Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.77 n.10 Johannesburg Nov. 2022
http://dx.doi.org/10.17159/2519-0105/2022/v77no10a5
CASE REPORT
Dentinogenic Ghost Cell Tumour, a rare case of an African young female patient: Review of literature and report of a case
M LamolaI; MMJ MasilelaII; TO AdedojaIII
IBDS. Limpopo Department of Health, WF Knobel Hospital, General Dentistry. South Africa. Orcid: 0000-0003-2136-990X
IIBDS, PG Dipl Dent (Oral Pathology), MDent (Oral Pathology). Sefako Makgatho Health Sciences University, Department of Oral and Maxillofacial Surgery, Pietersburg Academic Hospital, Oral Pathology Unit. South Africa. Orcid: 0000-0001-9702-5797
IIIBDS, FDS RCSEd, FWACS, Cert. Implantology. Mankweng Hospital, General Dentistry. South Africa. Orcid: 0000-0003-2441-5430
ABSTRACT
Dentinogenic ghost cell tumour (DGCT) is a very rare aggressive benign odontogenic tumour with high recurrence rate and a potential to transform into malignancy. It can render facial disfigurement. The tumour is most frequently encountered in males than females with a ratio of 2:1. The peak incidence is in patients aged 40 - 60 years and the posterior mandible is slightly more affected than the maxilla. Segmental resection is a recommended surgical treatment and long-term postsurgical follow up is essential. This paper discusses a case of a 20-year-old African female patient who was diagnosed with a DGCT.
Keywords: Dentinogenic ghost cell tumour, Ghost cells, Maxilla
INTRODUCTION
Dentinogenic ghost cell tumour (DGCT), is described as a benign but locally infiltrating odontogenic neoplasm of mixed epithelial and ectomesenchymal origin. It is the rarest of the ghost cell lesions accounting for <3% of all cases. As at 2017, only 47 cases have been reported in the literature with more than 50% of them occurring in Asian patients1. In 1962, the tumour was regarded as a solid variant of Calcifying Odontogenic Cyst (COC) 2 DGCT is, by virtue of histological presentation, characterized by ameloblastomatous odontogenic epithelium with aberrant keratinization in the form of ghost cells and is in association with dentinoid or osteodentin material. It can present either as a central (intraosseous) or peripheral (extraosseous) lesion. The tumour shows male predilection with a male to female ratio of 2:1. The peak incidence of occurrence is 40 -60 years with an age range of 11-79 years1. The aim of this article is to report a case of DGCT that was diagnosed in a relatively young African female patient and at an infrequent site.
CASE REPORT
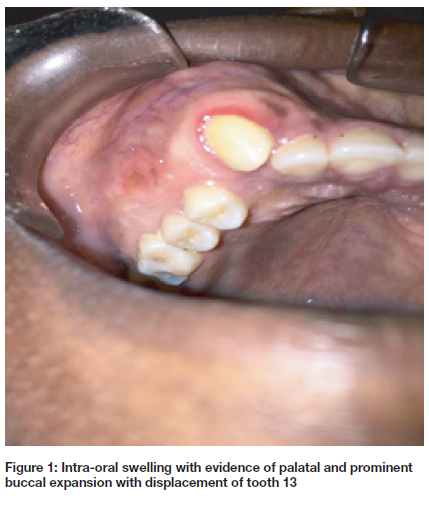
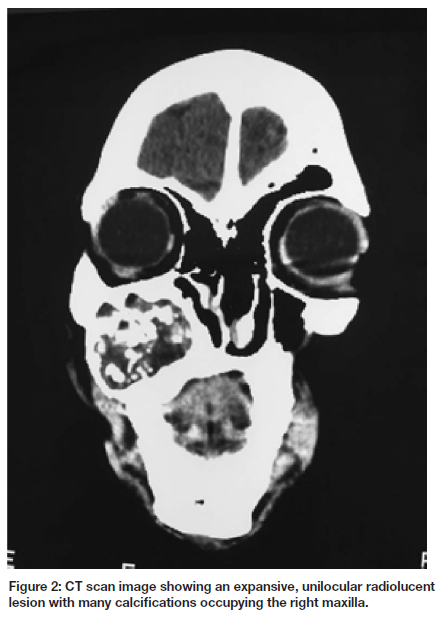
We describe a case of a 20 - year - old African female who presented with a painless progressive swelling of the right maxilla of approximately 2 years duration. The swelling was associated with buccal and moderate palatal expansion and it extended from tooth 12 to the right maxillary tuberosity (Fig. 1). The associated teeth were not significantly mobile. CT scan revealed a heterogenous, expansive, unilocular radiolucent lesion with many calcifications occupying the right maxilla (Fig. 2). The mass measured 44.9 x 41 x 35mm3.
The histopathological examination of the specimen revealed a solid odontogenic tumour characterized by numerous scattered islands of varying sizes of which some had undergone cystic degeneration. The cystic spaces were lined by ameloblastomatous epithelium that contains numerous ghost cells. The ghost cells were in greater proportion compared to the hyperchromatic epithelial cells. There was evidence of dentinoid material and increased basement membrane production within some islands and the stroma was fibrous (Fig. 3). The epithelium and ghost cells stained diffusely and strongly positive for AE1/AE3 and most ghost cells stained strongly positive for β-catenin (Fig. 4). The proliferative index as detected by Ki-67 was virtually 0%. The features were consistent with those of a Dentinogenic ghost cell tumour.
DISCUSSION
DGCT is an uncommon odontogenic tumour that was previously classified as a solid variant of COC2 however currently accepted as an entity. A comprehensive review was undertaken where 75 cases of DGCT were identified in a total of 48 articles that were published until July 2018. The articles demonstrated important details necessary to confirm the diagnosis of DGCT; however only 57 cases were analysed as a result of exclusion of 4 articles that lacked information on age and gender of the patients.3
In an analysis of 215 COCs, it was found that COC represents 1-2% of all odontogenic tumours and of these only 2-14% were solid tumours, considered to be DGCT. 4,5 DGCT is the rarest of the ghost cell lesions and it accounts for approximately <3% of all ghost cell lesions and is also considered to be one of the rare odontogenic tumours.1 This is supported by the findings reported in several studies worldwide. Comprehensive retrospective studies on odontogenic tumours were performed in various countries and in South Africa and Africa, there were no cases of DGCT reported.6'7,8,9 The largest study performed in Chinese population revealed nine (9) DGCT out of 1642 cases of odontogenic tumours10 and one (1) case out of 250 odontogenic tumour cases was reported in Indian.11 The tumour is found to be common in Asians.1,3 To the best of our knowledge, this is the first case to be reported in South Africa.
DGCT is believed to be derived from odontogenic epithelial cell remnants within the gingiva and /or the jaws12. The etiology is still unknown, however mutation in β-catenin was implicated to play a vital role in the tumorigenesis of DGCT by a process of inappropriate differentiation coordinated by the Wingless integrated (Wnt) pathway.4,13 Others reported that mutations in CTNNB1 gene, which encodes β-catenin, are the major drivers of COCs, and are associated with the formation of ghost cells,however, the status of CTNNB1 mutations in DGCT and the malignancy which DGCT may transform into, known as Ghost cell odontogenic carcinoma, remains largely unknown.14 Our present case revealed presence of β-catenin in association with the ghost cells which supports the statement mentioned by Yukimori et al. This could suggest that there is mutation of CTNNB1 in DGCT.
DGCT is predominantly seen in middle aged groups. Intraosseous DGCT tends to have a patient age range of 12-75 years with a mean of 40 years,3,15,16 whereas extraosseous lesions usually occur in the sixth decades of life, with an age range of 10-92 years.17 Few cases were reported in the second decade of life 18,19 and the youngest reported case was of a patient who was 2 - day old.20 These tumours have strong male predilection.3,10 Of the 7 individual cases reported, four were in males 5,18,19,20 and three in females 21-23. All the females were in the fifth and sixth decade of life. The recently published cases of DGCT in younger patients in the second decade of life were all in males.18,19 This makes our case peculiar because DGCT is encountered in a young female who is in the third decade of life.
The frequent site of occurrence is the posterior mandible and maxilla with slight predilection for mandible.1,3 Of the three cases reported in the literature post Pinheiro et al's bibliometric review of DGCT, two were in the mandible 18,21 and one in maxilla 19. While maxilla is regarded as an infrequent site of occurrence; we report a case that was encountered in the maxilla.
DGCT varies in size from 1 cm to more than 10 cm in diameter and is usually asymptomatic. The clinical signs of intraosseous DGCT variants may include expansion of the jaw, clinically visible swelling and obliteration of the maxillary sinus or infiltration of the soft tissues. Swelling can be painful or painless and occasionally accompanied by pus discharge, tooth displacement or mobility.1,3,22,24,25 In the present case, the patient presented with a painless maxillary lesion with a significant expansion extending from tooth 13 to the maxillary tuberosity causing gross facial disfigurement.
Radiographic findings of the present case were consistent with the previously reported cases of DGCT in which it appeared as a unilocular mixed radiolucent-radiopaque lesion with root resorption of an associated premolar. However, DGCT presenting as a completely radiolucent lesion has been reported.1 The appearance of either radiolucency or mixed radiolucent-radiopacity of the lesion directly depends on the degree of calcification.22 Majority of cases are unilocular but multilocular lesions may be observed 23 These tumours are typically well-defined, often expansile and may result in resorption and divergence of roots of adjacent teeth.24 Radiologic differential diagnosis of the current case (with mixed radiolucent-radiopacity) includes COC, Adenomatoid Odontogenic Tumour (AOT), Calcifying Odontogenic Epithelial Tumour (CEOT) and Ossifying Fibroma. The final diagnosis of the differentials can only be established on histology. Histopathologically, DGCT is characterized by sheets and islands of basaloid hyperchromatic cells associated with ghost cells and dentinoid/ osteodentin-like material. When the ghost cells come in contact with the connective tissue, they elicit an inflammatory foreign body reaction with multinucleated giant cells. Microcysts may be identified within the sheet of cells, however, there may be larger cystic spaces lined by ameloblastomatous epithelium. Mitotic figures are rare.1
The ghost cells are described as swollen, ellipsoidal keratinized epithelial cells characterized by loss of nuclei and preservation of basic cellular outlines. There are different theories on the origin of these ghost cells such as: transformation of epithelial cells, metaplastic transformation of odontogenic epithelium, squamous metaplasia with secondary calcification due to ischemia, degeneration of epithelial cells or as a result of apoptotic process.18,25
The presence of ghost cells alone is however not pathognomic of DGCT, since they can also be identified in other neoplasms such as Odontomas, Ameloblastomas and Ameloblastic fibroodontomas. The proportion of ghost cells of > 1-2% and the presence of dentinoid material are important features in establishing the diagnosis of DGCT.1
Several theories have also been documented regarding the presence of osteodentin or dentinoid material. Some authors considered this to represent an inflammatory response of the body towards masses of ghost cells.26 Others believe that the masses of "ghost cells" induce granulation tissue to lay down juxtraepithelial osteoid which may calcify.27 Meanwhile others were of the opinion that dentinoid represents a metaplastic change in the connective tissue without the participation of granulation tissue.28
Some authors considered dentinoid to be of mesodermal origin based on the finding that it is usually not found in the luminal proliferations unless there is a disintegration of the basement membrane with outgrowth of connective tissue between the epithelial ghost cells.14,18
Treatment of intraosseous lesions requires segmental resection as compared to conservative surgery (i.e. enucleation, curettage or simple excision). Segmental resection was found to yield less recurrence rates (33%) compared to conservative surgery which yielded high recurrence rates (73%).1 Long-term follow-up is recommended as recurrences have been reported not only following local excision/enucleation but also 1-5 years after segmental mandibular resection and partial maxillectomy.12,15 Extraosseous lesions require simple excision and recurrences are rare.15,29
This case was treated with an excision and peripheral osteotomy of more than 10mm normal bone which is judged to be adequate for a benign infiltrating tumour like DGCT. The need for long term follow up was emphasized to the patient.
In terms of prognosis, central DGCTs are aggressive neoplasms that show locally invasive behaviour and recurrence rates of up to 71%.30 Compared to central lesions, peripheral DGCTs are less aggressive in behaviour and are not thought to recur.5, 29
Malignant transformation of DGCT into Ghost cell odontogenic carcinoma (GCOC) has also been reported.18 It is important to distinguish the DGCT from GCOC because both lesions may exhibit ghost cells and infiltrative growth; however, gCoC displays nuclear pleomorphism and hyperchromasia, mitotic activity and necrosis microscopically. Expression of p53 and a high proliferative index favours the diagnosis of GCOC as it is known that the expression of these markers increases upon transformation. There were no malignant cytomorphological changes and there was virtually no increase in proliferation in our case as detected by a proliferative marker, Ki-67. 31 This is Arm evidence that our case is a benign neoplasm. Six-month post-surgical excision, there is no evidence of recurrence and this patient has been placed on long-term follow up.
CONCLUSION
DGCT is an aggressive tumour with significant tendency to recur and a potential for malignant transformation.
Segmental resection is the recommended surgical treatment and long-term postsurgical follow up is essential. The etiology of these lesions still needs to be further investigated and more cases are required elucidating the peculiar nature of this tumour.
Ethics
An informed consent was not obtained from the patient or relatives due to reasons explained to the Ethics committee and the Editor. A letter from the Ethics committee is attached.
Conflict of interest
None
REFERENCES
1. Carlos R, Ledesma-Montes C. Dentinogenic ghost cell tumour in The fourth edition of the head and neck World Health Organization. Adel K El-Naggar, John KC Chan, Jennfer R. Grandis, Takashi Takata, Pieter J Slootweg, WHO classification of head and neck tumours. LYON. IARC press, 2017: 226-227 [ Links ]
2. Gorlin RJ, Pindborg JJ, Odont, Clausen FP, Vickers RA. The calcifying odontogenic cyst-a possible analogue of the cutaneous calcifying epithelioma of Malherbe. An analysis of fifteen cases. Oral Surg Oral Med Oral Pathol. 1962; 15: 1235-43 [ Links ]
3. Pinheiro TN, de Souza APF, Bacchi CE, Consolaro A. Dentinogenic ghost cell tumor: A bibliometric review of literature. Journal of Oral Disease Markers. 2019; 3: 9-17 [ Links ]
4. Buchner A. The central (intraosseous) calcifying odontogenic cyst: An analysis of 215 cases. J Oral Maxillofac Surg. 1991; 49: 330-9. [ Links ]
5. Walia C, Kashyap B, Roy S. Disorganized histomorphology: Dentinogenic ghost cell tumor. J Oral Maxillofac Pathol. 2017; 21: 154 -77. [ Links ]
6. Mamabolo MM, Noffke C, Raubenheimer E. Odontogenic tumours in the first two decades of life in a rural African population sample: A 26 year retrospective analysis. Dentomaxillofacial Radiology. 2011; 40: 331-37 [ Links ]
7. Okoh DS, Akinshipo AO, Butali A, Omitola OG, Sigbeku OF, Soyele OO et al. Descriptive Epidemiology of Odontogenic Tumors in Nigeria: Ar African Oral Pathology Research Consortium Multicenter Study. Niger J Clin Prac. 2020; 23: 1695-701 [ Links ]
8. Singh K, Simon ENM, Mwalutambi S, Owibingire SS. A prospective Study on Odontogenic Tumours among Patients Attending Muhibili National Hospital, Dar Es Salaam, Tanzania. Oral Rehabilitation and Dentistry. 2020; 3(1): 2-6 [ Links ]
9. Ogundana OM, Effiom OA, Odukoya O. Pattern of distribution of odontogenic tumours in Sub-Saharan African. International Dental Journal. 2017; 67: 308-17 [ Links ]
10. Jing W, Xuan M, Lin Y, Wu L, Liu L, Zheng X et al. Odontogenic tumours: a retrospective study of 1642 cases in a Chinise population. Int. J. Oral Maxillofac Surg 2007; 36:20-25 [ Links ]
11. Ahire MS, Tupkari JV, Chettiankandyb TJ, Thakur A, Agrawal RR. Odontogenic tumors: A 35-year retrospective study of 250 cases in an Indian (Maharashtra) teaching institute. Indian J Cancer. 2018; 55: 265-72 [ Links ]
12. Regezi: Oral Pathology: Clinical Pathologic Correlations, 5th ed. An Imprint of Elsevier. WB Saunders, 2008: 745 [ Links ]
13. Kim SA, Ahn SG, Kim SG, Park JC, Lee SH, Kim J, et al. Investigation of the beta-catenin gene in a case of Dentinogenic ghost cell tumour. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103: 97-101 [ Links ]
14. Yukimori A, Oikawa Y, Morita K, Nguyen CTK, Harada H, Yamaguchi S. Genetic basis of calcifying cystic odontogenic tumors. PLOS One. 2017; 12: e0180224. [ Links ]
15. Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press, 2005: 314. [ Links ]
16. Juneja M, George J. Dentinogenic ghost cell tumor: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107: e17-22. [ Links ]
17. Praetorius F. Dentinogenic ghost cell tumour. In: Barnes L. editor. Surgical Pathology of the Head and Neck, Third Edition. CRC Press, 2009:1272-5. [ Links ]
18. Agrawal Y, Naidu GS, Makkad RS, Nagi R, Jain S, Gadewar DR et al. Dentinogenic ghost cell tumour - a rare case report with review of literature. Quant Imaging Med Surg 2017;7(5):598-604 [ Links ]
19. Patankar SR, Khetan P, Choudhari SK, Suryavanshi H. Dentinogenic ghost cell tumour: A case report. World J Clin Oncol. 2019; 10(4): 192-200 [ Links ]
20. Bafna SS, Joy T, Tupkari JV, Landge JS. Dentinogenic ghost cell tumor. J Oral Maxillofac Pathol. 2016; 20: 163 [ Links ]
21. Reddy V, Wadhwan V, Singh R, Bansal V. Dentinogenic ghost cell tumour: Case report of a rare central variant and literature review. J Oral Maxillofac Pathol. 2022; 26(1): 568-572 [ Links ]
22. Garcia BG, Ruiz Masera JJ, Zafra Camacho FM, Gutierrez CC. Intraosseous dentinogenic ghost cell tumor: Case report and treatment review. Rev Esp Cir Oral Maxillofac. 2015; 37: 243-6. [ Links ]
23. Salgado I, Vilares M, Nogueira R, Rito M, Rosa F. Dentinogenic ghost cell tumour - Case report of a rare entity. International Journal of Surgery Case Reports. 2021; 81:105651 [ Links ]
24. Konstantakis D, Kosyfaki P, Ebhardt H, Schmelzeisen R, Voss PJ. Intraosseous dentinogenic ghost cell tumor: A clinical report and literature update. J Craniomaxillofac Surg. 2014;42:e305-11 [ Links ]
25. Gupta S, Signh S, Anjum R, Sharma R, Dentinogenic ghost cell tumor of the maxilla: A case report and review of literature. J Oral Maxillofac pathol. 2019; 23 (3): 478. [ Links ]
26. Praetorius F, Hjcrting-Hansen E, Gorlin RJ, Vickers RA. Calcifying odontogenic cyst. Range, variations and neoplastic potential. Acta Odontol Scand. 1981; 39: 227-40. [ Links ]
27. Sonawane K, Singaraju M, Gupta I, Singaraju S. Histopathologic diversity of Gorlin's cyst: a study of four cases and review of literature. J Contemp Dent Pract 2011; 12: 392-397 [ Links ]
28. Singhaniya SB, Barpande SR, Bhavthankar JD. Dentinogenic ghost cell tumor. J Oral Maxillofac Pathol. 2009; 13: 97-100 [ Links ]
29. Kasahara K, Iizuka T, Kobayashi I, Totsuka Y, Kohgo T. A recurrent case of odontogenic ghost cell tumour of the mandible. Int J Oral Maxillofac Surg. 2002; 31: 684-7. [ Links ]
30. Sun G, Huang X, Hu Q, Yang X, Tang E. The diagnosis and treatment of Dentinogenic ghost cell tumor. Int J Oral Maxillofac Surg. 2009; 38: 1179-83. [ Links ]
31. de Arruda JAA, Monteiro JLGC, Abreu LG, de Oliveira Silva LV, Schuch LF, de Noronha MS, Callou G, Moreno A, Mesquita RA. Calcifying odontogenic cyst, dentinogenic ghost cell tumor, and ghost cell odontogenic carcinoma: A systematic review. J Oral Pathol Med. 2018; 47: 721-730. [ Links ]
 Correspondence:
Correspondence:
Dr Masilela MMJ
Sefako Makgatho Health Sciences University
Department of Oral and Maxillofacial Surgery
Pietersburg Academic Hospital, Oral Pathology Unit
South Africa.
Email: mmjmams@yahoo.com
Cell: 0824104225
Author contributions:
1 . M Lamola: 40%
2 . MMJ Masilela: 40%
3 . TO Adedoja: 20%














