Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.78 no.2 Johannesburg mar. 2023
CASE REPORT
Morphological variations of two cases of maxillary myofibromas
T GoundenI; RZ AdamII; L MdlaloseIII
IBChD (UWC), DipOralSurg (SA),PDD (UWC),MSc (UWC). Dentist, Department of Maxillofacial and Oral Surgery, Inkosi Albert Luthuli Central Hospital. Corresponding author. ORCID: 0000-0003-2714-6192
IIBChD (UWC), PDD (UWC), MSc (UWC), PhD (UWC). Associate Professor, Acting Head of Department of Conservative Dentistry, Faculty of Dentistry, University of the Western Cape. ORCID: 0000-0002-2645-9878
IIIBChD (UWC), MChD (UWC), FCMFOS (SA). Maxillofacial and Oral Surgeon, Head of Department Maxillofacial and Oral Surgery, Inkosi Albert Luthuli Central Hospital. ORCID: 0000-0003-0564-2304
INTRODUCTION
The aim of this case report is to depict the varied spectrum of clinical presentation of two cases of solitary myofibromas, one of which was intra-osseous whilst the other presented as a soft tissue lesion. This highlights the spectrum of the clinical presentation of the same pathology.
In the most recent World Health Organisation (WHO) 2022 classification of soft tissue tumours, myofibroma is included under the category of myopericytomas.
Myopericytoma is a distinctive perivascular myoid neoplasm that forms a morphological spectrum with myofibroma. Molecular evidence has revealed PDGFRB (platelet-derived growth factor receptor beta) mutations in myopericytoma and myofibroma as well as SRF-RELA gene fusions in both lesions confirming a common pathogenesis for both.1
Myofibromas are benign soft tissue neoplasms derived from myofibroblastic cells.2
The term myofibroma refers to a solitary lesion. Myofibromatosis refers to cases in which multiple lesions are present which may affect either one or multiple anatomical locations. Myofibromatosis is almost exclusively seen in young children under the age of 2-years. Myofibromas exhibit a wide age range of clinical presentation and may be present at birth or arise within the first two years of age, but may also present in adults with a significant male predominance. Solitary myofibromas have a predilection to occur in the oral cavity, skin or subcutis of the head, neck and trunk.
CASE 1
A 12-year-old male patient was referred to the Maxillofacial and Oral Surgery clinic in a central hospital in Kwa-Zulu Natal with no known medical co-morbidities or allergies and a previous history of general anaesthesia for an incisional biopsy of a left maxillary lesion. A 3-year history of a slow growing left maxillary mass causing a left lateralizing zygomatic deformity was reported (Figure 1). There were no palpable lymph nodes in the head and neck region noted on clinical examination.
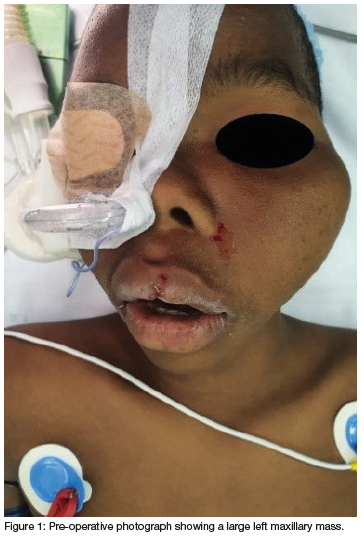
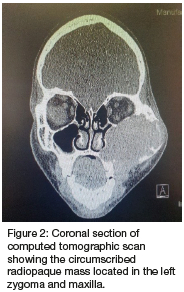
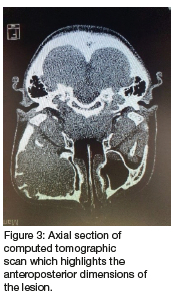
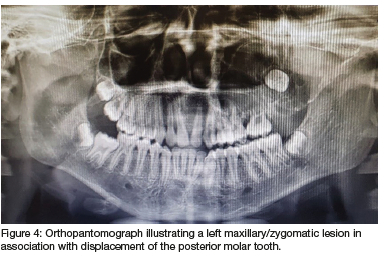
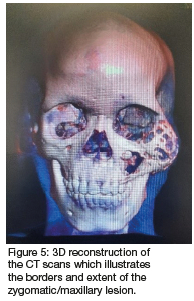
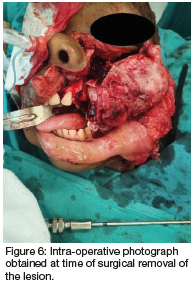
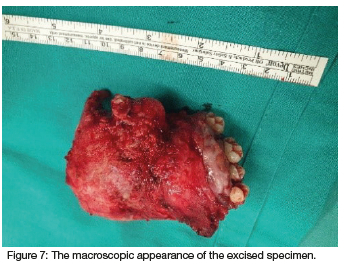
Computed tomography (CT) (Figures 2 & 3) and orthopantomography (OPG) (Figure 4), of the facial bones revealed an expansile mass involving the left maxilla/ zygoma complex/ infra-orbital and lateral orbital rims. Histology and immunostaining from an incisional biopsy confirmed a myofibroma. The left zygoma and maxilla was enlarged and firm and the lateral and infra-orbital rim showed signs of expansion. Surgical excision of the mass was completed under general anaesthesia followed by placement of an orbital mesh for globe support and function (Figures 6 & 7).
CASE 2 HISTORY
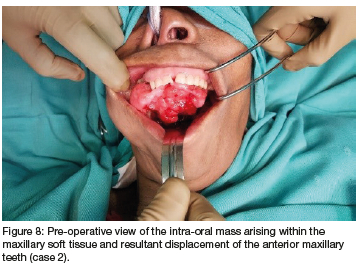
A 64-year-old female patient was referred from Obstetrics and Gynaecology (OBG) to the Maxillo-Facial and Oral Surgery department at a tertiary institution in Kwa-Zulu Natal. The patient presented with a large lobulated mass originating within the oral cavity. Intra-orally, it appeared as a whitish/ grey moveable lobulated mass which was firm in texture on palpation. The lesion appeared to originate from the right maxillary alveolar ridge. The patient's speech was negatively affected. There were no palpable lymph nodes to suggest lymph node involvement. Her main complaint was that she 'couldn't eat or talk properly' due to the size of the lesion, but had primarily come into the hospital due to a left inguinal abdominal pain. She provided a two-year history of recurrence following excision of a smaller lesion in the oral cavity at the same site in 2014. No chronic illnesses were reported, but acute constipation and vomiting was noted in the OBG ward. The patient had no known allergies, was a non-smoker and did not consume alcohol. The patient appeared malnourished on examination.
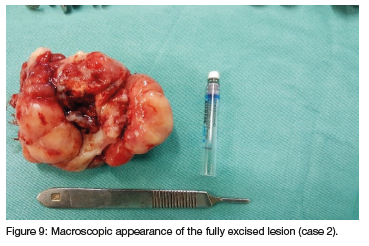
Plain Alms revealed no intra-osseous involvement, therefore no specialised radiography i.e., Computed tomography (CT) of the oral cavity was indicated. CT scan of the abdomen revealed an ovarian mass. The oral lesion was removed under general anaesthesia, a simple excision at the base of the lesion was done with a size 15 scalpel blade, a local soft tissue flap was raised to cover the soft tissue defect. Figure 8 shows the lesion in-situ prior to removal and figure 9 shows the excised lesion.
Histopathology and immunostaining revealed features of a spindle cell tumour favouring a myofibroma in the oral cavity. The histopathological diagnosis of the ovarian mass was that of leiomyomata (fibroids). There was no evidence of intra-oral recurrence at 10 month follow up and the patient declined dental rehabilitation. The ovarian lesion was thought to be co-incidental of the oral cavity lesion.
Discussion
Myofibromas occur predominantly in neonates and infants with few reported cases in adults.3 In a study done by Smith MH et al., 2017 the average age of clinical presentation for myofibromas was shown to be 23.1 years but approximately two-thirds of these tumours were reported to have been noted in the first two decades of life. The age range for myofibromas in this study showed clinical appearance ranging from birth to 84 years of age with a male predilection and a ratio of 1.2:1. The study interestingly also saw that most intra-osseous tumours were found in patients under 20 years and most patients over 40 had myofibromas of moveable tissue which is in keeping with this case report.2
Aetiology
Recent literature points to the mutations in the PDGFRB gene that appears to represent a common pathogenesis for myopericytoma and myofibroma.4
Myofibromas/ myopericytomas are associated with SRF-RELA gene fusions.
PDGFRB alterations are also seen in sporadic infantile /solitary adult myofibromas,5 Sporadic infantile myofibromatosis/ myopericytomatosis and conventional myopericytoma.4
PDGFRB mutation N666K is noted in myopericytomatosis but not in conventional myopericytoma.4
Clinical features
Essential features of myopericytoma include bland, myoid spindled cells growing in a concentric pattern around numerous small blood vessels.
Myopericytoma and myofibroma usually present as a painless, slow growing mass, which can be present for years.2
Grossly, most myofibromas are lobulated and on the cut surface there is a white, pink or grey rubbery appearance with whirling and may exhibit a gritty consistency. Displacement of teeth or developing tooth follicles, tooth mobility and/or expansion are seen in some intra-osseous lesions. There are variances in clinical features depending on the site and extent of the lesion.2
Clinically the tumour usually presents as a slow growing painless, distinct mass with intact overlying mucosa, however it has been noted that about a quarter of tumours showed rapid growth which may lead to the incorrect diagnosis of a malignancy.6 A small percentage of tumours do show atypical features and should be diagnosed as such as this is prognostically significant and may increase the risk of recurrence. Malignant transformation of myofibromas has been described previously, however, features of malignancy would be clinically, radiologically and pathologically noted.
Solitary myofibromas have a predilection for the head, while cases of myofibromatosis may involve soft tissues, bones and viscera, typically in the cardio-vascular, respiratory and GIT systems.7 In the head and neck region, the most frequent site is bone, followed by buccal mucosa and tongue.1
Radiology
Intra-osseous myofibromas present as solitary well-defined lesions. Soft tissue lesions may demonstrate focal bone erosion due to pressure effect radiographically.2
Haspel et al., 2012 suggests a full body CT with particular attention to the abdomen in order to rule out abdominal or visceral involvement.8
In case 2 it is noted that the histology referred to a leiomyomata (smooth muscle origin) for the ovarian lesion, which suggests the ovarian lesion to be unrelated to the myofibroma (myofibroblastic origin).
Histology
Myofibroma and myofibromatosis are histologically identical. Myofibroma is a benign mesenchymal neoplasm composed of myofibroblasts.8
Essential features of myofibroma include a biphasic growth pattern with primitive cellular zones often showing mitotic activity, necrosis, calcification and often surrounded by hyalinized nodules of myoid spindled cells.1
The centre of the lesion is composed of immature appearing, plump, spindled tumour cells associated with hemangiopericytoma-like branching blood vessels. Periphery of the lesion consists of nodules and fascicles of variably hyalinized, myoid appearing cells.1
Lesions are well circumscribed, encapsulated masses of spindled cells, often demonstrating a biphasic or zonal architecture. The periphery of the zones exhibits lighter-stained areas composed of short fascicles or whorls of myofibroblasts with pale pink cytoplasm and long, slender tapered nuclei. Myofibroblasts represent fibroblasts with contractile function and can often be identified by their spindled morphology and dendritic processes. These cells have plump oval nuclei often with open vesicular nucleoli.
The abrupt transition from central to peripheral zones may be an important feature in distinguishing myofibromas from other similar lesions. The central zone demonstrates darker-stained, more cellular areas composed of immature-appearing cells with less cytoplasm and larger, basophilic nuclei. This disease is often misdiagnosed as benign or malignant spindle cell lesions of nerve tissue or smooth muscle origin.
Morphological features of the cellular zones of myofibroma share some features with myopericytoma, suggesting that these are related entities. Myofibromatosis is defined by the presence of multiple myofibromas.1
Myofibroma/ myopericytoma with cellular/ atypical features show solid or focally infiltrative growth, increased cellularity Increased mitotic activity (more than 10 mitotic figures/ 10 high power fields), areas of infarction type necrosis no pleomorphism and no tumour necrosis.4
Pattern of zonation may be more haphazard or even reversed.9 Mitotic figures are variable in number and cellular zones may undergo necrosis and calcification.1 In infants, myofibromas may be composed almost entirely of primitive, cellular zones; such cases were historically labelled infantile hemangiopericytoma.10
Immunohistochemical staining of cells is required for an accurate diagnosis and in order to exclude similar spindled lesions. Vimentin is the most widely expressed intermediate filament protein thought to be involved in structural processes, however, is not diagnostic for this lesion. Specific markers of myoepithelial cells are alpha-SMA, muscle specific actin, and calponin, and negative markers include CD34, CD31, desmin, keratins and S-100 protein.2 Mitoses may be identified but are usually sparse. Increased cellularity, increased mitotic activity, cellular pleomorphism, areas of necrosis, infiltrative growth and focally staining perivascular orientation are features suggestive of an atypical myofibroma or malignant myopericytoma.
Differential diagnosis
Includes: reactive fibroblastic/ myofibroblastic lesions (nodular fasciitis, proliferative fasciitis, proliferative myositis), neurofibroma, fibrous histiocytoma, leiomyoma, leiomyosarcoma, hemangiopericytoma/ solitary fibrous tumour, fibromatoses, infantile fibrosarcoma, inflammatory myofibroblastic tumour, desmoplastic fibroma, cranial fasciitis and monophasic synovial sarcoma.1
Once the lesional cells react positively to alpha-SMA on immuno-histochemistry the differential diagnoses can be narrowed. Clinical presentation may vary amongst the different pathologies and clinicians should familiarise themselves with key differences in the varying morphologies and histopathology's.
Treatment
Factors influencing treatment include familial history of myofibromas, tumour location, cortical involvement, history of multiple lesions, aesthetic or functional concerns and the age of the patient. Simple excision is mainstay of treatment with local recurrence of 7%-31% according to Beck et al., 1999 however chemotherapy and radiation have been used on recurrent and non-resectable lesions.11
Conclusion
In conclusion clinicians must be wary of the array of soft tissue lesions that may present in the oral cavity. This case report highlights the importance of accurate diagnosis via histology with modern day techniques such as immunostaining. It also emphasises the wide age range that solitary myofibromas may appear and its morphological variations. Clinicians must be aware of the morphological similarities between benign and malignant spindle cell tumours as well as the possibility of possible pathologies at other sites of the body.
No conflict of interest
Informed consent taken was for images
REFERENCES
1. Alexiev BA. Myopericytoma / myofibroma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/softtissuemyopericytoma.html. Accessed March 10th, 2023. [ Links ]
2. Smith MH, Reith JD, Cohen DM, Islam NM, Sibille KT, Bhattacharyya I. An update on myofibromas and myofibromatosis affecting the oral regions with report of 24 new cases. Journal of oral and maxillofacial pathology. 2017; 124: 62-75. [ Links ]
3. Tajima N, Shirashi T, Ohba S, Fujita S, Asahina I. Myofibroma of the tongue: A case suggesting autosomal dominant inheritance. Oral and maxillofacial surgery. 2014; 117: 92-96. [ Links ]
4. Hung YP, Fletcher C. Myopericytomatosis. The American journal of surgical pathology. 2017;41: 1034-44. [ Links ]
5. Antonescu CR, Sung Y, Zhang L, Agaram NP, Fletcher C. Recurrent SRF-RELA Fusions Define a Novel Subset of Cellular Variants in the Myofibroma/Myopericytoma Spectrum: A Potential Diagnostic Pitfall with Sarcomas with Myogenic Differentiation. American Journal of surgical pathology. 2017; 41: 677-684. [ Links ]
6. Foss RD, Ellis GL. Myofibromas and myofibromatosis of the oral region: a clinicopathologic analysis of 79 cases. Oral surgery oral medicine oral pathology oral radiology endodontics. 2000; 89: 57-65. [ Links ]
7. Chung EB, Enzinger FM. Infantile myofibromatosis. Cancer. 1981; 48: 1807-1818. [ Links ]
8. Haspel AC, Coveillo VF, Stevens M, Robinson PG. Myofibroma of the mandible in an infant: case report, review of the literature and discussion. Journal of Oral and Maxillofacial Surgery. 2012; 70: 1599-1604. [ Links ]
9. Beham A, Badve S, Suster C, Fletcher C. Solitary myofibroma in adults: Clinicopathological analysis of a series. Histopathology. 1993; 22: 335-341. [ Links ]
10. Mentzel T, Calonje E, Nascimento AG, Fletcher C. Infantile Hemangiopericytoma Versus Infantile Myofibromatosis Study of a Series Suggesting a Continuous Spectrum of Infantile Myofibroblastic Lesions. The American Journal of Surgical Pathology. 1994; 18: 922-930. [ Links ]
11. Beck JC, Devaney KO, Weatherly RA, Koopman CF Jr, Lesperance MM. Paediatric myofibromatosis of the head and neck. Arch Otolaryngology Head neck surgery. 1999; 125: 39-44. [ Links ]
 Correspondence:
Correspondence:
Dr Tashen Gounden
Email: drtgounden@gmail.com
Author contributions:
Dr T Gounden Principal researcher and writing 40%
Dr RZ Adam Proof read and writing 30%
Dr L Mdlalose Proof read and specialist review 30%














