Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.77 no.7 Johannesburg Ago. 2022
http://dx.doi.org/10.17159/2519-0105/2022/v77no7a5
RESEARCH
In vitro antibacterial activity of three root canal sealers against Enterococcus Faecalis
TF MukoreraI; S AhmedII; E MabozaIII; F Kimmie-DhansayIV
IBDS, PDD, MSc, Maxillofacial Centre, Harare, Zimbabwe, ORCID: 0000-0002-7467-4230
IIBChD, PDD, MSc,Lecturer, Restorative Dentistry, Faculty of Dentistry, University of the Western Cape, ORCID : 0000-0001-8174-6928
IIIOral & Dental Research Institute Faculty of Dentistry, University of the Western Cape, ORCID : 0000-0003-0572-6591
IVBSc, BChD, PDD, PDD, MSc, Faculty of Dentistry, University of the Western Cape, ORCID : 0000-0003-2919-6193
ABSTRACT
The antibacterial activity of root canal sealers may contribute to the eradication of remaining bacteria in root canals following canal shaping and debridement.
AIM: The aim of the study is to assess the antimicrobial effect of 3 endodontic sealers: Sealapex™, EndoREZ™ and Guttaflow bioseal™ against Enterococcus faecalis
Materials and Methods: This was a laboratory-based comparative study testing the antimicrobial activity of three endodontic sealers against Enterococcus faecalis. The endodontic sealers were tested unset 20 minutes after mixing and after setting.
Testing after setting enabled the assessment of the antimicrobial activity of aged sealers after 7, 14, 21 and 28 days. A total of 150 samples were used for the study.
The tested sealers were divided into 3 groups:
• Group 1 (EndoREZ™) n = 45 plates, n = 5 control plates.
• Group 2 (Guttaflow bioseal™) n = 45, n = 5 control plates.
• Group 3 (Sealapex™) n = 45, n = 5 control plates.
RESULTS: All the materials exhibited some activity against the bacteria. The overall greatest antibacterial activity can be seen by Guttaflow bioseal™ (4.46, 0.01) on day 21, followed by Sealapex™ (5.12, 0.05) on day 7 and EndoREZ™ (6.37,0.08) on day 14.
CONCLUSION: Under the conditions of this study all the endodontic sealers exhibited some antimicrobial activity against E. faecalis with different behaviour patterns at different times.
INTRODUCTION
Endodontic treatment involves the optimum shaping and debridement of the canal system to gain a tapered centred canal ensuring that there is minimal transportation of the apex. This allows for optimal adequate cleaning through irrigation and placement of intracanal dressing.1
Complete removal of the microbial population from the root canal system of the tooth remains the overall goal of endodontic treatment.2 There is evidence, however, that no single method of root canal preparation is capable of completely eradicating the microbial population in root canal systems.3 Thus, materials used for obturation which have antibacterial properties are advantageous so that any residual microbial population remaining in the root canal system can be destroyed.4
Root canal preparation consists of two intimately related procedures namely mechanical preparation and disinfection, which remains an essential component of endodontic treatment. Several methods and instruments have been developed for root canal preparation. Nickeltitanium represents the latest metallurgy in endodontics for hand, rotary and reciprocating files.5
While a significant portion of microorganisms in dentine are removed during instrumentation, some areas within the canal remain untouched partly due to the complexity of the root canal system that encompasses lateral canals, fins, anastomoses and ramifications.5 Accordingly, in one study up to 53% of the canal walls were untouched by instrumentation.6 Utilizing new instruments like Self Adjusting File (SAF), TRUshape and XP-endo, that can deal with irregular canal anatomy is often advisable. Entombing bacteria in unprepared sites is not reliable and predisposes to poor treatment outcome.7 Therefore, it is essential that mechanical preparation shape the canal to facilitate irrigation of the canal.8
Irrigants used in endodontics should be able to destroy micro-organisms, neutralize endotoxin and remove organic tissue components.8 A variety of substances have been used as irrigants including; chlorhexidine, sterilox, sodium hypochlorite, EDTA and QMIX. Sodium hypochlorite possesses ideal antimicrobial properties and is still regarded by the profession as the gold standard irrigant.9
The method of action of sodium hypochlorite is related to its high pH which denatures proteins and the hydroxyl ion which destroys the bacterial lipid membrane, DNA amongst other things.10 On the other hand, the chloride ion is responsible for dissolving proteins through breakage of peptide bonds. Even though a significant number of microorganisms can be eradicated from the canal by irrigants alone or in combination with mechanical procedures, cultivable bacteria has been isolated in canals after root canal preparation before obturation.11 One such bacterial species isolated following shaping and disinfection of the canals is Enterococcus faecalis. Research done by Haapasalo et al.11 found that 1% sodium hypochlorite could not kill E. faecalis in the presence of dentine. In a study by Bystrom and Sundqvist12, necrotic root canals could not be rendered free of bacteria using different concentrations of sodium hypochlorite and EDTA.3,12,13
Following the complete debridement of the root canal system, obturation needs to be completed with non-toxic materials to ensure a full 3D obturation of the root canals.14 3D obturation should aim to provide a hermetic 'fluid-tight' seal that prevents reinfection of the canals.15 A positive correlation has been found between a good root canal seal and a positive outcome of the endodontic treatment.16 To obtain a hermetic fluid-tight seal, obturation is routinely performed with the combination of a solid core material and an endodontic sealer. Solid gutta percha is usually the core used in endodontic obturation. Different obturation techniques have been advocated although cold lateral and warm vertical compaction are most common.17 Endodontic infections can broadly be categorized into intra radicular or extra radicular infections.18 Intra radicular infections are further subdivided into:
• Primary or initial infection results when microorganisms enter and colonize non-vital pulpal tissue.
• Secondary infection is when microorganisms that were not part of the primary infection are then introduced into the canals of the tooth during endodontic treatment.
• Recurrent persistent infection results when the microbial population in the primary or secondary infections resists intracanal procedures and are able to survive in the treated root canal.19
Extra radicular infection in turn is a result of the colonization by microbes of the periradicular tissues, which is usually as a consequence of intra radicular infection. Extra radicular infections may be conditional on the intraradicular infection, or it can be completely independent thereof.19 Initial infections comprise of a multispecies community of bacteria dominated by anaerobes.20,21 The concentration and amount of bacterial species and cells determine the size of the apical periodontal lesion.22 During different phases of root canal infection, certain species may dominate over other bacterial species. The change in the microbial population makeup is most likely due to changes in the environmental conditions, especially oxygen tension and the availability of nutrients. Facultative bacteria dominate in the initial infectious stage and as there is depletion of oxygen within the root canal system; obligate anaerobes start increasing.18,23
The point of entry for microbes into the pulp is from the typical oral microbial population usually via the extension of a carious lesion from the tooth crown; dentinal tubules are opened enabling access to the bacterial population.24 The dentino-pulp complex is usually a sterile environment, and invasion with microorganisms only occur when there is a breach. Examples of this may be due to caries, trauma and/or restorative treatment. During endodontic intervention, the potential for entry of microorganisms also exist.25
Persistent intra radicular colonization is a result of bacteria that resist cleaning and disinfection of the canal thereby continuing to survive in obturated canals. These bacteria can be the remaining population of primary or secondary infections.26 Various studies have identified E. faecalis as one of the most frequently observed microorganisms in obturated root canals with an incidence of up to 90% of cases.27,28 In nonvital teeth, bacterial invasion occurs at a swift rate, conceivably due to the absence of host defence mechanism.28
Both persistent and secondary infections display various clinical symptoms, including:
• recurrent exudation
• persistent symptoms
• inter-appointment pain and flare-ups
• endodontic treatment failure, which is demonstrated by post treatment apical periodontitis lesion. 19
Several culture and molecular biology research projects concluded that E. faecalis is the most recurrent species in endodontically obturated teeth, with an incidence up to 90% of cases.12,28,29 Enterococcus faecalis in obturated canals can be thought to be a secondary invader capable of colonizing the canal and resisting treatment.30
Enterococcus faecalis belongs to the Enterococcus genus, which consist of catalase-negative, grampositive, non-spore forming, facultative anaerobes. These microorganisms may present as cocci or chains.31 Enterococcus faecalis can survive in very severe conditions like extreme alkaline pH, and salt concentrations. They can propagate in the range 10 - 45 °C.32
E faecalis is isolated in 24 to 90 % of the positive cultures.33,34 This may be due to the microorganism's ability to resist antimicrobial agents as well as the potential to adapt to a changing environment. This allows proliferation of the organism in the root canal system and may cause reinfection. Enterococcus faecalis binds to the dentine of root canals resulting in the formation of dental biofilm. The ecosystem of biofllms assists in resisting destruction by allowing the bacteria to become unaffected by phagocytosis, antibodies and antimicrobial measure. The antimicrobial resistance of this bacteria has been ascribed to the protective barrier provided by the extracellular polymeric matrix.35 This bacterium penetrates dentinal tubules thus evading mechanical instrumentation and chemical irrigation during endodontic treatment.19
Effective endodontic treatment requires a hermetic fluid-tight seal of the tooth, which is achieved by successful and complete obturation.36 Currently, the known method of endodontic Alling involves a solid or semi-solid core such as gutta percha and an endodontic sealer. The core like gutta percha has no sealing ability and antimicrobial activity, therefore, endodontic sealers are required to obtain a hermetic fluid-tight seal in the root canal. This is achieved through obturation of the lateral, accessory canals, voids, spaces and anomalies between gutta-percha and root dentine wall.37 Some root canal sealers have antibacterial properties and may help to entomb the remaining bacteria after endodontic preparation. Antibacterial activity is one of the prerequisites of an ideal endodontic sealer.38
Sealapex™ is a calcium hydroxide based endodontic sealers. It is one of the most studied endodontic sealers.39 The release of hydroxide ions and creation of an alkaline pH is responsible for the antimicrobial activity of Sealapex™. As the setting reacting takes place, the pH decreases and the efficacy of the endodontic sealer decreases.12,40,41 In a direct contact test (DCT) study, Fuss et al.41 tested two calcium hydroxide sealers including Sealapex™ and Zinc-oxide eugenol Roth™ cement against E faecalis. Roth™ cement was potent against the bacteria in the 1st hour and at 24 hours of aging while Sealapex™ showed better activity in 7 days of aging. In a study using Agar diffusion test (ADT) and direct contact test (DCT), Sealapex™ was found to have no antibacterial activity when ADT was used; while with the DCT; antimicrobial activity was found after 60 minutes of aging.42
A systematic review on calcium hydroxide based endodontic sealers showed conflicting results with some showing antibacterial activity while others showing no antibacterial activity. Therefore, the review noted that there was conflicting evidence regarding calcium hydroxide-based sealers.39 Regarding Sealapex™ in particular, another systematic review pointed out that there was no difference in antimicrobial efficacy of Sealapex™ and AH- Plus™ against E. faecalis. However, the review noted that the evidence was poor due to high risk of bias of the studies considered.43
EndoREZ™ is a urethane dimethacrylate resin based endodontic sealer. In a study by Eldeniz et al.44 using ADT and DCT, EndoREZ™ did not show any antimicrobial activity in 24 hours, 48 hours, 7 days, and 10 days. Later in a similar study Farmakis et al.45 found similar results as they noted that EndoREZ™ exhibited 0 mm exhibition zone using ADT while no antibacterial effect was also found with DCT. Heyder et al.46 also concluded the same for EndoREZ™. However, in an earlier study EndoREZ™ was found to be bactericidal against Enterococcus faecalis at 3 and 7 days after mixing.47
Guttaflow bioseal™ is the successor of silicone-based root canal sealers Guttaflow™ and Guttaflow2™. These are derived from polydimethylsiloxane with powdered gutta-percha and microsilver particles.48 Guttaflow bioseal™ is the latest material of the series which constitutes of polydimethylsiloxane, zirconium dioxide, gutta-percha powder, platinum catalyst, silver (preservative), bioactive glass ceramic and coloring. According to the manufacturer (Coltène/Whaledent, Altstatten, Switzerland); Guttaflow bioseal™ has improved biological properties including antibacterial activity compared to GuttaFlow™ and GuttaFlow 2™.49
Earlier studies involving Guttaflow™ and Guttaflow 2™ have indicated a lack of antibacterial activity. In a study comparing the antimicrobial activity against E. faecalis of Epiphany™, Guttaflow™ and AH-Plus™ endodontic sealers after 1- and 24-hours using ADT and DCT, Guttaflow™ was found to lack antimicrobial activity.50
However, a study by Anumula et al.51 showed slight antibacterial activity of Gutta Flow™ for the first 3 hours after mixing which reduced drastically. In a recent study RoekoSeal™, Guttaflow 2™, TotalFill BC™ sealer, AH Plus™ were tested against planktonic and 24-hour old Enterococcus faecalis biofllms. The authors concluded that RoekoSeal™ and Guttaflow 2™ had no antibacterial activity against E. faecalis in both forms.48
A recent study using Guttaflow 2™, found that it had no antimicrobial activity against E. faecalis using both ADT and DCT testing methods.52 Studies involving Guttaflow bioseal™ and E. faecalis are still very few due to its recent introduction in the market. However, in a recent study it was found that the antibacterial efficiency of Guttaflow Bioseal™ improved up to 4 weeks after placement. The calcium silicate particles in Guttaflow Bioseal™ are thought to provide an alkaline environment after setting through release of calcium ions and this results in antimicrobial activity.49
MATERIALS AND METHODS
A laboratory-based comparative study was conducted by testing the antimicrobial activity of three commonly used endodontic sealers against Enterococcus faecalis. The endodontic sealers were tested unset 20 minutes after mixing and after setting. Testing after setting enabled the assessment of the antimicrobial activity of aged sealers after 7 days, 14 days, 21 days and 28 days. A total of 150 samples were used for the study.
Sample size:
The tested sealers were divided into 3 groups:
Group 1 (EndoREZ™) n = 45 plates, n = 5 control plates.
Group 2 (Guttaflow bioseal™) n = 45, n = 5 control plates.
Group 3 (Sealapex™) n = 45, n = 5 control plates. Microorganism:
Enterococcus faecalis, American Type Cell Culture Collection (ATCC) 19434 was used as a test organism. The bacteria were cultured in air at 37° C on Tryptic Soy Agar plates for the experiments. For each experiment a 24-hour culture was used. A suspension of bacteria in phosphate-buffered saline (PBS) was made and adjusted to 0.5 McFarland scale equivalent to 1.5 x 10Λ8 CFU.
Endodontic Sealers:
Three sealers were used for the study. Sealapex™ (Kerr), EndoRez™ (Ultradent, South Jordan, UT) and Roeko Guttaflow Bioseal™ (Coltene/ Whaledent, Switzerland). All sealers were prepared according to manufacturers' guidelines. A 96-well microtiter plate was held perpendicular to the floor, and an area on the side wall of the wells was coated with an equal amount of each material. The sealers tested at day 0 (20 minutes after mixing) were regarded as fresh specimens while other specimens were allowed to set for 7,14,21 and 28 days in a humid atmosphere at 37 °C before testing.
A 250μ! bacterial suspension was placed in contact with each sealer. The bacterial solution placed in the uncoated wells served as the control. The incubation was done in 100% humidity at 37 °C for 2, 5, 20, and 60 minutes. After gently agitating with a pipette for 30 seconds, the bacterial suspension from each well was transferred and serially diluted in Phosphate Buffered Saline (PBS).
The evaluation of the DCT was assessed by culturing aliquots of 100 μL onto TSA plates after 10-fold serial dilutions. After incubation for 24 hours at 37°C, colonies on the plates were counted, and the CFU/m_ was calculated.
Direct contact test (DCT)
The Direct Contact Test method represents another way of assessing the antibacterial effect of endodontic sealers. This method of investigation was first introduced by Weiss et al.53 for the assessment of the antibacterial effect of root canal sealers and root-end filling materials.
Due to limitations of ADT, many studies advocate using the Direct Contact Test method. This method involves testing the antibacterial activity of the root canal sealer when there is a direct connection between the material under investigation and the specific bacterial organism. This method is able to measure antimicrobial activity, independent of whether the material is soluble or can diffuse through the medium. This is a quantitative and reproducible assay which allows for the investigation of insoluble materials resulting in a standardized assay. Thus, this method produces reliable results.53
Two methods of evaluating the results of the DCT have been used extensively in endodontic literature. Traditionally, colony forming units (CFU/ml) has been used to assess the results of the DCT, while recently the
Statistical analysis
All data was described with the mean and standard deviation. A one-way mixed measures ANOVA test with Bonferroni correction was used to determine statistical significance between the three materials. All tests were deemed statistically significant atp < 0.05. All tests were conducted in Stata Corp.2017. Stata Statistical Software Release 15. College Station, TX: StataCorp LLC
RESULTS & STATISTICAL ANALYSIS
Overview of results
All the materials exhibit some activity against E Faecalis and are presented as mean (SD). Guttaflow bioseal™ exhibited the overall greatest antibacterial activity, 4.46 (0.01) on day 21, followed by Sealapex™, 5.12; (0.05) on day seven and EndoREZ™, 6.37 (0.08) on day 14 (Figure 1). Between the materials investigated there was a difference in antibacterial activity over a period of time, where Guttaflow bioseal™ vs EndoREZ™ had the biggest difference, -1.12 (0.14) followed by Sealapex™ vs EndoREZ™, -0.805 (0.14). Guttaflow bioseal™ and Sealapex™ did not show much difference in activity, 0.32 (0.14).
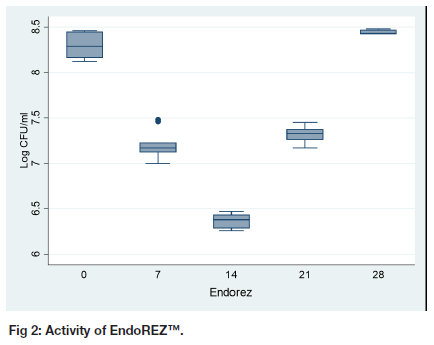
Antibacterial activity of EndoREZ™
The highest Log CFU count was 8.44 (0.02) at day 28 for EndoREZ™ (Table I, Figure 2). The second highest Log CFU count was at day 0, 8.30 (0.15). However, the difference between the activity on day zero and day 28 is not statistically significant (p = 0.124). The activity of EndoREZ™ gradually increases to reach its highest on day 14, 6.37 (0.08). After that, the activity gradually reduces to reach the highest Log CFU/ml on day 28.
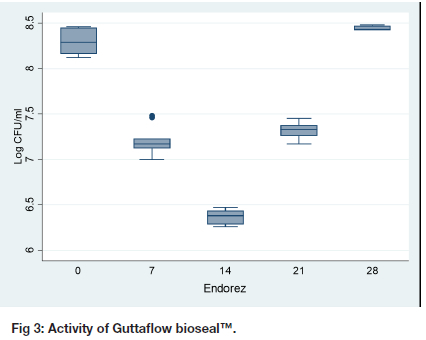
Antibacterial activity of Guttaflow bioseal™
The activity of Guttaflow bioseal™ increases from fresh samples to reach the maximum bactericidal activity on day 21, 4.46 (0.01) as shown by the lowest log CFU/ ml (Table I, Figure 3). After day 21, the activity reduced dramatically, 8.38 (0.10). The activity on day 28, 8.38 (0.10) was much less than the activity at day zero, 7.39 (0.07), p < 0.001. The difference between day 28 and day zero for Guttaflow was 0.974 (95% CI: 0.817 to 1.142).
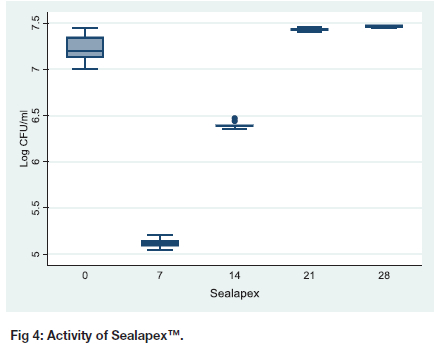
Antibacterial activity of Sealapex™
The activity of Sealapex™ increased and reached its peak on day seven, 5.12 (0.05), thereafter its potency dissipates, 6.40 (0.04) on day14 (Table I, Figure 4). On day 21 and 28 there is no difference in the activity of Sealapex as shown by the CI (-0.116 to 0.187), p =1.00. On comparing day 28 and day 0, there is a difference in the activity (CI 0.095 to 0.398) with day 28 sample being less potent, p < 0.001.
DISCUSSION
The goal of endodontic obturation is to achieve a permanent fluid tight hermetic seal of the pulp chamber and roots of the tooth in order to eliminate the risks of infection or reinfection of the root canal system. A sealer is usually employed to obtain a hermetic seal. Often failure of endodontic treatment is due to the spaces within the root canal system as a result of not being obturated properly. The interaction between the oral environment and root canal spaces as well as residual bacteria in canals (from inadequate debridement) may contribute to endodontic treatment failure. Thus, antibacterial activity of root canal sealers contributes to the success of endodontic treatment.
The antibacterial effect of root canal sealers may assist with the eradication of the remaining microbial organisms after root canal shaping and cleaning as it has been shown in previous studies that no root canal preparation technique is capable of eliminating all the microorganisms from the root canal system.47 Many root canal sealers have claimed to be antimicrobial against the common endodontic microorganisms. Enterococcus faecalis was the selected test microorganism in this study due to its high prevalence of isolation in cases of persistent apical periodontitis even after root canal treatment.55 E. faecalis is known to survive, grow within dentinal tubules and reinfect canals.56
Bacterial survival in root canals may be ascribed to their ability to penetrate the dentinal tubules where biofllm formation can take place. This protects these microorganisms from disinfecting agents cleaning the root canal system. Other authors advocate that Enterococcus faecalis in obturated teeth with post-treatment disease continues being viable as it adheres to collagen in the presence of human serum and form resistant biofilms.57
In this study the Direct Contact Test (DCT) was used, a method pioneered by Weiss et al.53 to evaluate the antimicrobial activity of endodontic sealers. The DCT evaluates the efficacy of direct and close contact between the material and the tested bacteria on microbial viability. Therefore, it enables measurement of whether the bacteria are viable regardless of the solubility and diffusibilty of the sealer's antibacterial mechanism.39 Standardization of root canal sealers antimicrobial testing protocols is lacking in literature. The DCT is a quantitative method which can be replicated to evaluate bacterial growth.
Sealapex™ is a calcium hydroxide based endodontic sealer. In this study fresh samples of Sealapex™ at day 0 have a weak activity against E. faecalis. This is similar to the study by Fuss et al.41 A study by Poggio et al.42 noted that fresh samples of Sealapex did not have any activity against E. faecalis. It is important to note that in the study by Poggio et al.42 the fresh samples were tested after 6 minutes. The antibacterial activity of Sealapex™ is derived from the release of OH ions. The study by Fuss et al.41 noted that fresh samples of Sealapex™ do not release OH ions in high concentrations hence explaining the weak activity against E. faecalis of these samples.41,42
Regarding aged samples, in this study the activity of Sealapex™ increased to reach the maximum on day 7, there after the activity started to decrease. This is in agreement with earlier studies which also recorded maximum activity of Sealapex™ on day 7.41,42,47 Most studies limit the evaluation time to 7 days, whereas in the current study the activity of Sealapex™ was evaluated for 28 days. The reduced activity of Sealapex™ after day 7 may be explained by the reduced concentration of the hydroxide ions which are vital for antimicrobial activity. Fuss et al.41 noted that the set material had a limited amount of the availability of the hydroxide ions.
Earlier on Bystrom and Sundqvist12 postulated that for a calcium hydroxide sealer to maintain antimicrobial effectiveness the pH must be around 12.5, a position which was also advocated by Mickel et al.40 As the material sets the pH drops to around 9 causing it to lose its effectiveness.12,40,41 A recent systematic review noted that there is loss of antibacterial activity against Enterococcus faecalis in calcium hydroxide sealers that were allowed to age. However, the evidence provided by the review is conflicting and may be due to the difference in methodologies of studies and time frames.39
EndoREZ™ is a methacrylate sealer which sets by chemical cure or light cure and can penetrate dentinal tubules. The fresh samples of EndoREZ™ showed weak antimicrobial activity against E. faecalis. The result agrees with the study by Eldeniz et al.44 which showed mild to no antibacterial activity for fresh samples though they used ADT in their study as opposed to the present study which used DCT. However, there is a contrast to the study by Zhang et al.47which recorded an efficient killing of E. faecalis using the DCT method.44,47
For aged samples of EndoREZ™ in this study, the antimicrobial activity increased to reach the peak on day 14, thereafter the material started to lose its activity against E. faecalis. The antimicrobial effect of EndoREZ™ is thought to occur as a result of the inhibitory effect of the oxygen layer limiting the setting reaction of EndoREZ™. This results in a greater quantity of non-reacted monomers killing E. faecalis.45
This may help to explain the weak activity of EndoREZ™ at day 21 and 28 as the material was fully set so there were no free monomers to exert the antibacterial activity against E. faecalis. Heyder et al.46 in their study noted that the antibacterial activity of EndoREZ™ was inferior to that of Zinc-oxide eugenol sealers and Sealapex™. This is in agreement with the present study which noted that the activity of EndoREZ™ was weaker than that of Sealapex™ and Guttaflow Bioseal™.
Guttaflow Bioseal™ is a recent addition to the market of the silicone based polymethyl hydrogen siloxane endodontic sealers.48,50 It is a successor to Guttaflow™ and Guttaflow 2™ and the manufacturer claims it has improved biological properties (Ruiz-Linares et al. 2019). Previous studies using either Guttaflow™ or Guttaflow 2™ showed that both materials had no activity against E. faecalis in fresh and aged samples.48,50,51 In this study the fresh samples had a weak antimicrobial activity against E. faecalis. Due to the recent introduction of Guttaflow bioseal™ studies investigating its antimicrobial activity are few.
In this study, the aging of Guttaflow bioseal™ resulted in increased antibacterial activity against Enterococcus faecalis reaching its peak on day 21 followed by sharp reduction of antimicrobial activity on day 28. This result partly agrees with the study by Ruiz- Linares et al.49 which showed increased antimicrobial activity with respect to the control as the material ages. In that study the assessment of antimicrobial activity was performed after day 1, 1 week and 4 weeks. In contrast to their results, the present study showed a marked decrease in the antimicrobial activity against E. faecalis on day 28. This can be attributed to the difference in methods of counting viable cells of E. faecalis after the DCT. The present study used CFU/ml (colony forming units); while the Ruiz-Linares et al.49 study used RLUs (relative luminescence intensities). Guttaflow bioseal™ is composed of a mixture of polydimethylsiloxane, platinum catalyzer, calcium silicate, gutta-percha powder and zirconium dioxide.
It is postulated that the calcium silicate particles are responsible for providing an alkaline environment through constant release of calcium ions after setting. This high pH environment is responsible for the antimicrobial properties.58 Guttaflow bioseal™ is a promising material in endodontics since its antimicrobial activity increases after setting, however further research is needed on this material.
CONCLUSION
It is clear from the study that all the endodontic sealers exhibited some antimicrobial activity against E. faecalis with different behaviour patterns at different times. Based on the results of this study Guttaflow Bioseal™ exhibited the greatest antibacterial activity on day 21, followed by Sealapex™ on day 7 and EndoREZ™ on day 14.
REFERENCES
1. Torabinejad M., Walton RE. Endodontics: Principles and Practice, 4th ed. St. Louis: Saunders, 2009: 264-265. [ Links ]
2. Hasheminia M, Razavian H, Mosleh H, Shakerian B. In vitro evaluation of the antibacterial activity of Ave sealers used in root canal therapy. J. Dent. R. 2017; 14(1):62. [ Links ]
3. Dalton BC, 0rstavik D, Phillips C, Pettiette M, Trope M. Bacterial reduction with nickeltitanium rotary instrumentation. J. Endod. 1998; 24(11):763-7. [ Links ]
4. Wainstein M, Morgental RD, Waltrick SB, Oliveira SD, Vier-Pelisser FV, Figueiredo JA, Steier L, Tavares CO, Scarparo RK. In vitro antibacterial activity of a silicone-based endodontic sealer and two conventional sealers. Braz oral research. 2016; 30(1). [ Links ]
5. Darcey J, Taylor C, Roudsari RV, Jawad S, Hunter M. Modern endodontic principles part 3: preparation. Dent. Update. 2015; 42(9):810-22. [ Links ]
6. Peters OA, Laib A, Göhring TN, Barbakow F. Changes in root canal geometry after preparation assessed by high-resolution computed tomography. J. Endod. 2001; 27(1):1-6. [ Links ]
7. Siqueira Junior JF, Rôças ID, Marceliano-Alves MF, Pérez AR, Ricucci D. Unprepared root canal surface areas: causes, clinical implications, and therapeutic strategies. Braz. oral res. 2018; 32(65): 1-7 [ Links ]
8. Hübscher W, Barbakow F, Peters OA. RootJ canal preparation with FlexMaster: canal shapes analysed by micro-computed tomography. Int ndod J. 2003; 36(11):740-7. [ Links ]
9. Holliday R, Alani A. Traditional and contemporary techniques for optimizing root canal irrigation. Dent Update. 2014; 41(1):51-61. [ Links ]
10. Darcey J, Jawad S, Taylor C, Roudsari RV, Hunter M. Modern endodontic principles part 4: irrigation. Dent Update. 2016; 43(1):20-33. [ Links ]
11. Haapasalo M. Current advances in irrigation. Endod Topics. 2013; 1(27):1-2. [ Links ]
12. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg, Oral Med, Oral Path, Oral Radiol. 1983; 55(3):307-12. [ Links ]
13. Rôças IN, Siqueira Jr JF, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004; 30(5):315-20. [ Links ]
14. Kokorikos I, Kolokouris I, Economides N, Gogos C, Helvatjoglu-Antoniades M. Long-term Evaluation of the Sealing Ability of Two Root Canal Sealers in Combination with Self-etching Bonding Agents. J Adhes Dent. 2009; 11(3). [ Links ]
15. Darcey J, Roudsari RV, Jawad S, Taylor C, Hunter M. Modern endodontic principles part 5: obturation. Dent Update. 2016; 43(2):114-29. [ Links ]
16. Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature-Part 2. Influence of clinical factors. Int Endod J. 2008; 41(1):6-31. [ Links ]
17. Khalil I, Naaman A, Camilleri J. Properties of tricalcium silicate sealers. J Endod. 2016; 42(10):1529-35. [ Links ]
18. Sakamoto M, Rôças IN, Siqueira Jr JF, Benno Y. Molecular analysis of bacteria in asymptomatic and symptomatic endodontic infections. Oral Microbiol Immunol. 2006; 21(2):112-22. [ Links ]
19. Siqueira JF, Rocas IN. Microbiology of Endodontic Infections. In: Berman LH, Hargraves KM, Rotstein I. 11th ed. Cohen's Pathways of the Pulp 2016 Missouri: Elsevier 599-629. [ Links ]
20. Blome B, Braun A, Sobarzo V, Jepsen S. Molecular identification and quantification of bacteria from endodontic infections using reaH time pol ymerase chain reaction. Oral Microbiol Immunol. 2008, 23(5):384-90. [ Links ]
21. Sundqvist, G. Endodontic microbiology. In Spangberg _SW, editors: Experimental endodontics, Boca Raton, CRC Press 1989; p 131. [ Links ]
22. Vianna ME, Horz HP, Gomes BP, Conrads G. In vivo evaluation of microbial reduction after chemoKI mechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J. 2006;39(6):484-92. [ Links ]
23. Gomes BPFA, Herrera DR. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz. Oral Res. 2018; 32(69):82-110 [ Links ]
24. Baumgartner JC, Falkler W. Bacteria in the apical 5mm of infected root canals. J Endod. 1991; 17(8) 380-383. [ Links ]
25. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root Alling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997; (30), 297306. [ Links ]
26. Waltimo T, Trope M, Haapasalo M, 0rstavik D. Clinical efficacy of treatment procedures in endodontic infection control and one-year follow-up of periapical healing. J Endod. 2005; (31) 863865. [ Links ]
27. Sedgley C, Nagel A., Dahlen G, Reit C, Molander A. Real-time quantitative polymerase chain reaction and culture analyses of Enterococcus faecalis in root canals, J Endod. 2006 (32)173-177. [ Links ]
28. Pinheiro ET, Gomes BPFA, Ferraz CCR, Sousa E_R., Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root filled teeth with periapical lesions. Int Endod J. 2003; 36(1):1-11. [ Links ]
29. Molander A, Reit C, Dahlen G. The antimicrobial effect of calcium hydroxide in root canals pretreated with 5% iodine potassium iodide. Dent. Traumatol. 1999; 15(5):205-9. [ Links ]
30. Siren, EK, Haapasalo MP, Ranta K, Salmi P, Kerosuo EN. Microbiological findings and clinical treatment procedures in endodontic cases selected for microbiological investigation. Int Endod J. 1997; 30(2): 91-95. [ Links ]
31. John G, Kumar KP, Gopal SS, Kumari S, Reddy BK. Enterococcus faecalis, a nightmare to endodontist: A systematic review. Afr J of Microbiol R. 2015; 9(13):898-908. [ Links ]
32. Flahaut S, Hartke A, Giard JC, Benachour A, Boutibonnes P, Auffray Y. Relationship between stress response towards bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiol Lett. 1996; 138(1):49-54. [ Links ]
33. Bhardwaj SB. Role of Enterococci faecalis in failure of Endodontic treatment. Int J Curr Microbiol App Sci. 2013; 2(8):272-7. [ Links ]
34. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg, Oral Med, Oral Path, Oral Radiol and Endod. 1998; 85(1):86-93. [ Links ]
35. Mallick R, Mohanty S, Behera S, Sarangi P, Nanda S, Satapathy SK. Enterococcus faecalis: A resistant microbe in endodontics. Int J Contemp Dent Med Rev. 2014 [ Links ]
36. Ingle JI, Simon JH, Machtou P, Bogaerts P. Outcome of endodontic treatment and re-treatment. Endodontics. 2002; (5)747-68. [ Links ]
37. Khandelwal D, Ballal NV. Recent advances in root canal sealers. Int J of Clin Dent. 2016; 9(3). [ Links ]
38. Mali R, Narangan RS, Dhamali D, Singh A, Thakur A, Patil A. Effect of placement techniques on sealing ability of root canal sealers. Int J oral Care Res. 2016; (4):201-203 [ Links ]
39. AlShwaimi E, Bogari D, Ajaj R, Al-Shahrani S, Almas K, Majeed A. In vitro antimicrobial effectiveness of root canal sealers against enterococcus faecalis: A Systematic Review. J Endod. 2016; 42(11):1588-97. [ Links ]
40. Mickel AK, Nguyen TH, Chogle S. Antimicrobial activity of endodontic sealers on Enterococcus faecalis. J Endod. 2003; 29(4):257-8. [ Links ]
41. Fuss Z, Weiss EI, Shalhav M. Antibacterial activity of calcium hydroxide-containing endodontic sealers on Enterococcus faecalis in vitro. Int Endod J. 1997; 30(6):397-402. [ Links ]
42. Poggio C, Trovati F, Ceci M, Colombo M, Pietrocola G. Antibacterial activity of different root canal sealers against Enterococcus faecalis. J Clin Exp Dent. 2017; 9(6): e743. [ Links ]
43. Parolia A, Nikolopoulou D, _im BS, Kanagasingam S. Comparison of antibacterial effectiveness between Sealapex and AH-plus sealer against Enterococcus faecalis: a systematic review of in vitro studies. G Ital Endod. 2020; 34(2). [ Links ]
44. Eldeniz AU, Erdemir A, Hadimli HH, Belli S, Erganis O. Assessment of antibacterial activity of EndoREZ. Oral Surg, Oral Med, Oral Path Oral Radiol. 2006; 102(1):119-26. [ Links ]
45. Farmakis ET, Kontakiotis EG, TselenHKotsovili A, Tsatsas VG. Comparative in vitro antibacterial activity of six root canal sealers against Enterococcus faecalis and Proteus vulgaris. J Investig Clin Dent. 2012; 3(4):271-5. [ Links ]
46. Heyder M, Kranz S, Völpel A, Pfister W, Watts DC, Jandt KD, Sigusch BW. Antibacterial effect of different root canal sealers on three bacterial species. Dent Mater. 2013; 29(5):542-9. [ Links ]
47. Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009; 35(7):1051-5. [ Links ]
48. Kapralos V, Koutroulis A, 0rstavik D, Sunde PT, Rukke HV. Antibacterial activity of endodontic sealers against planktonic bacteria and bacteria in biofilms. J Endod. 2018; 44(1):149-54. [ Links ]
49. Ruiz-Linares M, Baca P, Arias-Moliz MT, Ternero FJ, Rodríguez J, Ferrer-Luque CM. Antibacterial and antibioflim activity over time of GuttaFlow Bioseal and AH Pius. Dent Mater.J 2019; 38(5):701-6. [ Links ]
50. 50. Nawai RR, Parande M, Sehgai R, Naik A, Rao NR. A comparative evaluation of antimicrobial efficacy and flow properties for Epiphany, Guttaflow and AH-Plus sealer. Int Endod J. 2011; 44(4):307-13. [ Links ]
51. Anumula L, Kumar S, Kumar VS, Sekhar C, Krishna M, Pathapati RM, Venkata Sarath P, Vadaganadam Y, Manne RK, Mudlapudi S. An assessment of antibacterial activity of four endodontic sealers on Enterococcus faecalis by a direct contact test: an in vitro study. ISRN Dent 2012 [ Links ]
52. Huang Y, Li X, Mandal P, Wu Y, Liu L, Gui H, Liu J. The in vitro antimicrobial activities of four endodontic sealers. BMC Oral Health. 2019; 19(1):1-7. [ Links ]
53. Weiss EI, Shalhav M, Fuss Z. Assessment of antibacterial activity of endodontic sealers by a direct contact test. Dent Traumatol. 1996; 12(4):179-84. [ Links ]
54. Baer J, Maki JS. In vitro evaluation of the antimicrobial effect of three endodontic sealers mixed with amoxicillin. J Endod. 2010; 36(7):1170-3. [ Links ]
55. Siqueira Jr JF. Aetiology of root canal treatment failure: why wel-treated teeth can fail. Int Endod J. 2001; 34(1):1-0. [ Links ]
56. Love RM. Enterococcus faecalis-a mechanism for its role in endodontic failure. Int Endod J 2001; 34(5):399-405. [ Links ]
57. Van der Vyver PJ, Botha FS, De Wet FA. Antimicrobial efficacy of nine different root canal irrigation solutions. SADJ. 2014; 69(4):158-65. [ Links ]
58. Gandolfi MG, Siboni F, Prati C. Properties of a novel polysiloxane-guttapercha calcium silicate-bioglass-containing root canal sealer. Dent Mater. 2016; 32(5):113-26. [ Links ]
 Correspondence:
Correspondence:
Dr Suwayda Ahmed
Restorative Dentistry, Faculty of Dentistry
University of the Western Cape
Tel no.: (021)9373091
Email address: suahmed@uwc.ac.za
Author contributions:
1 . TF Mukorera: 50%
2 . S Ahmed: 35%
3 . E Mabuza: 7.5%
4 . F Kimmie-Dhansay: 7.5%














