Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.79 no.1 Johannesburg feb. 2024
http://dx.doi.org/10.17159/sadj.v79i01.16718
RESEARCH
Bacterial contamination of disinfectants: prevalence and students' compliance with infection control practices
NM MadzivaniI; SR MthethwaII; EM SekatiIII
IBSc, BSc Honours, BDS, Tshilidzini Hospital, Shayandima, Limpopo, South Africa ORCID: https://orcid.org/0009-0000-9190-9786
IIBDS, MPH, PhD, Sefako Makgatho Health Sciences University, Ga-Rankuwa, South Africa ORCID: https://orcid.org/0000-0003-0420-808X
IIIND Med Tech, BTech, BSc Med Honours, MSc ORCID: https://orcid.org/0009-0000-0395-940X
ABSTRACT
INTRODUCTION: Contaminated disinfectants have been occasional vehicles of healthcare associated infections.
AIMS AND OBJECTIVES: To determine the presence and level of bacterial contamination of disinfectants used to decontaminate suction devices and to assess the extent to which students comply with infection control practices.
DESIGN: A two-part cross-sectional descriptive study consisting of microbiological testing of disinfectants and a questionnaire-based observation of students.
METHODS: Unannounced observation of students disinfecting suction devices were recorded using a questionnaire. The process involved collecting a prepared disinfectant from a storage bin using a kitchen measuring jug. Specimens of disinfectants and swabs of jugs were collected for aerobic culture. Data pertaining to compliance with infection control practices was gathered.
RESULTS: Only 33.6% of the students were observed unannounced. An overwhelming majority (84.9%) of students disinfected suction devices; 52% cleaned and disinfected the external surface of suction hoses and the spittoon bowl; 18.6% allowed the disinfectant to remain in the system long enough, and 14% advised their patients not to close their lips around the suction device. The majority of disinfectant samples (56.3%) as well jugs (55.6%) were contaminated with bacteria.
CONCLUSION: Bacterial contamination of disinfectants was common in addition to poor compliance with infection control practices.
INTRODUCTION AND BACKGROUND
Healthcare-associated infections are a major global safety concern for both patients and healthcare professionals.1 The biofllm-derived microorganisms from contaminated hoses of dental chair suction devices - for example, the high-volume suction and saliva ejector - are a potential source of cross-contamination and cross-infection.2 Highvolume evacuation systems (HVE) prevent contaminated aerosols from escaping the immediate operating site.3 Studies have shown HVE to reduce more than 90% of aerosols arising from the operative site4,5 The efficiency of HVE is determined by the suction force of the appliance, the proximity of the HVE to the operating site and the number of evacuators used.6 Clinicians need to check the power and airflow volume of the HVE periodically.7 Saliva ejectors prevent contaminated aerosols from escaping the mouth.3 They may, however, create unsanitary conditions by allowing a backflow of previous patients' waste material or substances from the tubing into a patient's mouth. Three Interrelated predisposing factors for backflow have been identified. They are: simultaneous use of saliva ejector and HVE; the positioning of the suction tubing attached to the ejector above the patient's mouth; and the presence of less pressure in a patient's mouth than in the saliva ejector.8
I nternational Organization for Standardization (ISO) standards recommend manufacturers provide appropriate cleaning and disinfection directions to use on the suction devices unit which should be followed.9 Failure to clean suction devices daily leads to biofllm growth, a heavy bioburden and a greater risk of infections. Daily cleaning protects the equipment and maintains full suction power.10 Inadequate disinfection of suction hoses and bacterial contamination of disinfectants are additional potential sources of healthcare associated infections.11 Boyle and colleagues (2015) demonstrated that the method of disinfection influences the effectiveness of decontamination of suction hoses.12 They found that standard aspiration disinfection was more effective in decontaminating high volume suction hoses than low volume suction hoses and that standard aspiration was less effective than manual or automated flood disinfection.12 Contaminated disinfectants and antiseptics have been occasional vehicles of health-care associated infections and pseudo epidemics for more than 70 years.13 Two recent reviews (a scoping review and a systematic review) found glaring differences in risk factors for bacterial contamination between low- and middle-income countries and high-income countries. The differences were found at the level of container (reused, recycled or inadequate processing vs design and functioning, presence of cork and cotton, biofilm formation) preparation (place, utensils or tap water, high and incorrect dilutions vs nonsterile water, overdilution) and practices (topping up or too long use vs too long expiry dates, inappropriate container reprocessing, topping up of containers and deviations from procedures).13,14 The reviews found similarities in contaminating bacteria between low-and middle-income countries and high-income countries. Non-fermentative Gram negative rods and Enterobacterales were the most frequent isolates from contaminated antiseptics, disinfectants and hand hygiene products.13,14 Previous research reported that members of the genus Pseudomonas (P. aeruginosa, for example) were the most frequent isolates from contaminated disinfectants.15
It has been reported that contaminated disinfectants exhibit decreased efficacy and effectiveness.16 Also of therapeutic significance are reports that a number of bacterial contaminants isolated from disinfectants have exhibited resistance to commonly used antimicrobial agents.17
This study was designed to investigate the potential for healthcare-associated infections related to the process of disinfecting dental unit suction systems.
OBJECTIVES
To determine the presence and grade of bacterial contamination of disinfectants used to decontaminate suction devices.
To assess the extent to which students comply with infection control practices.
MATERIALS AND METHODS
Study design
This two-part cross-sectional descriptive study consisted of microbiological testing of disinfectants and a questionnaire-based observation of students during the process of disinfecting suction devices.
Target populations
The two populations studied were dental students who had clinical sessions and prepared disinfectant solutions, and jugs used to draw the solutions from storage bins. The study was conducted between June and August 2022 at a dental school in Gauteng, South Africa.
Dental students
Slightly more than one-third (33.6%) of the total population of 143 dental students had clinical sessions during the study period. At the clinics, students, in their classes, were organised into equal-sized groups and allocated dental chairs for the purposes of supervision. The number of groups and their size was dependent on class size.
Disinfectant solutions
A total of 16 60-litre capacity storage bins contained the disinfectant solutions. Nine one-litre capacity kitchen measuring jugs were used to draw from the bins.
Data collection
Microbiological testing
Five millilitre samples of disinfectants were collected in sterile universal containers using sterile pipettes from storage bins at the clinics over a period of one week during the 11am to 1pm clinic session while sterile swabs (premoistened with sterile saline) were used to collect samples for aerobic culture from the walls of the jugs. All samples reached the laboratory within 2 hours of collection and were processed Immediately upon arrival.
Disinfectant samples were cultured on blood agar, incubated at 37°C for 24-48 hours, using two different methods as specified by Danchaivijtr and colleagues (2005).18 Each labelled swab was uncapped and lightly rolled over the entire surface of a blood agar plate with the same label and incubated at 37°C for 48 hours. Resultant colonies were graded on a scale of 0 to 4+ based on the number of quadrants on each plate that showed positive growth according to the procedure used by Bible and colleagues (2009).19 They were classified according to the Gram stain procedure of Engelkirk and Duben-Engelkirk (2008).20 A selection of colonies was subcultured in blood agar and the bacteria identified in VITEK®2, an automated instrument used for the identification and antimicrobial susceptibility testing.
Observation-based survey
Unannounced observation of individual third, fourth and fifth-year dental students in their groups was performed by the researcher and co-supervisor using a questionnaire adapted from the Centers for Disease Control and Prevention's (CDC) Quick Observation Tools (QUOTs).21 The questionnaire consisted of a series of closed questions which could only be answered with a yes or no. The questions related to precautions, activities or practices which were necessary for infection control. The process of disinfecting dental unit suction systems involved collecting a prepared dental suction disinfectant from a storage bin using a jug. The dental suction disinfectant, Bacterex, was prepared i.e. 4 x 15 gram sachets of chlorine disinfectant cleaner powder were mixed in 60 litres of cold water, in cleaned and disinfected storage bins. It was stored out of direct sunlight. It was not freshly prepared on each workday. The storage bins were not labelled with the date prepared and the use-by date. The jugs used to draw the prepared dental suction disinfectant for aspiration disinfection of the suction devices were hygienic. They were stored in a dry, cool, clean environment. None of the nine jugs was graduated in units of volume i.e. millilitres and litres. The order in which the groups were observed was decided randomly - the groups were assigned numbers; these were thoroughly mixed and drawn at random without replacement. The third and fourth-year clinics were held separately in the same floor of the hospital. The agreement of the observations between the researcher and co-supervisor was assessed in one group of students in each class.
Definition of variables and terms
Overwhelming majority refers to a majority that is about 70% or more.
Vast majority refers to a majority that is 85% or more.
Ethical considerations
The study protocol was approved by the University Ethics Committee (SMREC/D/208/2020:PG). Permission to conduct the study was granted by the Chief Executive Officer (CEO) of the Oral Health Centre.
Statistical analysis/Hypothesis testing
Collected data was captured and analysed in SPSS software. Means and proportions (percentages) were calculated. The Chi-squared tests was performed to test for the statistical significance of the differences in proportions of the summary of observations. The chosen significance level for the tests was a p-value equal to or less than 0.05.
Results
The results of microbiological testing and the observation-based survey are presented separately.
Microbiological testing
Data obtained from microbiological testing of prepared disinfectant and swabs of the walls of the jugs were analysed.
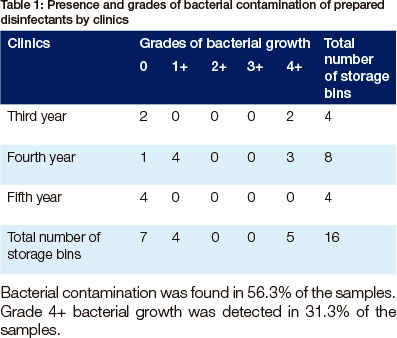
Bacterial contamination was found in 56.3% of the samples. Grade 4+ bacterial growth was detected in 31.3% of the samples.
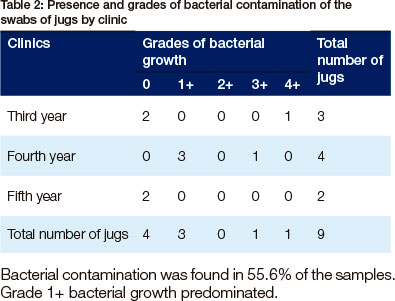
Bacterial contamination was found in 55.6% of the samples. Grade 1+ bacterial growth predominated.
Microscopy
The overwhelming majority of the bacteria were Gram positive. Cocci in pairs, clusters or chains predominated. Rod-shaped single cells were also seen.
Structured observations
Data gathered from structured observations of classes of third, fourth and fifth-year dental students were analysed.
None of the students: drew the recommended volume of the solution through the evacuation system lines; advised patients not to close their lips around the suction device; allowed the disinfectant to remain in the system for at least 10 minutes before they started working. Merely a third of the students disinfected the external surface of suction hoses during the time that they were disinfecting the suction lines.
None of the students drew the recommended volume of the solution through the evacuation system lines. An equal proportion (19.4%) of students advised patients not to close their lips around the suction device as allowed the disinfectant to remain in the system for at least 10 minutes before they started working.
The differences in the proportions of summary of observations between the classes by year of study was significant for the observations: the spittoon is cleaned and disinfected at the same time as the suction lines (p=0.007); the suction cleaning solution is allowed to remain in the system for at least 10 minutes (p=0.001) and patients are advised not to close their lips around the suction device (p=<0.001).
Of the three students observed, two allowed the disinfectant to remain in the system for at least 10 minutes. None of the students drew the recommended volume of the solution through the evacuation system lines.
DISCUSSION
This study set out to determine the presence and level of bacterial contamination of prepared suction system disinfectants and observe students' compliance with infection control practices.
Microbiological testing
The most interesting finding was that the majority of the samples of prepared disinfectants (56.3%) and of the jugs (55.6%) were contaminated with bacteria. Bacterial contamination of a disinfectant prepared by dissolving a known mass of solute in a known amount of solvent has not previously been described. A great deal of the previous research has been performed on disinfectant prepared by diluting a stock solution.11,13,16 The prevalence of contamination recorded in this study is 15% higher than the 40% range of published previous studies (3%,22 6.1 %,23 7.9%,24 34.4%25 and 43%11). It seems possible that the jugs played a significant role in the contamination of the disinfectants.
Another important finding was that the highest grade (4+) of bacterial growth was recorded in 31.3% samples of the prepared disinfectants. This rather disappointing finding suggests that the bacteria were able to adapt and multiply in solutions.26 This could be related to the reduced efficacy of the disinfectant.27 The factors that are known to affect the efficacy of disinfectants include: pH, concentration, temperature structure,15 nature, composition and condition of the organism,28 organic and inorganic load present, type and level of microbial contamination, presence of biofllms,15 overdilution of disinfectants, poor personal hygiene, non-adherence to proper techniques in their uses and reuse, and improper storage.29 The factors that may have played a role in this study are too long use and too long expiry dates related to the fact that storage bins were not labelled.
Microscopic identification
The results of this study show that gram-positive cocci were the predominant organisms. Although these results differ from some published studies,14,15 they are consistent with those Kgabi (2015) who found mainly gram-positive cocci and some gram-negative bacilli in samples of antiseptics and surface disinfectants.30
Biochemical identification
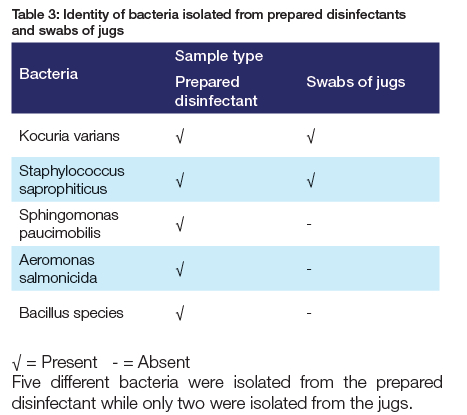
Bacteria cultured in this study were identified as Kocuria varians, Staphylococcus saprophiticus, Aeromonas salmonicida, Sphingomonas paucimobilis and Bacillus species. The findings of the current study do not support the previous research in that neither Enterobacterales nor P. aeruginosa were isolated from the disinfectants.13-15 It seems possible that the contaminants isolated in this study originated from hand contact or oral contamination as they naturally inhabit the skin and mucous membranes.31,32
OBSERVATION-BASED SURVEY
Response rate
The results of this study show that the response rate was low at an average of 34%. This finding was unexpected. There are several possible explanations for this result: students were away on offcampus rotations; patients did not honour appointments and the days of data collection for the study coincided with test dates. The data of the observation-based survey must be interpreted with caution because the sample is not representative of the population as a whole.
The results of this study show that a vast majority (95.3%) of students used personal protective equipment during the time that they were disinfecting the suction lines (Table 7). It is disappointing that a few students (almost five percent) disregarded safety precautions. The health effects of the chlorine-based disinfectant include the irritation and burning of eyes and hands, among others.
The results of this study indicate that an overwhelming majority (79.1%) of students disinfected the suction lines at the start of the clinic session (Table 7). The present finding confirms the existence of a disinfection policy at the clinics. This is consistent with the results of Shah and colleagues (2007) who found that 92% of orthodontics departments in the United Kingdom had a policy to disinfect waterlines and suction tubing.33 It is rather disappointing that 20.9% of the students did not follow the policy as it is well established that improperly disinfected suction apparatus provides a favourable environment for biofilm proliferation.12
The most interesting finding was that none of the students drew the recommended volume of the solution through the evacuation system lines (Tables 4-6). This finding is not surprising considering that none of the nine jugs used was graduated in the most common units of volume i.e. millilitres and litres. Failure to follow the manufacturer's recommendation may affect the efficacy of disinfection practices.34
Another important finding was that a little less than 20% (18.6%) of the students allowed the disinfectant to remain in the system for at least 10 minutes before they started working (Table 7). One unanticipated finding was that the contact time for the disinfectant used (Bacterex) was five minutes.35 This oversight makes it difficult to explain the results of this study. The oversight was due to the fact that most EPA-registered hospital disinfectants have a label contact time of 10 minutes.15
The results of this study show that the proportion of students who cleaned and disinfected the external surface of suction hoses together with the spittoon bowl at the same time as the suction lines varied widely i.e. the ranges were 66.7% and 48.4% respectively (Table 7). It is very concerning that not all students cleaned and disinfected the external surface of suction hoses and the spittoon bowl for the reason that Staphylococcus and Bacillus species have been isolated from these surfaces.36
The results of this study show that at most 14% (6 out of 43) of the students advised their patients not to close their lips around the suction device (Table 7). This result has not previously been described. This result may be explained by the fact that there were no notices in the clinics reminding student to comply with this recommendation. This finding is rather disappointing considering that the cross contamination potential of saliva ejectors has been investigated and reported on since 1990s. Although there is no direct proof of cross-contamination, a great deal of research has indicated that fluid can flow backward in low-volume suction lines when patients close their lips around the saliva ejector tip.37-39
LIMITATIONS CONCLUSION
The current study found that bacterial contamination of disinfectants was common in addition to poor compliance with infection control practices.
REFERENCES
1. Irek EO, Amupitan AA, Aboderin AO, Obadare TO. A systematic review of healthcare- associated infections in Africa: An antimicrobial resistance perspective. Afr J Lab Med 2018; 7(2): 1-9 [ Links ]
2. O'Donnell MJ, Tuttlebee CM, Falkiner FR, Coleman DC. Bacterial contamination of dental chair units in a modern dental hospital caused by leakage from suction system hoses containing extensive biofilm. J Hosp Infect 2005; 59(4):348-60 [ Links ]
3. Nagraj SK, Eachempati P, Paisi M, Nasser M, Sivaramakrishnan G, Verbeek JH. Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases. Cochrane Database Syst. Rev 2020: 10(10): CD013686 [ Links ]
4. Jacks ME. A laboratory comparison of evacuation devices on aerosol reduction. J Dent Hyg 2002; 76(3):202-6 [ Links ]
5. Remington WD, Ott BC, Hartka TR. Effectiveness of barrier devices, high-volume evacuators, and extraoral suction devices on reducing dental aerosols for the dental operator: A pilot study. J Am Dent Assoc 2022; 153(4):309-18 [ Links ]
6. Samaranayake LP, Fakhruddin KS, Buranawat B, Panduwawala C. The efficacy of bio-aerosol reducing procedures used in dentistry: a systematic review. Acta Odontol Scand 2021; 79(1):69-80 [ Links ]
7. Avasthi, A. High Volume Evacuator (HVE) in reducing aerosol - An exploration worth by clinicians. J. Dent. Health Oral Disord Ther 2018; 9(3):165-6 [ Links ]
8. CDC. Saliva Ejector and Backflow. Available https://www.cdc.gov/oralhealth/infectioncontrol/faqs/saliva.html Accessed [03 July 2023] [ Links ]
9. ISO 17664-1:2021. Processing of health care products - Information to be provided by the medical device manufacturer for the processing of medical devices - Part 1: Critical and semi-critical medical devices. https://www.iso.org/obp/ui/en/#iso:std:iso:17664:-1:ed-1:v1:en Accessed [03 July 2023] [ Links ]
10. DiGangi P. On stage with evacuation lines: Safety guidance for dental hygienists. Available: https://www.dentistryiq.com/dental-hygiene/clinical-hygiene/article/16367742/on-stage-with-evacuation-lines-safety-guidance-for-dental-hygienists Accessed [03 July 2023] [ Links ]
11. Oie S, Kamiya A. Microbial contamination of antiseptics and disinfectant. Am. J. Infect Control 1996; 24:389-95 [ Links ]
12. Boyle MA, O'Donnell MJ, Russel RJ, Galvin N, Swan J, Coleman DC. Overcoming the problem of residual microbial contamination in dental suction units left by conventional disinfection using novel single component suction handpieces in combination with automated flood disinfection. J Dent. 2015; 43:1268-79 [ Links ]
13. Lompo P, Heroes A-S, Agbobli E, et al. Bacterial Contamination of Antiseptics, Disinfectants and Hand Hygiene Products in Healthcare Facilities in High-Income Countries: A Scoping Review. Hygiene 2023; 3(2):136-75 [ Links ]
14. Lompo P, Agbobli E, Heroes A-S, et al. Bacterial Contamination of Antiseptics, Disinfectants, and Hand Hygiene Products Used in Healthcare Settings in Low- and Middle-Income Countries - A Systematic Review. Hygiene 2023; 3(2):93-124 [ Links ]
15. Rutala WA, Weber DJ, the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available: https://www.cdc.gov/infectioncontrol/guidelines. Accessed [14 February 2020] [ Links ]
16. Kajanahareutai S, Rahule S, Sirikulsatein P, Sangkasuwan S, Yospol P. Efficacy and contamination of in-use disinfectants in Rajavithi General Hospital. J Med Assoc Thai 1995; 78 Suppl 1: S36-9 [ Links ]
17. Randall LP, Cooles SW, Piddock LJ,Woodward MJ. Effect of triclosan or a phenolic farm disinfectant on the selection of antibiotic-resistant Salmonella enterica. J. Antimicrob. Chemother 2004; 54: 621-7 [ Links ]
18. Danchaivijitr S, Dhiraputra C, Rongrungruang Y, Srihapol N, Pumsuwan V. Microbial contamination of antiseptics and disinfectants. J Med Assoc Thai 2005; 88 Suppl 10: S133-9 [ Links ]
19. Bible JE, Biswas D, Whang PG, Simpson AK, Grauer JN. Which regions of the operating gown should be considered most sterile? Clin Orthop Relat Res 2009; 467(3):825-30 [ Links ]
20. Engelkirk PG, Duben-Engelkirk J. Laboratory Diagnosis of Infectious Diseases: Essentials of Diagnostic Microbiology. Baltimore: Wolters Kluwer/Lippincott Williams & Wilkins, 2008 [ Links ]
21. Centers for Disease Control and Prevention. Quick Observation Tools (QUOTs) for Infection Prevention, 2019. Available at: https://www.cdc.gov/infectioncontrol/tools/quots.html Accessed [05 March 2019] [ Links ]
22. Christensen EA, Jepsen OB, Kristensen H, Steen G. In-use tests of disinfectants. Acta Pathol Microbiol Immunol Scand B 1982; 90(2):95-100 [ Links ]
23. Gajadhar T, Lara A, Sealy P, Adesiyun AA. Microbial contamination of disinfectants and antiseptics in four major hospitals in Trinidad. Rev Panam Salud Publica 2003; 14(3):193-200 [ Links ]
24. Keah KC, Jegathesan M, Tan SC, et al. Bacterial contamination of hospital disinfectants. Med J Malaysia 1995; 50(4):291-7 [ Links ]
25. Olayemi AB, Obayan Y. Contaminated disinfectants in health clinics in Ilorin, Nigeria. Infect Control Hosp Epidemiol 1994; 15(9):581-2 [ Links ]
26. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control 1995; 23(4):251-69 [ Links ]
27. Setlow B, Loshon CA, Genest PC, Cowan AE, Setlow C, Setlow P. Mechanisms of killing spores of Bacillus subtilis by acid, alkali and ethanol. J Appl Microbiol 2002; 92(2):362-75 [ Links ]
28. Russell AD. Bacterial resistance to disinfectants: present knowledge and future problems. J Hosp Infect 1999; 43 Suppl: S57-68 [ Links ]
29. Bassett DC, Stokes KJ, Thomas WR. Wound infection with Pseudomonas multivorans. A water-borne contaminant of disinfectant solutions. Lancet 1970; 1(7658):1188-91 [ Links ]
30. Kgabi, SP. Bacterial Contamination of Disinfectants and Antiseptics at Medunsa Oral Health Centre [Master's Thesis]. Sefako Makgatho Health Sciences University. 2015 [ Links ]
31. Williams AN, MacLea KS. Draft Genome Sequence of Dermacoccus nishinomiyaensis TSA37, Isolated from Wood Ash. Microbiol Resour Announc 2019; 8(50): e01370-19 [ Links ]
32. Kandi V, Palange P, Vaish R, et al. Emerging bacterial infection: identification and clinical significance of Kocuria species. Cureus 2016; 8(8): e731 [ Links ]
33. Shah R, Collins JM, Hodge TM, Laing ER. A national study of cross infection control: "are we clean enough?". Br Dent J 2009; 207(6):267-74 [ Links ]
34. Boyce JM. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control 2016; 5:10 [ Links ]
35. Xtremexccessories.1 x 15-gram chlorine disinfectant cleaner powder with detergents & corrosion inhibitors (15g makes 5l of disinfectant). Available: https://xtremexccessories.co.za/products/15-gram-chlorine-disinfectant-cleaner-powder-with-detergents-corrosion-inhibitors-15g-makes-5l-of-disinfectant [Accessed 24 August 2022] [ Links ]
36. Deulkar S, Singh S, Tiwari D. Isolation of selected possible aerobic bacterial pathogens from dental environmental surfaces after use of disinfectants - A case study at a public dental clinic, in KwaZulu-Natal. SADJ 2020; 75(5): 241-6 [ Links ]
37. Watson CM, Whitehouse RLS. Possibility of cross-contamination between dental patients by means of the saliva ejector. J Am Dent Assn 1993; 124:77-80 [ Links ]
38. Mann GLB, Campbell TL, Crawford JJ. Backflow in low-volume suction lines: The impact of pressure changes. J Am Dent Assn 1996; 127: 611-5 [ Links ]
39. Barbeau J, ten Bokum L, Gauthier C, Prevost AP. Cross-contamination potential of saliva ejectors used in dentistry. J Hosp Infect 1998; 40: 303-11 [ Links ]
 Correspondence:
Correspondence:
Dr SR Mthethwa
Tel: (012) 521 5888
Email: rocky.mthethwa@smu.ac.za
Author's contribution
1 . D Madzivani - 30%
2 . SR Mthethwa - 40%
3 . EM Sekati - 30%














