Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.78 no.2 Johannesburg mar. 2023
RESEACH
Neoplastic tissue transfiguration in vivo by recombinant human transforming growth factor-β3
U RipamontiI; P SwartII; C DickensIII; R DuarteIV
IBone Research laboratory, School of Clinical Medicine Faculty of Health Sciences, University of the Witwatersrand, Johannesburg. Orchid: https://orcid.org/0000-0002-6167-3594
IIDivision of Anatomical Pathology, School of Pathology, University of the Witwatersrand, Johannesburg/National Health Laboratory Services
IIIMolecular and Cellular Biology, Department of Internal Medicine, School of Clinical Medicine, University of the Witwatersrand, Johannesburg
IVMolecular and Cellular Biology, Department of Internal Medicine, School of Clinical Medicine, University of the Witwatersrand, Johannesburg
Keywords: Human transforming growth factor-ß3, human squamous cell carcinoma, tissue transfiguration, de-differentiation, neoplastic transformation.
Human oral squamous cell carcinomas (hSCCs) are the most common head and neck cancers now presenting with more aggressive biological and clinical features due to smoking and alcohol together with widespread viremia. Transforming growth factor-ß (TGF-β) proteins are powerful morphogens that induce rapid and substantial induction of endochondral bone formation but in primates only.
Intramuscular heterotopic Implantation of 125 hTGF-ß3 generate organoids that show tissue transfiguration in vivo with rapid and substantial induction of mineralised bone by days 15 and 30 with large osteoid seams populated by contiguous osteoblasts, with rapid replacement and transfiguration of the rectus abdominis muscle into bone. Biopsies from hSCCs were implanted subcutaneously Into athymic nu/nu scid mice. Rapidly growing masses were injected with 250 hTGF-ß3 reconstituted with 300 Matrigel®Matrix kept fluid on ice. Stained sections showed poorly differentiated up to anaplastic hSCCs at the periphery of the transplanted masses with a more differentiated keratinised oncotype in the centre of the growing carcinomas. qRT-PCR showed significantly up-regulation of Keratin 17 with down-regulation of the Peptidase Inhibitor 3 gene. The results indicate that the transfiguration patterns seen in the centre of hTGF-ß3 -Matrigel®Matrix injected specimens activates the cellular memory of the transplanted carcinomas with the induction of differentiated oncotypes with keratinised pearls of tumour growth markedly contrasting with the peripheral anaplastic carcinomatous landscape.
Significance
Carcinomas survive by recapitulating mechanisms of normal development. The transfiguration mechanism(s) by hTGF-ß3 in Matrigel®Matrix set into motion gene expression pathways reintroducing a memory of developmental events already known to the altered cells bringing neoplastic cells back to their initial stage with keratinised pearls of a highly differentiated oncotype. The injections of 250 of hTGF-ß3 in Matrigel®Matrix re-introduce a memory of developmental pathways already known to the affected cells, bringing back neoplastic cells to its initial non-neoplastic and keratinised initial status.
Perspective
Malignant tumours are the leading cause of death across both developed and underdeveloped countries (https://www.cancer.gov/about-cancer/understanding/statistics). Combined chemo-, radio- and surgical treatments are not yet - if ever will be - biologically and surgically successful to therapeutically resolve human malignancies.1
Because of the combination of alcohol, smoking widespread viremia, and as yet unknown immunological and bacteriological causes, human oral squamous cell carcinomas (hSCCs) are now presenting with much more aggressive biological and rampant clinical features.2 Extant features present a morphological and clinical pattern of aggressive rapid growth with anaplastic invasion. 3-5
Experimentation in the Chacma baboon Papio ursinus has shown that the recombinant human transforming growth factor-ß3 (hTGF-ß3) is the most powerful osteoinductive morphogen so far tested in primates.6,7 Our systematic studies in heterotopic rectus abdominis sites reported the rapid and substantial induction of bone formation with newly formed ossicles comparable to organoids. Generated organoids show tissue transfiguration in vivo with rapid and substantial induction of mineralised bone by days 15 and 30 with large osteoid seams populated by contiguous osteoblasts.6,7
EMBEDDING MOLECULAR SIGNALS INTO NEOPLASTIC MASSES: TISSUE TRANSFIGURATION IN VIVO
Because of the pleiotropic multifaceted biological activity of hTGF-ß3 in primates' tissues and microenvironments, experiments were set to transfigure anaplastic human oral squamous cell carcinomas (hSCCs) by direct intra-tumoral injections of relatively high doses of hTGF-ß3. Human and animal ethics clearances were obtained from the University of the Witwatersrand, Johannesburg (Human Research Ethics Committee Clearance no. M150608; Animal Research Ethics Committee AREC no. 2014/39/C). Athymic scid mice were purchased from The Jackson Laboratories, US and kept in a sterile microenvironment at the Wits Research Animal Facility (WRAF).
Biopsies from harvested hSCCs at the time of surgical debridement (Figure 1) were implanted subcutaneously into scid mice over the lateral chest into the pectoralis' muscle opened by blunt dissection (Figure 1C). Histological analysis of transplanted hSSCs showed the classic hallmarks of highly differentiated anaplastic cells with hyperchromatic nuclei (Figures 2A,B).
Transplanted hSCCs required just over three weeks to "graft" into the host nude mice followed by growth for a further six to seven days to sizeable masses of 5/7 mm diameter (Fig. 1C). Half of the growing hSCCs in the subcutaneous space of the athymic scid mice were injected with 250 hTGF-ß3 reconstituted with 300 Matrigel®Matrix kept fluid on ice (Figures 1E). Transplanted masses were injected up to four times in selected animals. The remaining hSCCs were not injected, to monitor the carcinomatous growth of hSCCs without hTGF-ß3 injections in vivo (Figure 1C).
Due to a high mortality rate of the implanted mice, tissues for molecular and histological analyses were limited to two non-injected hSCCs harvested at 3 and 5 weeks after heterotopic implantation, and seven hSCCs injected and harvested at weekly intervals. Samples for molecular analysis were flash frozen in liquid nitrogen and stored at -80°C. Examination of resin-embedded sections cut at 3 to 4 (Morphisto AG, Germany) showed the development and growth hSCCs across the cut sections (Figures 2A,B).
The heterotopic subcutaneous growth of hSCCs is a fundamental result that shows the transplantation of viable hSCCs from bioptic surgical material (Figures 1A,B; 2A,B). Histological examination of the resin-embedded sections showed a reproducible recurrent histological pattern of undifferentiated anaplastic growth at the periphery of the transplanted hSCCs biopsies with a different yet reproducible pattern of a differentiated oncotype in the centre (Figures C,E; Figs. D,F). The morphological data showed reproducible patterns of growth spatio/temporally distributed, i.e. poorly differentiated up to anaplastic hSCCs at the periphery of the transplanted tumours (Figures D,F) with a more differentiated keratinised oncotype in the centre of the injected growing carcinomas (Figures 2C,E).
The oncotype pattern' variations are of great significance. The morphological data show reproducible patterns of growth spatio/temporally distributed, i.e. poorly differentiated anaplastic hSCCs at the periphery of the transplanted biopsies vs. more differentiated with keratinised oncotype in the centre of the injected growing carcinomas, thus less malignant with a more differentiated oncotype in the centre following injections of doses of hTGF-ß3 in Matrigel®Matrix. Injected hSCCs thus induced an oncotype characterised by a shift into highly differentiated oncotypes with multiple pearls of keratinisation (Figures 2C,E). Molecular analyses were later performed on the flash frozen harvested tissues sampled according to origin. The shift into a different oncotype characterised by multiple pearls of keratinisation is mechanistically highlighted by overexpression of the human Keratin 17 gene in hTGF-ß3 injected samples when compared to untreated hSCCs control (Figure 3).
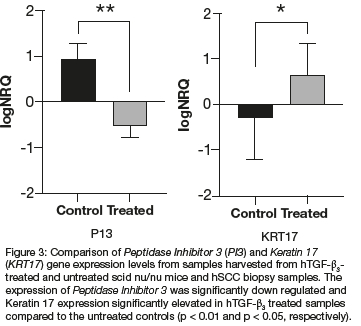
RNA was extracted using the RNeasy Micro Kit (Qiagen, GmbH, Hilden, Germany). RNA quantification, cDNA synthesis and quantitative real time polymerase chain reaction (qPCR) were as performed as previously described.8 Expression levels of Keratin 17 and Peptidase Inhibitor 3 normalised using three reference genes, were compared between hTGF-ß3 treated and untreated samples harvested from the scid mice and sections of the original hSCC biopsies used for the implantation. Peptidase Inhibitor 3 was significantly down-regulated and Keratin 17 expression significantly elevated in hTGF-ß3 treated samples compared to the untreated controls (p < 0.01 and p < 0.05, respectively) (Figure 3). The above tested genes were genes of interest identified in a genome wide expression profiling of oral squamous cell carcinoma.9
Non-injected hSCCs specimens showed a reproducible pattern of anaplastic growth throughout the transplanted hSCCs in the subcutaneous tissues of the operated athymic mice (Figure 2). hTGF-ß3 injected specimens showed a reproducible pattern of neoplastic growth with anaplastic differentiation at the periphery of the transplanted and injected SCCs. In the centre of the injected lesions, there was the differentiation of a highly differentiated oncotype with keratinised pearls of tumour growth markedly contrasting with the peripheral anaplastic carcinomatous landscape (Figures 2C,E). Cancers survive by recapitulating mechanisms of normal development.10 The transfiguration mechanism(s) by hTGF-ß3 in Matrigel®Matrix set into motion gene expression pathways reintroducing a memory of developmental events already known to the altered cells bringing neoplastic cells back to their initial stage with keratinised pearls of a highly differentiated oncotype.
Endogenous TGF-β suppresses tumorigenesis in a breast cancer xenograft model by affecting cancer stem cells or early progenitors.11 The paper reported that endogenous TGF-β has the potential to function as a tumour suppressor in carcinomas by "depleting the putative cancer stem cells or early progenitors cell population and by promoting differentiation of the more committed progeny".11 The findings that endogenous TGF-β is promoting differentiation of the more committed progeny, is also shown morphologically and molecularly in our study embedding hTGF-ß3 in fluid Matrigel®Matrix on ice resulting in the induction of differentiated oncotypes.
The injections of the 250 of hTGF-ß3 in Matrigel®Matrix re-introduce a memory of developmental pathways already known to the affected cells, bringing back neoplastic cells to its initial non-neoplastic and keratinised initial status.
ETHICS APPROVAL
Human and animal ethics clearances were obtained from the University of the Witwatersrand, Johannesburg (Human Research Ethics Clearance no. M150608; AREC 2014/39/C).
CONSENT FOR PUBLICATION
The authors agree with the contents of the manuscript and provide consent for publication. Availability of data and materials: Data are available upon request.
FUNDING
The University of the Witwatersrand, Johannesburg, for the Wits Seed Fund WSF15/04, and the SA NRF for a 2015 award of The Blue Skies Funding Instrument, Grant no. 93117.
COMPETING INTERESTS
The authors confirm that there are no conflicts of interest.
AUTHORS' CONTRIBUTIONS
Ugo Ripamonti conceptualised, designed the study and surgically implanted the human biopsy material in scid nu/ nu mice; Peter Swart analysed the histological sections; Caroline Dickens and Raquel Duarte prepared the material for molecular analyses, designed primers and performed and analysed qRT-PCR. Ugo Ripamonti wrote the manuscript and all authors commented, edited, and approved the final manuscript.
ACKNOWLEDGMENTS
The University of the Witwatersrand, Johannesburg, for the continuous support of our studies on the hTGF-ß3 isoform, kindly supplied by Novartis AG, Zurich. Dr Lisa Burnell for the supply of surgical bioptic material of hSCCs.
REFERENCES
1. Weinberg RA. Coming full circle - From endless complexity to simplicity and back again. Cell 2014;157:267-271. [ Links ]
2. Garrana RM, Shangase SL, Mohangi GU. Oral squamous cell carcinoma, a growing problem. SADJ. 2018;73(10):491-496. [ Links ]
3. Irfan M, Delgado RZR, Frias-Lopez J. The oral microbiome and cancer. Front Immunol. 2020;11:5911088. https://doi/org/doi:103389/fimiTiu.2020.591088 [ Links ]
4. Chen S-H, Hsiao S-Y Chang K-Y Chang J-Y New insights into oral squamous cell carcinoma: From clinical aspects to molecular tumorigenesis. Int J Mol Sci. 2021;22, 2252. https://doi.org/doi:10.3390/ijms22052252 [ Links ]
5. Sasahira T, Kirita T. Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcimoma. Int J Mol Sci. 2018;19,2413; https://doi.org/doi:10.3390/ijms19082413 [ Links ]
6. Ripamonti U, Ramoshebi LN, Teare J, Renton L, Ferretti C. The induction of endochondral bone formation by transforming growth factorß3: Experimental studies in the non-human primate Papio ursinus. J Cell Mol Med. 2008;12(3):1029-1048. doi:10.1111/J.1582-4934.2008.00126.x. [ Links ]
7. Ripamonti U. Rapid induction of bone formation by the transforming growth factorß3 isoform. In: Induction of bone formation in primates. In: Ripamonti U, editor. The Transforming growth factor-beta3. Ripamonti U (Ed), CRC Press Boca Raton, USA, 2016. p. 47-74. [ Links ]
8. Ripamonti U, Dix-Peek T, Parak R, Milner B, Duarte R. Profiling bone morphogenetic proteins and transforming growth factor-ßs by hTGF-ß3 pre-treated coral-derived macroporous bioreactors: the power of one. Biomaterials 2015;35:90-102. [ Links ]
9. Kengkarn S, Petmitr S, Boonyueni U, Reamtong O, Poomsawat S, Sanguansin S. Identification of novel candidate biomarkers for oral squamous cell carcinoma based on whole gene expression profiling. Path & Oncol Res. 2020;26:2315-2325. [ Links ]
10. Arias J-I, Aller M-A, Prieto I, Arias A, de Julian Z, Yang H, Arias J.The amazing power of cancer cells to recapitulate extraembryonic functions: The cuckoo's tricks. J Oncol. 2012, doi:10.1155/2012/521284. [ Links ]
11. Tang B, Yoo N, Vu M, Arias A, Namura M, Nam J-S, Ooshima A, Du Z, Desprez P-Y Anver MR, Michalowska AM, Shih J, Parks WT, Wakefield LM. TGF-ß can suppress tumorigenesis through effects on the putative cancer stem or early progenitor cell and committed progeny in a breast cancer xenograft model. Cancer Res. 2007;67(18):8643-8652. [ Links ]
 Correspondence:
Correspondence:
U Ripamonti
Bone Research Laboratory Address: University of the Witwatersrand, Johannesburg
Email: ugo.ripamonti@wits.ac.za














