Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.77 n.8 Johannesburg Sep. 2022
http://dx.doi.org/10.17159/2519-0105/2022/v77no8a5
RESEARCH
Assessing the antibacterial properties of eggshell-titanium powder
SC OnwubuI; MU MokgoboleII; PS MdluliIII; TH MokhothuIV
IPhD, Chemistry, Durban University of Technology (DUT), Durban, South Africa, ORCID: 0000-0002-4499-1534
IIMHSc, Somatology, Durban University of Technology (DUT), Durban, South Africa , ORCID: 0000-0001-6530-7079
IIIPhD, Chemistry, Durban University of Technology (DUT), Durban, South Africa
IVPhD, Chemistry, Durban University of Technology (DUT), Durban, South Africa
ABSTRACT
INTRODUCTION: The global distribution of oral diseases caused by bacterial and the severity of their consequences constitute a pandemic condition.
AIMS AND OBJECTIVES: The present paper reports on the antibacterial properties of a modified eggshell and titanium dioxide using the mechanochemical method.
METHODS: EB@TiO2 was modified and characterized using X-ray diffraction (XRD) while the degradation condition was studied using Thermogravimetric analysis (TGA). The inhibitory properties of EB@TiO2 at different concentrations (2:1; 3:1 and 4: 1) against both Gram-positive (E. coli) and negative bacterial (B. Cereus) strain were studied the using disk diffusion method
RESULTS AND CONCLUSIONS: The XRD result confirmed the presence of a thermodynamically stable calcite structure, which is indicative of calcium carbonate. The diffraction peak of the XRD at 2θ = 29.5o suggests the deposition of TiO2 on the surface of CaCo3. TGA curves shows the decomposition of anatase form of titanium dioxide and calcium carbonate. The study evidently shows the antibacterial activities of EB@TiO2 against Escherichia coli and B. Cereus. The salient feature of the study finding is that modifying eggshells with titanium dioxide improves the antibacterial properties, and thus offers a promising role for the development of potent dental materials in the management of oral diseases.
Keywords: Antibacterial; eggshell; Mechanochemical; titanium dioxide
INTRODUCTION
Although the oral status of the general population globally has improved, oral disease such as dental caries and periodontitis remains a significant public concern posing a grave challenge for the oral health care provider in mitigation and prevention1. From an epidemiological perspective, the World Health Organisation (WHO) underlines that oral diseases like dental caries affect around 60 to 90% of the general population 2,3. The aforementioned oral disease is mostly caused by bacterial activities in the mouth. Bacteria such as Streptococcus mutans, Streptococcus sobrinus, and lactobacilli are the main oral cariogenic pathogens due to their ability to produce high levels of lactic acid following sugar fer-mentation and their resistance to the adverse effects of low pH 4. Modern molecular analyses and microbial culture techniques have also demonstrated that an entire range of bacteria can contribute to the caries process at different stages 5. Besides the aforementioned bacteria species, some classes of fungi like the candida albicans significantly enhance the cariogenic virulence of plaque biofllms 6.
To address and help prevent oral diseases, published literature suggests that brushing regularly with well-formulated fluoride toothpaste can prevent the onset of this disease 7. Concerning, however, young children may accidentally swallow enough amount of fluoridated dentifrice to produce levels of fluoride consumption associated with a risk of developing dental fluorosis 8. More so, fluoride is an incessant non-biodegradable, hazardous, and persistent pollutant, which is of concern to the environment. Lacson et al.9 note that the uncontrolled concentration of fluoride can subsequently cause fluorosis, which is a common fluoride- and waterborne disease. To counteract this looming concern, providing safe, effective, environmentally friendly and affordable material in toothpaste formulation becomes highly important.
Ultimately, eggshells could be an effective environmental alternative in toothpaste formulation. Xia and his team, for example, recently developed a simple defluoridation by boiling eggshell with the addition of acetic acid and sodium phosphate intended for household application 10. This suggests that eggshells could be a viable alternative to fluoride as it is safe for household use. Moreover, eggshells are utilised in various industries such as dentistry, the pharmaceutical industry and medicine 11. In dentistry, various studies have illustrated the effective use of eggshells such as Onwubu et al.12 found that eggshell powder has been successful in reducing the surface roughness of acrylic dentures and Mony et al.13 concluded that eggshells are capable of re-mineralizing enamel carious lesions. Furthermore, recent studies by Onwubu et al.14 demonstrate that eggshells modified with titanium dioxide provide robust resistance to enamel demineralization against erosive acids.
Besides this, other studies have reported that titanium dioxide (TiO2) is an effective antimicrobial agent, and it is compatible with the human body or environment 15, 16. Given the usefulness of eggshells as a biomaterial, this study envisages that assessing the antibacterial properties of eggshell-modified titanium dioxide (EB@TiO2) will help in validating the material as an alternative dental material in the prevention of oral diseases. To our knowledge, there is limited research that has investigated the antibacterial properties of a modified eggshell and titanium dioxide. The purpose of this study was to assess in vitro the antibacterial properties of a modified eggshell and titanium dioxide against Escherichia coli and B. Cereus in comparison with commercially available toothpaste.
MATERIALS AND METHODS
Preparation of Eggshell-Titanium dioxide composite (EB -TO
Modification of eggshell with titanium dioxide was achieved in two steps in accordance with the techniques reported by Onwubu et al. 17. In the first step, eggshells were ball-milled by placing 30g of the eggshell in a 500ml stainless jar (inner diameter of 100 mm), together with 10 stainless steel balls of 10 mm diameter and dry-milled in a planetary ball mill (Retsch ® PM 100) at 400 rpm for 20 min. The collected powder was sieved to a particle size of < 25μιτι using a mechanical sieving shaker (Retsch AS 200, Germany). The eggshell powder and titanium dioxide mixing ratio were optimized following the procedure reported by 18, 20g of the fine eggshell powder obtained in step 1 were modified by adding 5g of anatase titanium dioxide (< 15μηη). The mixture was subsequently ball-milled for 150 min to obtain eggshell-titanium dioxide composite. The prepared powder was subsequently charisterised to determine the phyisco-chemical properties.
Characterization of EB@TiO2
• Fourier Transform Infrared Spectroscopy Analysis
• The infrared spectra were measured using a Perkin Elmer Universal ATR spectrometer to identify the functional group constituents of EB@TiO2.
• Transmission Electron Microscopic Analysis
• A Transmission Electron Microscope (TEM) was used to observe the particle size, shape and distribution of EB@TiO2. Very small quantities of EB@TiO2 were dispersed in 10ml ethanol and sonicated at 10kv for 10 min. Subsequently, thin cross-sections of cryo-microtomed specimens were prepared using a Leica microtome (South Africa) and placed on carbon copper grids. Analysis was conducted using a transmission electron microscope (TEM-Philips CM 120 model) at 120 kV.
• Thermogravimetric analysis
• Thermal degradation and stability of the modified EB-TiO2 were studied using thermo-gravimetric analysis (TGA). The thermal stability was measured using a TA instrument (Thermal Universal Analyser V4.5A). The test was performed under a dry nitrogen gas flow at the rate of 100mL/min from 20oC, at a heating run of 10oC/min.
Antibacterial Assessment of EB@TiO2
The inhibitory properties of EB@TiO2 against both Gram-positive and negative bacterial strains were studied the using disk diffusion method. The Bacterial strain was obtained from Anatech supplies, South Africa. Thereafter, pure colonies from freshly grown strain E.coli (ATC 25922), and B. Cereus (ATC 10876) were isolated and subsequently trans-ferred from the plates into a sterile normal saline solution and vortexed to form bacterial homogenous suspensions. The turbidity was then adjusted to 0.5 McFarland standard units, and the suspensions were poured over Mueller-Hinton agar (MHA) plates.
Sterile filter paper disks with a diameter of 6 mm were placed over these plates. The sterile disks were impregnated with 20 of the tested compounds (10 mg/ mL dissolved in DMSO). Positive control (Ciprofloxacin) and negative control (sterile distilled water) were used. The impregnated plates were incubated at 37°C for 24 hours. in line with the procedure described by Wiegand et al. 19, the inhibition zones were calculated and the value estimated in millimetres.
RESULTS
Characterization
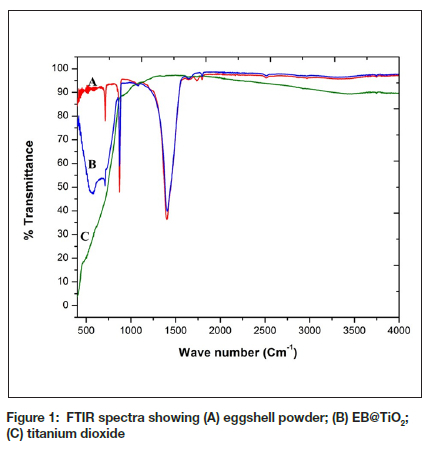
The FT-IR spectra of eggshell powder, titanium dioxide, and EB@TiO2 is presented in Figure 1. The band aspect of the FT-IR spectra shows the difference between the eggshell powder (Figure 1 (A)), EB@TiO2 (Figure 1(B)), and titanium dioxide (Figure 1(C)). The prominent absorption peak of carbonate at 1411 cm-1 was observed for both eggshell powder (Figure 1A) and EB@TiO2 (Figure 1B) suggesting that both materials contain a thermodynamically stable form of calcium carbonate. In addition, the broad stretching displayed for EB@TiO2 above 1000 cm-1 suggests the surface medication of calcium carbonate structure in eggshell powder with titanium dioxide (Figure 1B).
From Figure 2 the TGA curves show the incidence of two thermal events within the temperature range of 50-900oC. The first phase (600oC) is attributed to the decomposition of the anatase form of titanium dioxide, which caused a small weight loss (~ 1.061%). The second phase (699.14oC) is endothermic and is linked to the decomposition of calcium carbonate into carbon dioxide and calcium oxide 20.
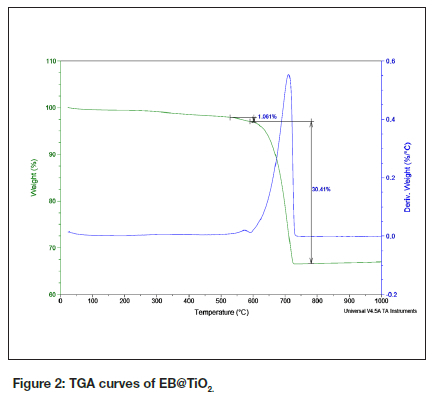
This weight loss equated to approximately 30.41% of the total mass.
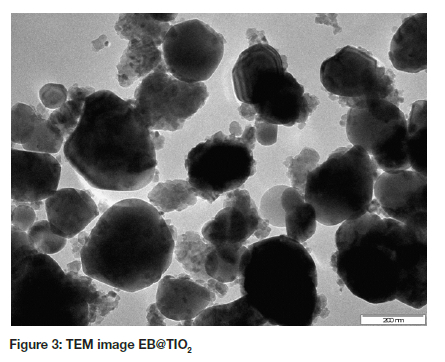
The TEM image of EB@TiO2 is shown in Figure 3. The image revealed a nonhomogeneous structure of spherical shape particles and irregular particles with different sizes distribution. The irregular shape particles are indicative of eggshell powder while the spherical particles typified the titanium dioxide particles. It can be observed that the pure titanium dioxide particles were scattered on the surface of the composite.
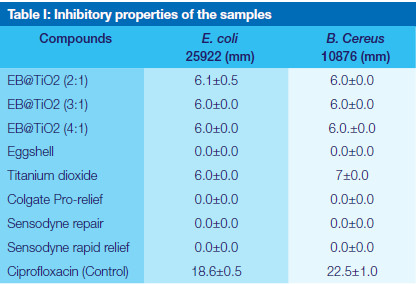
Antibacterial properties
The antibacterial screening of the EB@TiO2 compounds at different ratios of eggshell and titanium dioxide modification (2:1, 3: 1, and 4:1) was determined using the disc diffusion method against E. coli and B. Cereus. The results obtained have shown that these compounds displayed a slight inhibition against E. coli and B. Cereus (Table I). It was also found that irrespective of the ratio of eggshell and titanium dioxide used, the inhibitory properties observed for EB@TiO2 were more or less the same. On the contrary, tested toothpaste (Colgate Prorelief, Sensodyne Repair, Sensodyne rapid relief), and eggshell alone didn't display any inhibition against the bacteria used.
The inhibitory zone observed for EB@TiO2 at different ratio compositions is given in Figure 4. The image visibly confirmed the inhibitory properties of EB@ TiO2 against E. coli and B. Cereus. The inhibitory properties of Ciprofloxacin (Control) demonstrated the maximum zones of inhibition with a mean diameter of 18.6 and 22.5 mm for Escherichia coli and B. Cereus, respectively when compared to EB@TiO2 which showed maximum zones of 6 mm.
DISCUSSION
Oral disease such as dental caries continues to pose a significant public health concern due to the associated negative impacts on the quality of life for individual sufferers. Flouride containing toothpaste has largely been the most common effective oral hygiene practice that aids in the prevention of oral disease 7, particularly those caused by bacterial. However, the risks of dental fluorosis, as well as the environmental concern posed by fluoride has necessitated an alternative ingredient in toothpaste formulation. The purpose of this study was to assess in vitro the antibacterial properties of a modified eggshell and titanium dioxide (EB@TiO2) against Escherichia coli and B. Cereus. As reported in the literature, some common oral bacteria responsible for dental caries formation include Staphylococcus aureus 21, Escherichia coli22, Streptococcus mutans 23, Streptococcus sobrinus 24 Lactobacilli sp. 25, and Porphyromonas gingivalis 22. The finding of this study showed the EB@TiO2 at different concentrations tested in the study exhibited antimicrobial activity against both Escherichia coli and B. Cereus (Table 1).
Comparison of the zones of inhibition formed showed evidence of antibacterial activities (Figure 4). This may be attributed to the modification of eggshells with titanium oxide.
Further to the above, TiO2 has been reported to be an effective antimicrobial agent that is compatible with the human body or environment 15 16. In particular, and in a more recent study, it was demonstrated that titania reduces the ability of bacterial such as Staphylococcus aureus (S. aureus) to adhere to surfaces by rupturing their cell membrane 26.
As such, one could attribute the inhibitory properties of EB@TiO2 to the attachment of titanium oxide (TiO2) on the surface of the eggshell powder. This is can be supported by the fact eggshell powder alone showed no antibacterial activities whilst the antibacterial activities of TiO2 was comparable to EB@TiO2 (Table 1). The plausible explanation for this may be attributed to the fact that the calcium carbonate which is a constituent of eggshells is a poor source of nutrients for microbes to colonise. This is corroborated by Sepehrnia et al. 27, that calcium carbonate inhibits the colonisation of bacteria.
While the slimy extracellular polymeric substances (EPS) produced by dental caries causing bacteria offers it protection and mechanical supports 28, it can be said the abrasiveness of eggshells (since it is mainly calcium carbonates) and the antibacterial properties of TiO2 could offer robust protection through mechanical removal of biofilms, as well as, rupturing of the cell walls of the bacterial. This is relevant as the calcium carbonates in eggshells will aid in providing the mechanical removal of the biofilms.
Although the inhibitory zones found for EB@TiO2 against both Escherichia coli and B. Cereus were lower than that measured Ciprofloxacin (Control), it is, however, higher when compared to the commercially available toothpaste which showed no antimicrobial activities (Table I). The probable reason for this could be that the main active ingredients in the tested toothpaste function mainly act as anti-sensitivity. In reviewing the literature on antibacterial properties of commercially available toothpaste, it was found that the inhibitory zones measured for EB@TiO2 against Escherichia coli, for example, was higher when compared to the 2.22mm reported for Colgate Salt (Neem) by Dhakal et al. 29. This thus indicates the antibacterial properties of EB@TiO2.
While this study provides insight into the antimicrobial activity of the tested EB@TiO2, certain limitations exist such as the limited number of tested microorganisms selected for this study. Hence, it may be premature to dismiss the antibacterial properties of commercially available toothpaste. Future study needs to comprehensively assess the antibacterial properties of EB@TiO2 in conjunction with other ingredients in toothpaste formulations.
CONCLUSION
Oral diseases caused by bacterial is a serious public health concern to the general public. The study showed that modifying eggshell with titanium dioxide increases its antibacterial properties. This is vital towards the use of eggshells as an ingredient in toothpaste formulation. From a public health perspective and environmental sustainability, the study envisages that using eggshell-titanium dioxide in toothpaste formulation will offer a robust strategy in the prevention of oral diseases caused by bacterial and at the same time, acting as a catalyst towards a greener environment.
Acknowledgements
This work was supported the National Research Foundation of South Africa (Grant Number: 129492).
Declaration: No conflict of interest declared
REFERENCES
1. Onwubu S, Mduli P, Singh S, Bharuth V. An in vitro examination on the effectiveness of commercial toothpastes in the prevention of tooth decay, using eggshell as a substitute for human tooth material. S. Afr. Dent. J. 2018;73(7):446-51. [ Links ]
2. Zanella-Calzada LA, Galván-Tejada CE, Chávez-Lamas NM, Gracia-Cortés M, Carmen D, Moreno-Báez A, et al. A Case-Control Study of SocioEconomic and Nutritional Characteristics as Determinants of Dental Caries in Different Age Groups, Considered as Public Health Problem: Data from NHANES 2013-2014. Int. J. Environ. Res. Public Health. 2018;15(5):957. [ Links ]
3. Petersen PE, Ogawa H. Prevention of dental caries through the use of fluoride-the WHO approach. Community Dent. Health. 2016;33(2):66-8. [ Links ]
4. Nurelhuda NM, Al-Haroni M, Trovik T, Bakken V. Caries experience and quantification of Streptococcus mutans and Streptococcus sobrinus in saliva of Sudanese schoolchildren. Caries Res. 2010;44(4):402-7. [ Links ]
5. Tanner A, Kressirer C, Rothmiller S, Johansson I, Chalmers N. The caries microbiome: implications for reversing dysbiosis. Adv. Dent. Res. 2018;29(1):78-85. [ Links ]
6. Koo H, Bowen WH. Candida albicans and Streptococcus mutans: a potential synergistic alliance to cause virulent tooth decay in children. Future Microbiol. 2014;9(12):1295-7. [ Links ]
7. Clark-Perry D, Levin L. Comparison of new formulas of stannous fluoride toothpastes with other commercially available fluoridated toothpastes: A systematic review and meta-analysis of randomised controlled trials. Int. Dent. J. 2020;70(6):418-26. [ Links ]
8. Beltran E. Fluoride in toothpastes for children: suggestion for change. Pediatr Dent. 1988;10:185-8. [ Links ]
9. Lacson CFZ, Lu M-C, Huang Y-H. Fluoride-containing water: A global perspective and a pursuit to sustainable water defluoridation management-An overview. J. Clean. Prod. 2021;280:124236. [ Links ]
10. Xia Y, Huang X, Li W, Zhang Y, Li Z. Facile defluoridation of drinking water by forming shell@ fluorapatite nanoarray during boiling egg shell. J. Hazard. Mater. 2019;361:321-8. [ Links ]
11. Waheed M, Yousaf M, Shehzad A, Inam-Ur-Raheem M, Khan MKI, Khan MR, et al. Channelling eggshell waste to valuable and utilizable products: a comprehensive review. Trends Food Sci Technol. 2020;106:78-90. [ Links ]
12. Onwubu SC, Vahed A, Singh S, Kanny KM. Reducing the surface roughness of dental acrylic resins by using an eggshell abrasive material. J Prosthet Dent. 2017;117(2):310-4. [ Links ]
13. Mony B, Ebenezar AR, Ghani MF, Narayanan A. Effect of Chicken Egg Shell Powder Solution on Early Enamel Carious Lesions: An Invitro Preliminary Study. J. Clin. Diagnostic Res. 2015;9(3):ZC30. [ Links ]
14. Onwubu SC, Mdluli PS, Singh S, Lawrence M, Ngombane Y. Characterization and in vitro evaluation of an acid resistant nanosized dental eggshell-titanium dioxide material. Adv Powder Technol. 2019:1-8. [ Links ]
15. Rana S, Misra R. The anti-microbial activity of titania-nickel ferrite composite nanoparticles. JOM. 2005;57(12):65-9. [ Links ]
16. Rawat J, Rana S, Srivastava R, Misra RDK. Antimicrobial activity of composite nanoparticles consisting of titania photocatalytic shell and nickel ferrite magnetic core. Mater. Sci. Eng. C. 2007;27(3):540-5. [ Links ]
17. Onwubu SC, Mdluli PS, Singh S. Evaluating the buffering and acid-resistant properties of eggshell-titanium dioxide composite against erosive acids. J. Appl. Biomater. Funct. Mater. 2019;17(1):1-7. [ Links ]
18. Lin H, Dong Y-b, Jiang L-y. Preparation of calcium carbonate particles coated with titanium dioxide. Int. J. Miner. Metall. Mater. 2009;16(5):592-7. [ Links ]
19. Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163-75. [ Links ]
20. Chaudhuri B, Mondal B, Modak D, Pramanik K, Chaudhuri B. Preparation and characterization of nanocrystalline hydroxyapatite from egg shell and K2HPO4 solution. Mater. Lett.. 2013;97:148-50. [ Links ]
21. Torlak E, Korkut E, Uncu AT, Kener Y. Biofilm formation by Staphylococcus aureus isolates from a dental clinic in Konya, Turkey. J. Infect. Public Health. 2017;10(6):809-13. [ Links ]
22. Patra JK, Kim ES, Oh K, Kim H-J, Kim Y, Baek K-H. Antibacterial effect of crude extract and metabolites of Phytolacca americana on pathogens responsible for periodontal inflammatory diseases and dental caries. BMC Complement Altern Med. 2014;14(1):1-6. [ Links ]
23. Kawada-Matsuo M, Komatsuzawa H. Role of Streptococcus mutans two-component systems in antimicrobial peptide resistance in the oral cavity. Jpn Dent Sci Rev. 2017;53(3):86-94. [ Links ]
24. Liu Y, Liu P, Wang L, Shi Y, Chen J, Wang H, et al. Inhibitory effects of citrus lemon oil and llmonene on Streptococcus sobrinus-Induced dental caries in rats. Arch. Oral Biol. 2020;118:104851. [ Links ]
25. Caufleld P, Schön C, Saraithong P, Li Y, Argimón S. Oral lactobacilli and dental caries: a model for niche adaptation in humans. J. Dent. Res. 2015;94(9_ suppl):110S-8S. [ Links ]
26. Depan D, Misra R. On the determining role of network structure titania in silicone against bacterial colonization: mechanism and disruption of biofllm. Mater. Sci. Eng. C. 2014;34:221-8. [ Links ]
27. Sepehrnia N, Mahboubi AA, Mosadeghi MR, Khoda KG, Safari SA. Effect of calcium carbonate and calcium sulfate on E. coli survival in fine sand mixtures. J. Environ. Sci. Stud. 2012;38(62):37-9. [ Links ]
28. Saini R, Saini S, Sharma S. Biofllm: A dental microbial infection. J Nat Sci Biol Med. 2011;2(1):71. [ Links ]
29. 29. Dhakal A, Sundaram S, Rajesh V, Rajan RJAP. Comparative antimicrobial efficacy study of different commercially available toothpaste in In-dia: An in vitro study. dRc Sustainable Future: Journal of Environment, Agriculture, and Energy 2021;2(2):122-31. [ Links ]
 Correspondence:
Correspondence:
Onwubu Stanley Chibuzor
Dental Sciences Department, Dental Technology Programme
Durban University of Technology (DUT)
Durban, South Africa
Email: stanleyo@dut.ac.za
Author contributions:
1 . Stanley Chibuzor Onwubu 50%
2 . Mokgadi Ursula Mokgobole 20%
3 . Phumlane Selby Mdluli: 15%
4 . Thabang Hendrica Mokhothu 15%














