant for hypertension. The patient denied any drug and alcohol use in the past. No history of smoking and weight loss were reported by the patient. The patient was not febrile nor in any acute distress.
Head and neck examination revealed a nontender, rubbery firm, freely mobile, well-circumscribed mass in the right submandibular region extending to the lower right side of the neck to the level of the clavicle in the ipsilateral side. The mass was not warm to touch and measured approximately 8.5cm x 7.5cm, with no regional omolateral or contralateral lymphadenopathy. The swelling was lobulated, freely mobile and not fixed to the underlying tissues. The overlying skin was normal in colour and texture, except for what appeared to be a scar from the previous incision but was not ulcerated or fixed to the swelling.
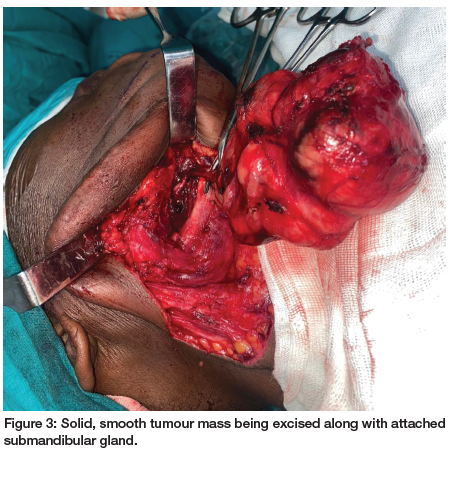
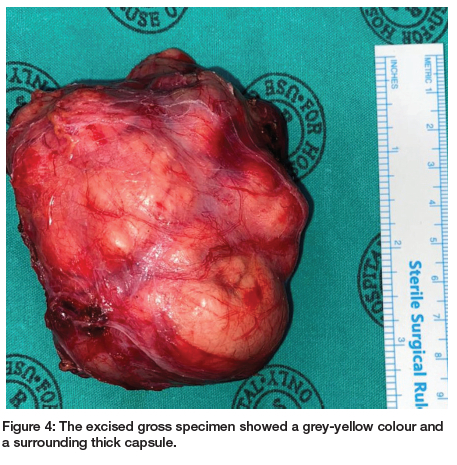
On ultrasonographic examination, a solid tumour with heterogenic low echo areas and multiple calcifications was observed. A contrast-enhanced computed tomographic (CT) scan confirmed an 8.5cm x 6cm solid mass extending from the right submandibular region to neck midline. The lesion contained multiple dense calcifications and low-density necrotic areas (Figures 2A, B). After clinical and CT examinations, a differential diagnosis of pleomorphic adenoma was made. An incisional biopsy was performed, which confirmed the diagnosis of a pleomorphic adenoma. The patient was operated on via a submandibular approach under general anaesthesia. The tumour was bounded by the anterior belly of digastric muscle anteriorly; the retromandibular fossa and the sternocleidomastoid posteriorly, the lower border and lateral surface of the mandible superiorly; and the submandibular triangle and lateral aspect of neck inferiorly. Medially, the lesion extended toward the submandibular fossa and the inferior pole of the parotid gland. The cortical bone on the lateral side of the mandible was intact, with a minimal defect due to pressure resorption. The 8.5cm x 7.5cm x 4cm solid, smooth mass was completely excised along with adjacent glandular tissue (Figure 3). The excised gross specimen showed a gray-yellow colour and a surrounding thick capsule (Figure 4). Microscopic examination of the lesion confirmed the diagnosis of pleomorphic adenoma with clear margins. Sections showed a well-circumscribed neoplasm surrounded by a thin rim of fibrous connective tissue with adjacent seromucous salivary gland parenchyma and fibroadipose tissues. There were proliferating epithelial and myoepithelial cells with minimal pleomorphism arranged in tubules, cords and small groups in a myxochondroid matrix with a surrounding capsule which was not infiltrated by the tumour (Figure 5). On serial sectioning, no atypical mitoses, necrosis or malignant change were observed.
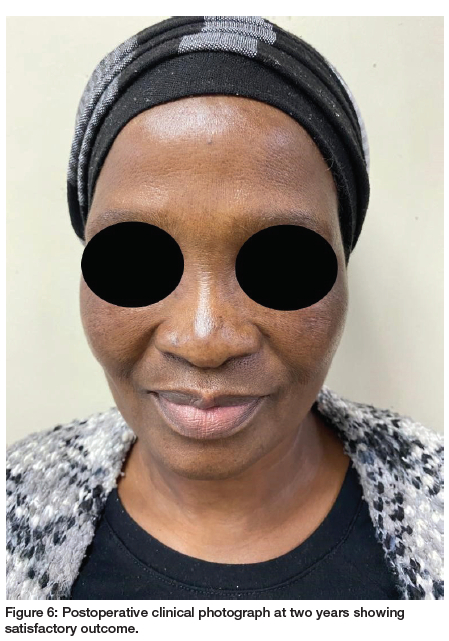
No complications were observed in the postoperative period. Two years after surgery, there were no clinical signs of recurrence of the tumour and the patient is under periodic examination (Figure 6).
DISCUSSION
PA accounts for 60% of all benign salivary glands.8,11,12,13 PA of the submandibular gland is rare and represents about 5-10% of affected cases. It generally presents as an asymptomatic, slow growing tumour which seldom exceeds 6cm in diameter.14 However, if left untreated for many years, it can reach large and grotesque proportions, as exemplified by the present case, which comprised a circumscribed multilobulated tumour mass measuring 8.5cm x 7.5cm x 4cm, with attached salivary gland measuring 2.5cm x 1.5cm x 0.8cm.
Clinical guidelines recommend that a histological sample be obtained for histopathological confirmation of salivary gland tumours prior to deciding on the treatment modality, especially when a high index of suspicion of malignancy exists. Traditionally, fine-needle aspiration (FNA) biopsy has been used for histopathological confirmation of salivary gland tumours. Fine needle aspiration biopsy of the present case was suggestive of a benign tumour of salivary gland origin. FNA biopsy is a readily available and inexpensive diagnostic tool for evaluating neoplastic and non-neoplastic lesions, especially in superficial or easily palpable masses. FNAs are, however, unable to diagnose the exact pathology without architectural context for morphology and further staining techniques for molecular and genomic profiling.15 The major drawbacks of FNA include a lower sensitivity than specificity and a relatively high rate of non-diagnostic results.16
Because of concerns about its limited sensitivity and significant false negative ratio, and the purported risk of seeding the tumour along the needle tract, some clinicians do not support its widespread usage.15 For these reasons, FNA must be seen as an additional tool in the evaluation of salivary glands or cervical masses rather than a diagnostic procedure on which therapeutic decisions can be based.
Accuracy of ultrasound-guided core-needle biopsy in salivary gland tumours is high, with a very high sensitivity and a reliable diagnosis of malignancy. It should be considered the technique of choice when a nodule is detected in the salivary glands.17 The size of the present case and ease of access made an incisional tissue biopsy a tissue sampling of choice, which confirmed the diagnosis of pleomorphic adenoma, considered to be a recurrent lesion based on previous surgical history.
The dominant histologic feature of the pleomorphic adenoma is its great heterogeneity. The present case consisted of lobules composed of spindled myoepithelial cells, chondromyxoid stroma, plasmatoid myoepithelial cells and bilayered ductal structures lined by bland cuboidal epithelium. There were no focal areas of necrosis or atypical mitoses suggestive of possible malignant transformation. When malignant transformation of a pleomorphic adenoma occurs, it is usually in the form of moderate to poorly differentiated adenocarcinoma referred to as carcinoma within a pleomorphic adenoma (carcinoma ex pleomorphic adenoma). Longevity and recurrence are considered risk factors for malignant transformation.18 The risk of malignant transformation is reported to be between 1.8% and 6.2%.19,20,21 Carcinoma ex pleomorphic adenoma appears to be more aggressive and lethal than either adenocarcinoma or adenoid cystic carcinoma.22
Early stages of PA in the submandibular triangle may clinically be indistinguishable from malignant submandibular gland tumours and enlarged submandibular lymph node. Our patient's presentation with an enlarging asymptomatic mass on the right submandibular region extending down into the neck necessitated a differential diagnosis from other cervical masses. The rule of thumb when evaluating neck masses is the "rule of 80", which pertains to adults over the age of 40, as described by Skandalakis et al.23 This rule states that 80% of non-thyroid neck masses are neoplastic. Of the neoplastic masses, 80% are malignant. Of the malignant masses, 80% are secondary. Of the secondary masses, 80% occur above the clavicle. They also described a secondary way of predicting the diagnosis based on the duration of the lesion using the "rule of 7s". If a mass has been present for 7 days, it is more likely inflammatory in nature; if present for 7 months, it is likely neoplastic in nature; and if present for 7 years, it would most likely be developmental. These generalisations are, however, no substitutes for detailed history taking and thorough clinical examination in reaching a correct diagnosis. Acuity of onset, location in the neck, duration, rate of growth, presenting symptoms, gender and age are other important considerations towards establishing accurate diagnosis.
Cervical masses are thus categorised into the three following categories: inflammatory, neoplastic (benign, malignant) and congenital/developmental.24 In children and young adults, neck masses are mostly due to inflammation.24 These can either be acute or chronic, and may either be of viral origin, bacterial, parasitic or manifestation of granulomatous diseases (such as sarcoidosis). The non-febrile nature and 10 years' duration of the mass in the present case ruled out an inflammatory origin. Polymerase chain reaction (PCR) result of aspirates from the lesion also yielded a negative GeneXpert MTB/RIF ultra-assay.
The slow rate of growth over a 10-year period of the mass in the present case was suggestive of a benign lesion. These lesions are usually unaesthetic over the years and may in addition result in mass effect on important structures of the neck.
CT scans with contrast in the present case revealed a large well-defined mass in the right submandibular space extending to the neck with no evidence of fixation to the surrounding structures. The mass was mobile but firm.
Also, there was no facial paralysis or other neurologic deficits associated with the mass. Cervical lymph node examination did not reveal any fixed lymph node suggestive of metastatic cervical lymphadenopathy from primary head and neck squamous cell carcinoma (SCC), oropharyngeal SCC or distant metastasis. There was also no history of alcohol or smoking on this patient. The non-smoking history, however, should be interpreted with caution as it is now common knowledge that human papilloma virus (HPV) causes oropharyngeal SCC in adult patients with zero smoking history.
In addition, the patient didn't present with symptoms such as fever, weight loss and night sweats suggestive of lymphoma, the second most common head and neck malignancy which also presents as an enlarged neck mass commonly in the posterior triangle.24 Lymphomas also commonly affect the paediatric population.24 FNA flow cytometry also excluded diagnosis of lymphoma.
The most common submandibular gland malignant tumours - first, mucoepidermoid carcinoma and second, adenoid cystic carcinoma - can also present as single, unilateral Arm neck masses. However, unlike in the present case, these malignant diseases have limited mobilities because of their location within the parenchyma of the gland.25
Although PAs are encapsulated, expansible growth-producing protrusions into the surrounding gland can be caused by incomplete pseudocapsule, pseudopodia of PA tissue and extracapsular extension.26
Surgical extirpation remains the primary choice of treatment of PA, with excellent prognosis and low rate of recurrence when clear margins are achieved. In our case, the tumour was excised without rupture of the capsule. Recurrence after excision has been reported in 1%-5% of cases.27 Risk factors for recurrence include pseudopodia, capsular penetration of the tumour, incomplete excision and tumour rupture or spillage during surgery.28 Simple enucleation is also associated with high recurrence rates (2%-25%) and is thus not recommended.29 Postsurgical adjuvant therapies such as chemotherapy and radiotherapy are not routinely indicated for pleomorphic adenoma. They may, however, play a role in inoperable tumours, treatment of local recurrences, tumours with nerve involvement, tumour spills and multifocal diseases.8
Though PA is associated with good prognosis postoperatively, follow-up with regular US monitoring is mandatory. After a two-year follow-up, no recurrence was observed in the clinical case described.
Ethics approval
This study was approved by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand and was performed in accordance with the principles of the Declaration of Helsinki on medical protocols and ethics (Approval number M220969).
Acknowledgement
Gratitude is conveyed to Dr F Mahomed (Department of Oral and Maxillofacial Pathology) for her help with the pathological diagnosis.
REFERENCES
1. Benedetti A, Popovski V, Popovik-Monevska D, et al. Giant Pleomorphic Adenoma of the Submandibular Gland: Case Report and Therapeutic Challenge. Oral Maxillofac Pathol J 2021; 12(2): 78-80 [ Links ]
2. Lau RP, Yee-Chang M, Rapkiewicz A. Educational Case: Head and Neck Neoplasia Salivary Gland Tumours. Acad Pathol. 2018; 5:1-5 [ Links ]
3. Lorena Pingarrón-Martín LJ, Arias-Gallo G, et al. Remarkable Triple Pleomorphic Adenoma Affecting both Parotid and Submandibular Glands. Craniomaxillofac Trauma Reconstr. 2015; 8(2): 129-131 [ Links ]
4. La Macchia R, Stefanelli S, Lenoir V, et al. Pleomorphic Adenoma Originating from Heterotopic Salivary Tissue of the Upper Neck: A Diagnostic Pitfall. Case Rep Otolaryngol 2017; 2017:5767396 [ Links ]
5. Tamba B, Diatta M, Kane M, et al. Management of a giant pleomorphic adenoma of the palate: A case report. Advances in Oral and Maxillofac Surg. 2021; 4: 100168 [ Links ]
6. Neville B, Damm D, Allen C, Bouquot J. Oral and Maxillofacial Pathology. Chapter 11. Salivary Gland Pathology, Second edition. WB Saunders Company. 2002. p. 410 [ Links ]
7. Zbären P, Vander Poorten V, Witt RL, et al. Pleomorphic adenoma of the parotid: formal parotidectomy or limited surgery? Am J Surg. 2013; 205 (1):109-118 [ Links ]
8. Lingam RK, Daghir AA, Nigar E, et al. Pleomorphic adenoma (benign mixed tumour) of the salivary glands: its diverse clinical, radiological, and histopathological presentation. Br J Oral Maxillofac Surg. 2011; 49 (1):14-20 [ Links ]
9. Bordoy-Soto MA, Velez-Gimon HJ, Hernandez MF, et al. Giant pleomorphic adenoma of the palate: case report and literature review. Rev Odontol Mexic. 2016; 20: 252-257 [ Links ]
10. Zbären P, Vander Poorten V, Witt RL, et al. Woolgar JA, Shaha AR, Triantafyllou A, et al. Pleomorphic adenoma of the parotid: formal parotidectomy or limited surgery? Am J Surg. 2013; 205 (1): 109-118 [ Links ]
11. Ejeil AL, Moreau N, Le Pelletier F. A rare ectopic localisation of pleomorphic adenoma. J Stomatol Oral Maxillofac Surg. 2019; 120: 373-374 [ Links ]
12. Zbären P, Vander Poorten V, Witt RL, Woolgar JA, Shaha AR, Triantafyllou A, et al. Pleomorphic adenoma of the parotid: formal parotidectomy or limited surgery? Am J Surg. 2013; 205 (1): 109-118 [ Links ]
13. Jorge J, Pires FR, Alves FA, Perez DE, Kowalski LP, Lopes MA, et al. Juvenile intraoral pleomorphic adenoma: report of five cases and review of the literature. Int J Oral Maxillofac Surg.2002; 31 (3):273-275 [ Links ]
14. Silva MN, Kosgodab KMS, Tilakaratnea WM, Murugadas P. A case of giant pleomorphic adenoma of the parotid gland. Oral Oncology Extra. 2004; 40 (3): 43-45 [ Links ]
15. Roth L, Moerdler S, Weiser D, Douglas L, Gill J, Roth M. Otolaryngologist and pediatric oncologist perspectives on the role of fine needle aspiration in diagnosing pediatric head and neck masses. Int J Pediatr Otorhinolaryngol. 2019; 121: 34-40 [ Links ]
16. Edizer DT, Server EA, Yigit Ö, Yildiz M. Role of fine-needle aspiration biopsy in the management of salivary gland masses. Turk Arch Otorhinolaryngol. 2016; 54: 105111 [ Links ]
17. Del Cura JL, Coronado G, Zabala R, Korta I, López I. Accuracy andeffectivenessof ultrasound-guided core-needle biopsy in the diagnosis of focal lesions in the salivary glands. Eur Radiol. 2018; 28: 2934-2941 [ Links ]
18. Key S, Chia C, Hasan Z, et al. Systematic review of prognostic factors in carcinoma ex pleomorphic adenoma. Oral Ocology. 2022; 133: 106052 [ Links ]
19. Andreasen S, Therkildsen MH, Bjorndal K, Homoe P. Pleomorphic adenoma of the parotid gland 1985-2010: a Danish nationwide study of incidence, recurrence rate, and malignant transformation. Head Neck. 2016; 38(1): e1364-e1369 [ Links ]
20. Valstar MH, de Ridder M, van den Broek EC, et al. Salivary gland pleomorphic adenoma in the Netherlands: a nationwide observational study of primary tumour incidence, malignant transformation, recurrence, and risk factors for recurrence. Oral Oncol. 2017; 66: 93-99 [ Links ]
21. Chooback N, Shen Y, Jones M, et al. Carcinoma ex pleomorphic adenoma: case report and options for systemic therapy. Curr Oncol. 2017; 24: e251-e254 [ Links ]
22. Suzuki M, Matsuzuka T, Saijo S, et al. Carcinoma ex pleomorphic adenoma of the parotid gland: a multi-institutional retrospective analysis in the Northern Japan Head and Neck Cancer Society. Acta Otolaryngol 2016 Nov; 136 (11):1154-8 [ Links ]
23. Skandalakis J, Colborn G, Weidaran T, et al: Surgical Anatomy: The Embryologic and Anatomic Basis of Modern Surgery. Paschalidis Medical Publications. McGraw-Hill Publishing, Greece, 2004 [ Links ]
24. Weiss A, Shnayder G, Tagliareni J, Wun E, Clarkson E, Dym H. Large unilateral mass in submandibular region. J Oral Maxillofac Surg. 2012; 70:842-850 [ Links ]
25. Sheedy TM. Evaluation and management of adult neck masses. Physician Assist Clin. 2018; 3: 271-284 [ Links ]
26. Li Y Xiao N, Dai Y Guo S, Zhang Y, Wang D, Cheng J. Comprehensive characterization of pleomorphic adenoma at intraoral unusual sites. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022; 133: 21-27 [ Links ]
27. Andreasen S, Therkildsen MH, Bjorndal K, Homoe P. Pleomorphic adenoma of the parotid gland 1985-2010: a Danish nationwide study of incidence, recurrence rate, and malignant transformation. Head Neck. 2016; 38 (Suppl 1):E1364-E1369 [ Links ]
28. Valstar MH, de Ridder M, van den Broek EC, et al. Salivary gland pleomorphic adenoma in the Netherlands: a nationwide observational study of primary tumour incidence, malignant transformation, recurrence, and risk factors for recurrence. Oral Oncol. 2017; 66: 93-99 [ Links ]
29. Zbären P, Tschumi I, Nuyens M, Stauffer E. Recurrent pleomorphic adenoma of the parotid gland. Am J Surg. 2005; 189 (2):203-207 [ Links ]
 Correspondence:
Correspondence:
Name: RE Rikhotso
Email: erikhotso@gmail.com














