Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.78 n.1 Johannesburg Feb. 2023
CASE REPORT
The oral presentation of secondary syphilis among men: the evolving interplay between syphilis, HIV and prophylactic strategies
J FourieI; L MukuchaII; L MasukaIII
IBChD, DipOdont(Perio), DipOdont(Rest), MScOdont, MChd. University of Pretoria, Faculty of Health Sciences, ORCID: 0000-0002-8674-8145
IIUniversity of Pretoria, Faculty of Health Sciences, Department of Periodontics and Oral Medicine. Registrar
IIIUniversity of Pretoria, Faculty of Health Sciences, Department of Periodontics and Oral Medicine. Registrar
ABSTRACT
Syphilis has been intricately linked with HIV because of shared transmission pathways and because these infections promote each other's transmission. In addition, HIV infection may change the clinical presentation and management of syphilis lesions.
Initially, the HIV epidemic had improved safe sex practices among men who have sex with men (MSM), but subsequent effective treatment and prophylaxis strategies, have resulted in behavioural disinhibition and a resurgence of syphilis.
AIMS AND OBJECTIVES: Here, we report on three cases of oral secondary syphilis and explore the relationship between oral syphilis and sexual practices, HIV and prophylactic measures that MSM employ
DESIGN/METHODS: Three men, who presented to the University of Pretoria Oral Health Centre (UPOHC), complaining of oral lesions, were diagnosed by histopathology with secondary syphilis. The clinical appearance of the lesions, HIV status, treatment and prophylaxis employed by the men were documented
RESULTS: The clinical presentation, sexual practices, HIV status and prophylactic measures among these men differed and demonstrate the complexity of oral secondary syphilis diagnosis and management
CONCLUSIONS: Syphilis presents variably in the oral cavity, and this may be linked to the sexual practices and HIV status of the patient
Keywords: secondary syphilis, Human Immunodeficiency virus (HIV), anti-retroviral treatment (ART), pre-exposure prophylaxis (PrEP), oral lesions, oral sex, men who have sex with men (MSM)
INTRODUCTION
Syphilis is a sexually transmitted disease (STD) caused by the spirochete bacterium Treponema pallidum, subspecies pallidum.'
Genital syphilitic lesions significantly increase the risk of HIV transmission.2-3 This resulted in an initial curb in syphilis prevalence, especially among men who have sex with men (MSM), due to safer sex practices.4-5 However, oral sex may wrongly be considered a 'safe sex' practice, and subsequently, result in the oral transmission of other sexually transmitted diseases, such as syphilis. Barrier protection remains the most effective way to reduce the sexual transmission of diseases. And although pre-exposure prophylaxis (PrEP) is now offered as an additional precaution against HIV transmission, it may inadvertently result in behavioural disinhibition or riskier sexual practices.6
When syphilis is acquired through oral sex, a painless ulcer, known as a chancre, may develop at the site of inoculation. However, due to its short-lived and painless nature, the primary infection often goes unreported.1 The secondary stage, however, has a varied clinical presentation and duration,7 during which patients may search for treatment from dental clinicians.7-8 Oral lesions of secondary syphilis have frequently been reported in the literature.9-12 The variation in clinical presentation makes it difficult to make a clinical diagnosis,12 and we therefore have to rely on histology and serology to reach a final diagnosis.13-14 It is possible that concurrent HIV infection may further alter the oral presentation and management of secondary syphilis.15
Here, we report on three cases of oral secondary syphilis and explore the relationship between oral syphilis and sexual practices, HIV and prophylactic measures that may be employed.
CASE PRESENTATIONS
Patients and methods: three male patients with oral secondary syphilis were identified at the Oral Medicine Clinic of the University of Pretoria Oral Health Centre (UPOHC) in 2021. The Research Ethics Committee granted a waiver of the need for written informed consent (University of Pretoria, Faculty of Health Sciences, Research Ethics Committee clearance number 379 2022). The data were anonymized at the stage of extraction from the patient charts.4
Case 1
A 26-year-old, white male presented with a complaint of an intra-oral 'rash' that had been present for the past four weeks. A saltwater rinse has helped to alleviate some of the sensitivity associated with the lesion. During the anamnesis, the patient reported being healthy, only smoking hubbly-bubbly and consuming alcohol socially. The patient practices sex with other men. Besides the palpable, rubbery, left submandibular lymph node, there were no other extra-oral abnormalities detected or reported by the patient. Intra-orally a large and irregularly shaped erythematous erosion was present on the left soft palate, with other, smaller and circular lesions starting to develop on the hard palate (Figure 1).
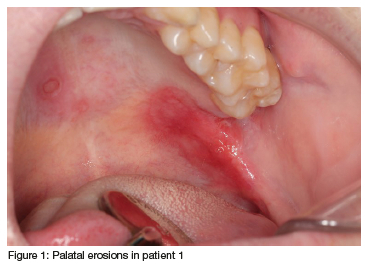
A differential diagnosis of an aphthous ulcer was considered, but the new lesions that started to occur on the hard palate, as well as the absence of a true break in the epithelium, discredited this differential diagnosis. Other possibilities, such as a deep fungal infection such as histoplasmosis or bacterial infection, such as tuberculosis, or oral syphilis, were also considered.
An incisional biopsy was performed on the lesion in the soft palate and the histopathological examination revealed hyperplastic stratified squamous epithelium with extensive neutrophilic exocytosis. Parts of the epithelium were ulcerated and covered by a fibrinopurulent membrane. The lamina propria consisted of a deep and dense plasmacytic infiltrate. Special stains with PAS did not demonstrate any fungal elements, and the Warthin-Starry stain showed isolated spirochetes at the basement membrane. Immunohistochemistry (IHC) for T. pallidum showed numerous spirochetes intra-epithelially as well as at the basement membrane. Overall, the features were in keeping with a syphilitic ulcer. The patient elected to have further laboratory investigations and treatment performed by his private medical doctor.
Case 2
A 29-year-old, African male complained of a lesion that moves around his mouth. Similar, painless, lesions developed 3 months earlier. These have all regressed with only the lesion of the upper lip remaining. The patient only experienced a tingling sensation associated with the lesion. The patient reports being completely healthy and occasionally consuming alcohol and smokes cigarettes. The patient has sex with men and had only a single recent sexual partner. He uses PrEP due to his concern for HIV infection.
No extra-oral abnormalities were noted or reported, and only a solitary, regular, and well-defined white plaque was noticed on the upper right labial mucosa (Figure 2).
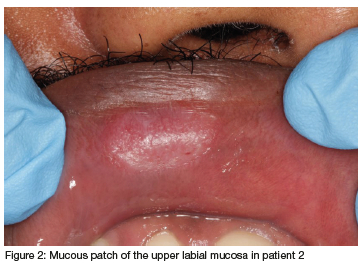
Differential diagnoses for a white plaque such as discoid lupus, leukoplakia, chemical burn and a mucous patch of secondary syphilis were considered.
An incisional biopsy was performed and the histopathological examination revealed the presence of a mucosa surfaced by hyperplastic parakeratinising stratified squamous epithelium with marked acanthosis and severe inflammatory exocytosis. Dense lymphoplasmacytic inflammation was present in the lamina propria, which extended deeper around vascular channels. IHC staining for T. pallidum demonstrated the corkscrew-like spirochetes within the epithelium and the adjacent connective tissue and was therefore deemed representative of a mucous patch of secondary syphilis.
Subsequent serology (ELISA) confirmed that the patient is HIV naïve, while serology for syphilis confirmed the diagnosis with a Rapid Plasma Reagin titre of 16:1. Treatment was initiated with a single IM dose of 2.4 million units of Benzathine penicillin G (BPG).
Case 3
The third patient was a 46-year-old, African male who was referred by his local clinic. The patient complained about painful 'blisters' of his tongue that limited his tongue movement and had been present for more than a year. He believed this disease may have been sexually transmitted or due to his broken-down dentition. He practices sex with women and has many different partners. Earlier treatment with antifungal agents at his local clinic was not successful. The patient is HIV positive and on treatment with ART (absolute CD4 count = 878 cells/uL). He smokes cigarettes and consumes alcohol occasionally.
No extra-oral abnormalities were noted. Multiple intra-oral lesions were present which could all be characterised as white plaques, which varied in regularity and definition, and were sometimes bordered by a red rim (Figure 3).
Based upon the clinical presentation of numerous white plaques, differential diagnoses such as human papilloma virus associated lesions, leukoplakia, oral squamous cell carcinoma (especially of the irregular plaque of the left buccal mucosa), hyperplastic candidiasis and a mucous patch of secondary syphilis were considered.
An incisional biopsy was performed of the lower labial mucosa and the left buccal mucosa. The histopathological examination revealed that both specimens had similar histological features consisting of hyperplastic stratified squamous epithelium with extensive neutrophilic exocytosis. The lamina propria contained a dense and deep plasmacytic infiltrate. Special stains with PAS did not demonstrate any fungal elements, while the Warthin-Starry stain showed isolated spirochetes at the basement membrane. IHC for T. pallidum showed numerous spirochetes intra-epithelially as well as at the lamina propria, confirming the diagnosis of syphilis infection. The patient was lost to recall, and serological confirmation could not be obtained, as the patient refused further management.
LITERATURE REVIEW AND DISCUSSION
History of syphilis
Syphilis has always been a stigmatized, contemptible disease. Countries have blamed other countries, as has been done with COVID-19, for outbreaks.16 At first, syphilis behaved more aggressively, spread more rapidly, and evolved atypically, frequently resulting in death. But, over time, as immunity in the community grew, and certain strains of T. pallidum evolved, the disease became milder and more predictable.16
Syphilis was first recognised as a separate disease to other sexually transmitted infections (STIs) in 1831, and the bacterial aetiology established in 1905. Direct identification of the bacterium was made possible through dark-field microscopy shortly thereafter. The first serologic test, the T. pallidum immobilization test (TPI), only became available in 1949.16
Epidemiology
The epidemiology of syphilis and HIV is intricately linked by shared transmission pathways and risk factors. Particularly in MSM, patients with multiple sexual partners, sex workers, intravenous (IV) drug users and patients with a previous history of STIs.3 So that HIV status became a significant predictor of syphilis prevalence.17
South Africa continues to see some of the highest rates of STIs. The adult population prevalence of syphilis has declined since 1990, likely due to improved treatment coverage, and was estimated at 0.50% for women and 0.97% for men in South Africa in 2017.18 While the estimated overall HIV prevalence rate is approximately 13,0%, with the total number of people living with HIV estimated at approximately 7,8 million in 2020. For adults aged 15-49 years, an estimated 18,7% of the population is HIV positive.19
In England, Germany and the USA, the HIV epidemic resulted in a reduced prevalence of syphilis between 1980 and 2000, as MSM changed their sexual behaviour.5 4,20 However, a sudden and significant increase was subsequently seen from the beginning of 2000 with infection rates among MSM almost doubling.5,4,20-21 The incidence of syphilis increased drastically among HIV infected MSM,3 resulting in a 45.5% prevalence of syphilis, compared to only 8.8% of HIV infected men who have sex with women.20 Furthermore, in a recent review of patients with secondary syphilis, 98% of patients were MSM, and almost a third of the population were co-infected with HIV.12
The increase in syphilis among MSM has been attributed to reduced condom use, more effective treatment of HIV and more recently, the use of PrEP, which have all resulted in riskier sexual behaviour.22
PrEP consists of a once-daily dose of tenofovir (TDF), with or without emtricitabine (FTC),6 but can also be taken as an 'event-driven' approach.23 PrEP has significantly reduced the incidence of HIV among MSM. However, by reducing the use of other primary prevention methods,6,23 it may increase the risk of other STIs.24 Therefore, it is imperative that MSM who start PrEP routinely (3 monthly) be tested for STIs.6, 25, 22 In fact, bacterial STIs in MSM have reached almost the same numbers as was seen before HIV infection appeared in the late 1970s.25 These infections can be addressed by the prophylactic use of doxycycline.26, 27 Although PrEP does not necessarily result in risk compensation and is usually not the only preventive method employed, a gradual decline in condom use has been noted,23 resulting in a subsequent increase in STIs.24-25 Therefore, individuals using PrEP should receive ongoing education and counselling to emphasize the importance of condom use and safe sexual behaviour to ensure that risk compensation does not occur.6 Pre-treatment, as well as continuous HIV screening, is essential during PrEP use, because undiagnosed infection may result in the development of drug resistance mutations, placing the cornerstone of ART at risk.27
Aetiopathogenesis and transmission
T. pallidum is an obligate human pathogen and spreads via infected blood, predominantly, through all means of sexual contact (vaginal, anal, and oral) when mucocutaneous lesions are present, but may also spread from mother to child.1,28
At the site of inoculation, T. pallidum replicates and enters the circulation, to disseminate systemically, resulting in three stages of infection: primary, secondary, and tertiary.1,28 Syphilis is only transmissible during the first few years of infection, with sexual transmission being rare after 2 to 3 years of infection.1 Syphilis infection is the result of unsafe sexual practices among both MSM and heterosexual individuals, suggesting either a lack of knowledge about transmission risks or that individuals have become complacent about the risk of acquiring STIs.5
HIV and syphilis are both acquired infections and often appear together as a co-infection. Besides the epidemiological relationship between HIV and syphilis mentioned earlier, there is also a plausible mechanistic relationship whereby these two infections increase the transmission of each other. Syphilis, because of its ulcerative nature which disrupts the barrier provided by the skin and mucous membranes, will increase the portal of entry and exit for HIV and therefore increase the chances of contracting HIV.2-3,29 In addition, there is an influx of immune cells at the site of a syphilitic lesion, especially CD4+ cells, which increases the target cells for HIV.3,30-31 T. pallidum itself increases the expression of HIV co-receptors on macrophages and other dendritic cells (CCR5) allowing efficient entry of HIV into target cells.32 Syphilis may also change the course of HIV disease by inducing a decrease in the CD4 cell count and an increase in the HIV viral load in HIV infected patients.33, 3
Screening for HIV and other STIs should be done at the time of syphilis diagnosis as well as 3 months later, while HIV-infected patients should undergo regular screening for syphilis.3,5,34-35 Although syphilis may be transmitted through oral intercourse,36-37 oral sex is generally considered a low-risk sexual activity for contracting HIV, and therefore usually not protected through barrier use.38 Yet, HIV can be transmitted through receptive oral intercourse,39-40 and should therefore be included in safer sex counselling.41 The risk of HIV transmission is increased when the oral mucosa is compromised by dental procedures, allergies, pharyngitis, chemotherapy or periodontal disease.42
Subsequently, and because high-risk sexual practices are normally not isolated,42 patients may choose to take PrEP against HIV, yet, unwittingly expose themselves to other STIs, such as syphilis.
Oral presentation
Acquired oral syphilis presents as primary, secondary and tertiary infections, most commonly among men (78,9%) in the 3rd and 4th decades of life, favouring in order of frequency the tongue, palate, lips, buccal mucosa, labial commissure and gingiva,8,43 similar to our patients.
The chancre is the hallmark feature of primary syphilis, appearing 2-3 weeks after exposure at the site of inoculation and healing within 2-10 weeks.1,37 The oral cavity is the most common extra-genital site to be affected,10-11 and then mostly affects the tongue, lips, and palate.44 The oral sites of involvement used to show a gender predilection according to the sexual acts performed,9 but sexual orientation has changed this arbitrary association.
Given its painless and self-limiting nature, chancres are often not reported by patients, or may even go unnoticed.1,45 Yet, with its deep, red, purple or brown base and the irregularly raised border, oral squamous cell carcinoma and traumatic ulcers should be excluded.9 Neither of our patients reported the original ulcer and did not even recall it upon questioning.
The secondary stage of syphilis is characterised by systemic symptoms such as pharyngitis, myalgia, arthralgia, lassitude, headache and generalised lymphadenopathy, but these are only variably present.10 It is mostly the secondary stage of syphilis that is associated with oral mucosal lesions7-8 where it may be seen in up to 30% of patients and it may even be the sole manifestation.9-12
The literature paints varied pictures of the oral lesions of secondary syphilis and the terminology is not uniformly applied. Essentially, there may either be a sensitive white plaque known as a mucous patch (note the dichotomy of terms by naming a plaque as a patch), which may ulcerate, or papillary to nodular lesions which resemble viral papillomas and have therefore been named 'condyloma lata'.9,45-46 The hyperplastic epithelium of condyloma lata may be mistaken for condyloma acuminatum and other papillomatous lesions,45 but these are much more frequently found on the skin than the oral mucosa.47
The traditional snail track pattern is created by the merging of adjacent mucous patches. When necrosis and sloughing of the epithelium from a mucous patch occurs, the underlying, red, connective tissue is exposed7,9 leaving a clean based, non-purulent ulcer as in our first patient.37 This appearance is sometimes known as a 'syphilitic rosette', is generally painless, well defined and commonly involves the tongue, gingiva, soft palate and lips.37 Some authors wish to separate the ulcerative aspect from the plaque-like aspect of mucous patches because these are so dissimilar.15
Mucous patches may also take on a 'leukoplakia-like' or 'leukokeratotic' appearance, which appears as a well-defined, corrugated, and non-homogenous plaque as seen in our second and third cases.7,12,48-49 Other, less frequently encountered oral lesions include plaques en prairie fauchée (shallow, painful, round to oval erosions/ depapillation on a background of a whitish, non-removable hyperkeratotic thickening of the posterior dorsal aspect of the tongue) as well as fausse perleche when a mucous patch creates a painful split papule a the angle of the mouth.11
Although lesions are not usually symptomatic, 8,12,37 when the tongue is involved, the patient may complain of an altered taste sensation as well as a burning sensation of the tongue.11
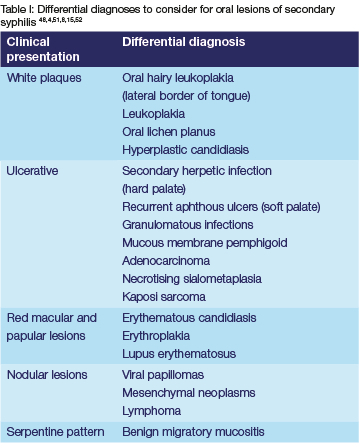
The oral mucosal lesions of secondary syphilis often mimic other diseases and have therefore become known as the "great imitator",1,7 from mimicking oral herpes infection8,50 to lymphoma.46 This varied clinical appearance makes a clinical diagnosis challenging, especially for clinicians who do not frequently encounter the disease. Differential diagnoses that may be considered for secondary lesions of oral syphilis will vary depending on the clinical characteristics (Table I);43 but syphilis should always be considered in the presence of non-specific oral ulcers and erosions, where there is a discrepancy between clinical and histological findings, and especially when the systemic symptoms and social history are suspicious.4,8 However, in a high-risk population of HIV+ MSM, the oral lesions are often conspicuous enough to make a diagnosis.4,15
It is not infrequent that patients will have been treated by different clinicians and by various means before a final diagnosis is established,10-12,45,53 especially when only isolated oral lesions are present.12 The diagnostic delay increases the risk of transmission from these highly contagious lesions because of the high number of spirochetes.4,28 However, even in the absence of an accurate diagnosis and successful therapy, the lesions will eventually resolve, committing the patient to a latent, non-infective state, until the tertiary stage is reached.53
The relative prevalence of the different oral lesions of secondary syphilis varies in the literature. Among an HIV+ population, mucous patches accounted for 85.5% of lesions.15 However, others found that ulcerative lesions are seen slightly more frequently10,12 and that when oral lesions were the sole presentation, 86% of lesions were erosive or ulcerative.12 Nodular (10%) and leukokeratotic lesions (5%) of the tongue are seen much less frequently.12
Tertiary syphilis is the most serious of all the stages of syphilis as it may involve the central nervous system and cardiovascular system.1 Oral features of tertiary syphilis include gumma, atrophic luetic glossitis and syphilitic leukoplakia.8 The opportunity to successfully diagnose and treat a patient during the secondary and last clinically evident phase of the disease, should therefore not be missed.
Some authors have suggested that the clinical manifestations of syphilis, and response to treatment, may differ in people living with HIV.1,15 Yet, it appears that for genital lesions, at least, there are only minor differences: the primary infection may be accompanied by multiple ulcers and the secondary infection with a greater likelihood of concomitant genital ulcers.54 When oral lesions were the sole manifestation of syphilis, the prevalence of individual lesions was similar between HIV-infected and uninfected individuals.12 Yet, it is not clear from this study if the distribution or number of lesions differed between these populations. Among our patients, the third patient who was HIV positive, presented with a wider variety and distribution of lesions, as well as a much longer duration of lesions. However, given his sexual history, repeated re-infection may have been responsible for his persistent disease.
Diagnosis and special investigations
As oral medicine clinicians, and given the relatively infrequent and varied presentation of oral syphilis, our first instinct had been to perform biopsies for histopathological analysis.10,12-13,43 This strategy allowed for the identification of the spirochete through immunohistochemistry (IHC) and/ or a silver stain (Warthin-Starry). Which in all instances still required serological confirmation. However, histology is not a primary requirement in the diagnoses of oral secondary syphilis lesions, as a more astute clinician can make the diagnoses through clinical findings, sexual history,5 and serology alone.15,43
T. pallidum, cannot be cultivated in the laboratory, therefore other laboratory investigations are necessary.14,55 These may be through the direct identification of the spirochete in tissue samples or, indirectly, by measuring the host's immune response to the organism or its components.14 Yet, there is no single test with adequate sensitivity or specificity that can diagnose syphilis with 100% accuracy during all stages of the disease.55
Darkfield microscopy (DFM) can demonstrate the presence of spirochetes in lesion exudate, which makes it most suitable for primary lesions,56-57 but it is hardly used for oral lesions due to the risk of nosocomial infection and confusion with other oral treponemes.9 A histology specimen allows for the direct identification of the tissue spirochetes. This is usually done through silver staining of specimens but this technique is difficult and non-specific due to the presence of non-treponemal spirochetes in the oral cavity, has low sensitivity, and is time-consuming.9,13-14 In fact, a recent review estimated silver staining to have a sensitivity as low as 0-41%.55 The oral cavity harbours a multitude of spirochetes, the most common of which is Treponema denticola, known for its association with periodontal disease.58 The oral, non-pathologic spirochetes, should only be demonstrated on the surface of the epithelium and should not be seen invading the mucosa,45 while T. pallidum can be seen in the superficial epithelium, next to blood vessels, macrophages and endothelial cells.9,13 Direct fluorescent anti-T,pallidum antibodies (DFA) and PCR can also be used, with immunohistochemistry (IHC) having improved upon the sensitivity and specificity of silver staining, locating the spirochete in the precise location in the lesion.14,55 PCR has the greatest sensitivity when the sample was obtained from the primary lesion exudate (75-95%).55 These direct detection methods were developed to address the shortcomings of serological assays, particularly for the diagnosis of primary disease.55
The histologic features of syphilis lesions are mostly nonspecific but one hallmark of primary and secondary syphilis is plasma cell infiltration.9-10,13 While this is a common occurrence in oral mucosal biopsies when this infiltrate extends more deeply and in a band-like distribution into the submucosa, syphilis should be suspected (Barrett 2004).13 The plasma cells may even infiltrate the walls of blood vessels and nerve bundles, consistent with plasma cell arteritis, peri-arteritis, and plasma cell neuritis, and should be considered pathognomonic of oral syphilitic lesions.10
Intra-epithelial micro-abscesses and unusual epithelial hyperplasia7,10 may also be seen. These histological features may be sufficient to direct the clinician in performing a serologic syphilis screen.10 If special stains are not performed due to stronger consideration being given to other clinical diagnoses, histopathology may not be sufficient to make a diagnosis.7
Besides the difficulty of distinguishing other oral spirochetes from T pallidum when direct detection methods like DFM and silver stains are used,14,57 three other pathogenic spirochetes can cause human treponemal diseases so that even serology is not entirely specific for syphilis.1 The bacterial family members cannot be distinguished from one another, either by morphological, chemical or immunological means.16
The indirect detection methods have excellent sensitivity during secondary and later stages of the disease (>95%) but are very unreliable during primary infection,9,59 with false-negative results obtained in up to 46% of patients.55
Serologic tests are divided into treponemal and non-treponemal tests. Non-treponemal tests are non-specific and are often used for screening purposes, these include the Venereal Disease Research Laboratory (VDRL) and more commonly, the Rapid Plasma Reagin (RPR) tests which detect IgG and IgM antibodies against synthetic cardiolipin, cholesterol, and lecithin antigen complexes.14,28,60 But because these antibodies are not specific for syphilis, the results need to be confirmed with a treponemal test.59 Non-treponemal test reactivity regresses after successful treatment of syphilis,55,60 although false positives may sometimes be seen after successful treatment.55 It is less likely for HIV naïve patients to have false-negative titers, while HIV infected patients are more likely to have false-positive RPR results34 - yet, the impact of HIV on serologic titers probably has minimal clinical significance.3 Serologic tests remain accurate and reliable for diagnosing and monitoring the response to treatment, in patients with HIV.34 Refer to Figure 4 for a flow diagram of diagnostic procedures that are followed to confirm a syphilis diagnosis after performing a biopsy.
Treponemal tests include T. pallidum particle agglutination (TPPA), fluorescent treponemal antibody absorption test (FTA-ABS), and T. pallidum hemagglutination assay (TPHA) which detects IgG and IgM antibodies against T. pallidum or their proteins.14 Treponemal tests become reactive shortly after a new infection and remain reactive regardless of treatment.9,60
While traditionally, the non-treponemal tests have been used for screening purposes, recently, with the increasing availability of immunoassays, a reverse algorithm has been suggested whereby screening is performed by treponemal immunoassay and then confirmed by non-treponemal or treponemal serology. The benefit is that immunoassays are more sensitive than non-treponemal tests during secondary and tertiary syphilis and eliminate the risk of biological false positives of anti-cardiolipin antibodies from other diseases.59
Treponema, point-of-care tests for syphilis are now available and recommended in resource-limited settings, providing results in 15 - 20 minutes, and are more cost-effective in screening and treating syphilis than laboratory-based methods, such as RPR. However, as with other treponemal tests, it is not possible to distinguish between current and past infections. Dual syphilis and HIV infection point-of-care tests can be used in populations at high risk of dual infection, hopefully paving the way towards home-based self-testing.25
Treatment and measuring response to treatment
The treatment of syphilis has evolved from the earliest use of purgatives to mercury, and finally, since its introduction in the 1940s, penicillin, which continues to be the treatment of choice.16
The treatment of syphilis depends on the stage of the disease,62 either early or late (including unknown) stage syphilis (Table II). Cefixime, a 3rd generation cephalosporin, has shown promising results in an HIV+ population when given a dose of 400mg twice daily for 10 days during early-stage disease.63 But evolving resistance to the macrolide antibiotics, makes this a dubious choice among penicillin-allergic patients.64 The extended protocols of late-stage syphilis are suggested due to the probable slower replication rate of T pallidum.34
Clinical and serological evaluation should be performed at 6 and 12 months after treatment, or more frequently (3 monthly) if re-infection is a concern, especially among patients with HIV.34 Re-infection is particularly likely if clinical signs and symptoms persist or when there is a fourfold increase in non-treponemal test titre.34 Successful treatment should result in a 4 fold decline in RPR and VDLR titres,34-35 failure of which will require additional clinical and serological follow-up and screening for HIV infection.34
Initially, it was believed that the dose and duration of treatment of syphilis should be adjusted among HIV-infected patients.5 Some report that serological failure is more likely, and that serological success may take twice as long to reach within an HIV-infected population65-66 but that it does not affect the cure of lesions.66
Yet, the CDC recommends that the same treatment of early syphilis be employed in both HIV-infected and uninfected populations,34 admittedly, even though some feel that the evidence for this strategy is not optimal nor found in objective data.67-68
Neither increasing the single dose of BPG, to 3 weekly doses nor the addition of a 10-day course of amoxicillin with probenecid, improves serological outcomes beyond what is achieved with a single dose35,66,69-70 regardless of the CD4 count.35 Although a faster serological response has been reported in patients with higher pretreatment titres and CD4 counts.70 Serological failure may be attributed to re-infection,35 necessitating serological monitoring and retreatment if a failure occurs.67
Partner notification or contact tracing is essential for the management of syphilis.5,71 Health care providers should routinely obtain sexual histories from patients to address risk reduction and offer to counsel as needed.34,72 Clinicians should continue to encourage safe sexual practices and the use of condoms by those having sex with unknown partners.5
Case-control management is an integral part of an STI control strategy because early treatment can disrupt onward transmission if treatment and partner notification are successful. Most patients are willing to self-notify partners of STIs.25 However, the sex partners of persons with syphilis who are deemed at risk of infection, especially within the first year of diagnosis, may confidentially be notified, and pre-emptively treated if deemed necessary.34
CONCLUSION
The three presented cases not only highlight the diversity of oral lesions associated with syphilis, but also the diverse male population that it affects. From the naïve patient who unwittingly puts himself at risk by having sex with men, to the HIV naïve patient who conscientiously uses PrEP, but inadvertently exposes himself to other STIs, and the HIV-infected patient who knowingly participates in high-risk sexual behaviours. This historic disease continues to burden men with high-risk sexual behaviours. Despite the earlier decline in syphilis numbers that the risk of HIV has caused, the successful prevention and management of HIV have resulted in a behavioural disassociation, even though the risk of STIs remains. The use of barrier protection remains essential in the prevention of STIs.
REFERENCES
1. Hook EW, 3rd. Syphilis. Lancet. 2017; 389(10078):1550-7. doi:10.1016/s0140-6736(16)32411-4 [ Links ]
2. Greenblatt RM, Lukehart SA, Plummer FA, Quinn TC, Critchlow CW, Ashley RL, et al., Genital ulceration as a risk factor for human immunodeficiency virus infection. Aids. 1988; 2(1):47-50. doi:10.1097/00002030-198802000-00008 [ Links ]
3. Farhi D, Dupin N. Management of syphilis in the hiv-infected patient: Facts and controversies. Clin Dermatol. 2010; 28(5):539-45. doi:10.1016/j.clindermatol.2010.03.012 [ Links ]
4. Hertel M, Matter D, Schmidt-Westhausen AM, Bornstein MM. Oral syphilis: A series of 5 cases. J Oral Maxillofac Surg. 2014; 72(2):338-45. doi:10.1016/j.joms.2013.07.015 [ Links ]
5. Doherty L, Fenton KA, Jones J, Paine TC, Higgins SP, Williams D, et al., Syphilis: Old problem, new strategy. Bmj. 2002; 325(7356):153-6. doi:10.1136/ bmj.325.7356.153 [ Links ]
6. Mayer KH, Ramjee G. The current status of the use of oral medication to prevent hiv transmission. Curr Opin HIV AIDS. 2015; 10(4):226-32. doi:10.1097/coh.0000000000000170 [ Links ]
7. Compilato D, Amato S, Campisi G. Resurgence of syphilis: A diagnosis based on unusual oral mucosa lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108(3):e45-9. doi:10.1016/j.tripleo.2009.05.013 [ Links ]
8. Schuch LF, da Silva KD, de Arruda JAA, Etges A, Gomes APN, Mesquita RA, et al., Forty cases of acquired oral syphilis and a review of the literature. Int J Oral Maxillofac Surg. 2019; 48(5):635-43. doi:10.1016/j.ijom.2018.10.023 [ Links ]
9. Leão JC, Gueiros LA, Porter SR. Oral manifestations of syphilis. Clinics (Sao Paulo). 2006; 61(2):161-6. doi:10.1590/s1807-59322006000200012 [ Links ]
10. Czerninski R, Pikovski A, Meir K, Casap N, Moses AE, Maly A. Oral syphilis lesions-- a diagnostic approach and histologic characteristics of secondary stage. Quintessence Int. 2011; 42(10):883-9. [ Links ]
11. Eyer-Silva WA, Freire MAL, Horta-Araujo CA, Almeida Rosa da Silva G, Francisco da Cunha Pinto J, Raphael de Almeida Ferry F. Secondary syphilis presenting as glossodynia, plaques en prairie fauchée, and a split papule at the oral commissure: Case report and review. Case Rep Med. 2017; 2017:1980798. doi:10.1155/2017/1980798 [ Links ]
12. Lampros A, Seta V, Gerhardt P, Isnard C, Husson C, Dupin N. Oral forms of secondary syphilis: An illustration of the pitfalls set by the great imitator. J Am Acad Dermatol. 2021; 84(2):348-53. doi:10.1016/j.jaad.2020.04.089 [ Links ]
13. Barrett AW, Villarroel Dorrego M, Hodgson TA, Porter SR, Hopper C, Argiriadou AS, et al., The histopathology of syphilis of the oral mucosa. J Oral Pathol Med. 2004; 33(5):286-91. doi:10.1111/j.0904-2512.2004.00099.x [ Links ]
14. Buffet M, Grange PA, Gerhardt P, Carlotti A, Calvez V, Bianchi A, et al., Diagnosing treponema pallidum in secondary syphilis by pcr and immunohistochemistry. J Invest Dermatol. 2007; 127(10):2345-50. doi:10.1038/sj.jid.5700888 [ Links ]
15. Ramírez-Amador V, Anaya-Saavedra G, Crabtree-Ramírez B, Esquivel-Pedraza L, Saeb-Lima M, Sierra-Madero J. Clinical spectrum of oral secondary syphilis in hiv-infected patients. J Sex Transm Dis. 2013; 2013:892427. doi:10.1155/2013/892427 [ Links ]
16. Tampa M, Sarbu I, Matei C, Benea V, Georgescu SR. Brief history of syphilis. Journal of medicine and life. 2014; 7:4-10. [ Links ]
17. Hoque M, Hoque ME, van Hal G, Buckus S. Prevalence, incidence and seroconversion of hiv and syphilis infections among pregnant women of south africa. Southern African journal of infectious diseases. 2021; 36(1):296-. doi:10.4102/sajid.v36i1.296 [ Links ]
18. Kularatne RS, Niit R, Rowley J, Kufa-Chakezha T, Peters RPH, Taylor MM, et al., Adult gonorrhea, chlamydia and syphilis prevalence, incidence, treatment and syndromic case reporting in south africa: Estimates using the spectrum-sti model, 1990-2017. PLoS One. 2018; 13(10):e0205863. doi:10.1371/journal. pone.0205863 [ Links ]
19. SA S. 2020 mid-year population estimates. 2020. South African Census, Statistics South Africa. [ Links ]
20. Prevention CfDCa. Sexually transmitted disease surveillance 2017. Atlanta: US Department of Health and Human Services, 2018. [ Links ]
21. Abara WE, Hess KL, Neblett Fanfair R, Bernstein KT, Paz-Bailey G. Syphilis trends among men who have sex with men in the united states and western europe: A systematic review of trend studies published between 2004 and 2015. PLoS One. 2016; 11(7):e0159309. doi:10.1371/journal.pone.0159309 [ Links ]
22. Spiteri G, Unemo M, Márdh O, Amato-Gauci AJ. The resurgence of syphilis in high-income countries in the 2000s: A focus on europe. Epidemiol Infect. 2019; 147:e143. doi:10.1017/s0950268819000281 [ Links ]
23. Vuylsteke B, Reyniers T, De Baetselier I, Nöstlinger C, Crucitti T, Buyze J, et al., Daily and event-driven pre-exposure prophylaxis for men who have sex with men in belgium: Results of a prospective cohort measuring adherence, sexual behaviour and sti incidence. Journal of the International AIDS Society. 2019; 22(10):e25407-e. doi:10.1002/jia2.25407 [ Links ]
24. Traeger MW, Cornelisse VJ, Asselin J, Price B, Roth NJ, Willcox J, et al., Association of hiv preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of hiv infection. Jama. 2019; 321(14):1380-90. doi:10.1001/jama.2019.2947 [ Links ]
25. Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, et al., Sexually transmitted infections: Challenges ahead. Lancet Infect Dis. 2017; 17(8):e235-e79. doi:10.1016/s1473-3099(17)30310-9 [ Links ]
26. Bolan RK, Beymer MR, Weiss RE, Flynn RP, Leibowitz AA, Klausner JD. Doxycycline prophylaxis to reduce incident syphilis among hiv-infected men who have sex with men who continue to engage in high-risk sex: A randomized, controlled pilot study. Sex Transm Dis. 2015; 42(2):98-103. doi:10.1097/olq.0000000000000216 [ Links ]
27. Molina JM, Charreau I, Chidiac C, Pialoux G, Cua E, Delaugerre C, et al, Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: An open-label randomised substudy of the anrs ipergay trial. Lancet Infect Dis. 2018; 18(3):308-17. doi:10.1016/s1473-3099(17)30725-9 [ Links ]
28. Soares AB GH, Jorge MA, Barraviera SRCS. Oral manifestations of syphilis: A review. J. Venom. Anim. Toxins incl. Trop. Dis. 2004; 10(1):2-9. [ Links ]
29. Wu MY Gong HZ, Hu KR, Zheng H-y, Wan X, Li J. Effect of syphilis infection on hiv acquisition: A systematic review and meta-analysis. Sexually Transmitted Infections. 2021; 97(7):525-33. doi:10.1136/sextrans-2020-054706 [ Links ]
30. Lafond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev. 2006; 19(1):29-49. doi:10.1128/cmr.19.1.29-49.2006 [ Links ]
31. Radolf JD, Deka RK, Anand A, Smajs D, Norgard MV Yang XF. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nat Rev Microbiol. 2016; 14(12):744-59. doi:10.1038/nrmicro.2016.141 [ Links ]
32. Salazar JC, Cruz AR, Pope CD, Valderrama L, Trujillo R, Saravia NG, et al., Treponema pallidum elicits innate and adaptive cellular immune responses in skin and blood during secondary syphilis: A flow-cytometric analysis. J Infect Dis. 2007; 195(6):879-87. doi:10.1086/511822 [ Links ]
33. Kofoed K, Gerstoft J, Mathiesen LR, Benfield T. Syphilis and human immunodeficiency virus (hiv)-1 coinfection: Influence on cd4 t-cell count, hiv-1 viral load, and treatment response. Sexually Transmitted Diseases. 2006; 33(3):143-8. doi:10.1097/01.olq.0000187262.56820.c0 [ Links ]
34. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015; 64(Rr-03):1-137. [ Links ]
35. Costa-Silva M, Azevedo C, Azevedo F, Lisboa C. Early syphilis treatment in hiv-infected patients: Single dose vs. Three doses of benzathine penicillin g. J Eur Acad Dermatol Venereol. 2016; 30(10):1805-9. doi:10.1111/jdv.13766 [ Links ]
36. Edwards S, Carne C. Oral sex and transmission of non-viral stis. Sex Transm Infect. 1998; 74(2):95-100. doi:10.1136/sti.74.2.95 [ Links ]
37. de Andrade RS, de Freitas EM, Rocha BA, Gusmão ES, Filho MR, Júnior HM. Oral findings in secondary syphilis. Med Oral Patol Oral Cir Bucal. 2018; 23(2):e138-e43. doi:10.4317/medoral.22196 [ Links ]
38. Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary hiv infection. Ann Intern Med. 1996; 125(4):257-64. doi:10.7326/0003-4819-125-4-199608150-00001 [ Links ]
39. Rothenberg RB, Scarlett M, del Rio C, Reznik D, O'Daniels C. Oral transmission of hiv. Aids. 1998; 12(16):2095-105. doi:10.1097/00002030-199816000-00004 [ Links ]
40. Gilbart VL, Evans BG, Dougan S. Hiv transmission among men who have sex with men through oral sex. Sex Transm Infect. 2004; 80(4):324. doi:10.1136/sti.2004.009217 [ Links ]
41. Cohen MS, Shugars DC, Fiscus SA. Limits on oral transmission of hiv-1. Lancet. 2000; 356(9226):272. doi:10.1016/s0140-6736(00)02500-9 [ Links ]
42. Wood LF, Chahroudi A, Chen HL, Jaspan HB, Sodora DL. The oral mucosa immune environment and oral transmission of hiv/siv. Immunol Rev. 2013; 254(1):34-53. doi:10.1111/imr.12078 [ Links ]
43. Leuci S, Martina S, Adamo D, Ruoppo E, Santarelli A, Sorrentino R, et aí., Oral syphilis: A retrospective analysis of 12 cases and a review of the literature. Oral Dis. 2013; 19(8):738-46. doi:10.1111/odi.12058 [ Links ]
44. Zhou X, Wu MZ, Jiang TT, Chen XS. Oral manifestations of early syphilis in adults: A systematic review of case reports and series. Sex Transm Dis. 2021; 48(12):e209-e14. doi:10.1097/olq.0000000000001538 [ Links ]
45. Carbone PN, Capra GG, Nelson BL. Oral secondary syphilis. Head Neck Pathol. 2016; 10(2):206-8. doi:10.1007/s12105-015-0623-3 [ Links ]
46. Dai T, Song NJ. An unusual case of oral condyloma lata. Int J Infect Dis. 2021; 105:349-50. doi:10.1016/j.ijid.2021.02.051 [ Links ]
47. Pourang A, Fung MA, Tartar D, Brassard A. Condyloma lata in secondary syphilis. JAAD case reports. 2021; 10:18-21. doi:10.1016/j.jdcr.2021.01.025 [ Links ]
48. Aquilina C, Viraben R, Denis P. Secondary syphilis simulating oral hairy leukoplakia. J Am Acad Dermatol. 2003; 49(4):749-51. doi:10.1067/s0190-9622(03)00484-5 [ Links ]
49. de Paulo LF, Servato JP, Oliveira MT, Durighetto AF, Jr., Zanetta-Barbosa D. Oral manifestations of secondary syphilis. Int J Infect Dis. 2015; 35:40-2. doi:10.1016/j.ijid.2015.04.007 [ Links ]
50. Araujo JP, Jaguar GC, Alves FA. Syphilis related to atypical oral lesions affecting an elderly man. A case report. Gerodontology. 2015; 32(1):73-5. doi:10.1111/ger.12047 [ Links ]
51. Kelner N, Rabelo GD, da Cruz Perez DE, Assunção JN, Jr., Witzel AL, Migliari DA, et al., Analysis of nonspecific oral mucosal and dermal lesions suggestive of syphilis: A report of 6 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 117(1):1-7. doi:10.1016/j.oooo.2012.04.028 [ Links ]
52. Leone C, Sugaya N, Migliari D. An intriguing case of ectopic benign migratory glossitis resembling secondary syphilis: A case report. Case Rep Dermatol. 2020; 12(3):262-5. doi:10.1159/000510776 [ Links ]
53. Dybeck Udd S, Lund B. Oral syphilis: A reemerging infection prompting clinicians' alertness. Case Rep Dent. 2016; 2016:6295920. doi:10.1155/2016/6295920 [ Links ]
54. Rompalo AM, Joesoef MR, O'Donnell JA, Augenbraun M, Brady W, Radolf JD, et al., Clinical manifestations of early syphilisbyhivstatus and gendenResultsofthe syphilis and hiv study. SexTransmyis.2001;28(3):15SR5.aoi:1SJ097Sa0007435-200103000-00007 [ Links ]
55. Theel ES, Katz SS, Pillay A. Molecelar anS ditect sletactionSestsfor treponensa pallidum subspecies pallidum: A reviewoft helitxrature, 1964-2017. Clin Infect Dis. 2020; 71(Suppl 1):S4-s12. doi:10.1093/cid/ciaa176 [ Links ]
56. Hook EW, 3rd, Roddy RE, Lukehart SA, Hom J, Holmes KK, Tam MR. Detection of treponema pallidum in lesion exudate with a pathogen-specific monoclonal antibody. Journal of clinical microbiology 1985; 22(2):241-4. doi:10.1128/ jcm.22.2.241-244.1985 [ Links ]
57. Pierce EF, Katz KA. Darkfield microscopy for point-of-care syphilis diagnosis.MLO Med Lab Obs. 2011; 43(1):30-1 [ Links ]
58. Yousefi L, Leylabadlo HE, Pourlak T, Eslami H, Taghizadeh S, Ganbarov K, et al., Oral spirochetes: Pathogenic mechanisms in periodontal disease. Microb Pathog. 2020; 144:104193. doi:10.1016/j.micpath.2020.104193 [ Links ]
59. Loeffelholz MJ, Binnicker MJ. It is time to use treponema-specific antibody screening tests for diagnosis of syphilis. J Clin Microbiol. 2012; 50(1):2-6. doi:10.1128/jcm.06347-11 [ Links ]
60. Marra CM, Ghanem KG. Centers for disease control and prevention syphilis summit: Difficult clinical and patient management issues. Sex Transm Dis. 2018; 45(9S Suppl 1):S10-s2. doi:10.1097/olq.0000000000000851 [ Links ]
61. Who guidelines approved by the guidelines review committee. Who guidelines for the treatment of treponema pallidum (syphilis). Geneva: World Health Organization © World Health Organization 2016.; 2016. [ Links ]
62. Thakrar P, Aclimandos W, Goldmeier D, Setterfield JF. Oral ulcers as a presentation of secondary syphilis. Clin Exp Dermatol. 2018; 43(8):868-75. doi:10.1111/ced.13640 [ Links ]
63. Stafylis C, Keith K, Mehta S, Tellalian D, Burian P, Millner C, et al., Clinical efficacy of cefixime for the treatment of early syphilis. Clin Infect Dis. 2021; 73(5):907-10. doi:10.1093/cid/ciab187 [ Links ]
64. Beale MA, Marks M, Sahi SK, Tantalo LC, Nori AV, French P, et al., Genomic epidemiology of syphilis reveals independent emergence of macrolide resistance across multiple circulating lineages. Nat Commun. 2019; 10(1):3255. doi:10.1038/s41467-019-11216-7 [ Links ]
65. Ghanem KG, Erbelding EJ, Wiener ZS, Rompalo AM. Serological response to syphilis treatment in hiv-positive and hiv-negative patients attending sexually transmitted diseases clinics. Sexually transmitted infections.2007; 83(2):97-101. doi:10.1136/sti.2006.021402 [ Links ]
66. Rolfs RT, Joesoef MR, Hendershot EF, Rompalo AM, Augenbraun MH, Chiu M, et al., A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The syphilis and hiv study group. N Engl J Med. 1997; 337(5):307-14. doi:10.1056/nejm199707313370504 [ Links ]
67. White AC, Jr. Treatment of early syphilis in hiv: What do we really know? Clinical Infectious Diseases. 2016; 64(6):765-6. doi:10.1093/cid/ciw866 [ Links ]
68. Blank LJ, Rompalo AM, Erbelding EJ, Zenilman JM, Ghanem KG. Treatment of syphilis in hiv-infected subjects: A systematic review of the literature. Sex Transm Infect. 2011; 87(1):9-16. doi:10.1136/sti.2010.043893 [ Links ]
69. Andrade R, Rodriguez-Barradas MC, Yasukawa K, Villarreal E, Ross M, Serpa JA. Single dose versus 3 doses of intramuscular benzathine penicillin for early syphilis in hiv: A randomized clinical trial. Clin Infect Dis. 2017; 64(6):759-64. doi:10.1093/cid/ciw862 [ Links ]
70. Ganesan A, Mesner O, Okulicz JF, O'Bryan T, Deiss RG, Lalani T, et al., A single dose of benzathine penicillin g is as effective as multiple doses of benzathine penicillin g for the treatment of hiv-infected persons with early syphilis. Clin Infect Dis. 2015; 60(4):653-60. doi:10.1093/cid/ciu888 [ Links ]
71. Çakmak SK, Tamer E, Karadag AS, Waugh M. Syphilis: A great imitator. Clin Dermatol. 2019; 37(3):182-91. doi:10.1016/j.clindermatol.2019.01.007 [ Links ]
72. Achterbergh RCA, Hoornenborg E, Boyd A, Coyer L, Meuzelaar SJA, Hogewoning AA, et al., Changes in mental health and drug use among men who have sex with men using daily and event-driven pre-exposure prophylaxis: Results from a prospective demonstration project in amsterdam, the netherlands. EClinicalMedicine. 2020; 26:100505. doi:10.1016/j.eclinm.2020.100505 [ Links ]
 Correspondence:
Correspondence:
Dr J Fourie
Tel: 082 460 8368, 012 319 2312; Email: Jeanine.fourie@up.ac.za
Author contribution:
Dr J Fourie: abstract, introduction, epidemiology, concept design and execution of the research paper (60%).
Dr L Mukucha: aetiopathogenesis, oral presentation, case 2 and 3 (20%).
Dr L Masuka: diagnosis and special investigations, treatment and measuring response to treatment, social strategies, case 1 (20%).
Acknowledgement: No funding was received. No financial interests to declare. No conflict of interests.














