Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.77 no.5 Johannesburg 2022
http://dx.doi.org/10.17159/2519-0105/2022/v77no5a3
RESEARCH
http://dx.doi.org/10.17159/2519-0105/2022/v77no5a3
Demography and COVID-19 Symptoms of South African Oral Health Workers in an Academic Hospital
SM NemutandaniI; Y Malele-KolisaII; E BlignautIII
ISchool of Oral Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. President, Health Professions Council of South Africa Health. ORCID: 0000-0001-73354414
IIHead of Clinical Unit, Department of Community Dentistry, Wits School of Oral Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. ORCID: 0000-0003-3368-9193
IIIDepartment of Oral Biological Sciences, Wits School of Oral Health Sciences; 7 York Road, Parktown, 2193, Johannesburg. ORCID: 0000-0002-9937-9844
ABSTRACT
INTRODUCTION: Oral health care workers constitute a high-risk profession to contract COVID-19. The aim of this study was to determine the prevalence and clinical experience of COVID-19 infected oral health workers at an academic hospital in Gauteng, South Africa
METHODS: A cross-sectional, questionnaire-based study was conducted among staff who contracted COVID-19 between May and December 2020. Data was captured in Excel and analyzed with Stata (StataCorp, USA
RESULTS: COVID-19 prevalence among 219 members of staff was 22.4%, and 46 participated. The majority ranged in age between 31- 40 years (n=18, 39%, 95% CI 25.78-54.32) and 41 - 50 years (n=19, 41%, 95% CI 7.88-56.4). Clinicians and dental assistants constituted 48%, while 76% perceived to be infected at work, with 72.7% sharing an office with > 3 persons. Twenty-four staff members received post-test counselling, of whom 21.7% were counselled at work. Sixteen participants remained asymptomatic while most prevalent self-reported COVID-19 symptoms included cough (47.7%), sore throat (27.3%) and shortness of breath (20.5%). Significantly more females (55%) reported no COVID-19 symptoms than males (Chi2 test, p = 0.01
CONCLUSION: The COVID-19 prevalence in this study was much higher than previously reported for oral health workers in an academic setting. The high percentage of staff who remained asymptomatic raises the possibility of more staff being infected without being tested. Infection prevention and awareness training of all staff should be routinely provided and mitigating measures instituted to reduce office occupancy, including adequate post-test counselling
INTRODUCTION
The COVID-19 pandemic continues to spread at an alarming and unrelenting rate across the globe and at the time of writing on 11 May 2021 more than 159 665 632 people have been infected and more than 3,3 million have succumbed to the infection world-wide1. South Africa has not been spared the ravages and to date recorded more than 1,6 million infections and 54 825 deaths. The country ranks 20th out of 222 countries where the disease has been recorded and is the country with the highest number of infections and deaths on the African continent1. The country has been placed under various levels of lockdown since a national state of disaster and initial complete lockdown (Level 5) was declared on 15 and 23 March 2020 respectively2-4.
Health care workers (HCWs) and support staff constitute members of the essential workforce, working to contain the spread of the SARS-CoV-2 virus and managing COVID-19 patients. They are vital resources for every country and their health and safety are crucial, not only for continuous and safe patient care but also for the smooth running of health care systems5,6. Staff shortages occurred, either directly due to staff being infected by the SARS-CoV-2 virus or indirectly because of compulsory quarantine due to high risk exposures7. Additionally, some HCWs in Africa themselves fall into the category of 'high-risk' groups for COVID-19, given the high rates of certain communicable diseases such as tuberculosis and HIV, and non-communicable conditions like hypertension and diabetes7-10.
Despite healthcare workers accepting an increased risk of infection as part of their chosen profession, caring for patients with COVID-19 nevertheless causes considerable mental stress, resulting in high levels of anxiety and post-traumatic stress disorders11-12. This is exacerbated by anxiety about spreading the virus to their families and friends, especially those who are elderly or have chronic medical conditions13-15. The rapid onset of the pandemic caught everyone off guard and there was little time to sufficiently prepare staff with the result that in the initial stages of the pandemic the lack of a clear understanding about SARS-CoV-2 transmission and disease also contributed to anxiety16. In addition, some HCWs feared returning to work following recovery from the infection. This further highlights the need for psychological support in the workplace to deal with the many concerns17,18.
The risk of HCWs being infected can be mitigated with adequate precautions within health facilities19,20. Primarily, this involves training and the use of personal protective equipment (PPE), including a gown, gloves, face mask and a face shield or goggles. Careful donning and doffing of this equipment constitute a key defence and requires supervision. It was anticipated that the risk of infection would be the highest at the beginning of the outbreak when healthcare workers may not have been familiar with PPE use. PPE was also in short supply, even in high-income countries, and it was to be expected that limited supplies thereof would be experienced in lesser resourced countries19-21. The lack or a shortage of good quality PPE can also contribute to anxiety22. From a moral perspective it was hoped that these scarce PPE resources would be appropriately used and distributed equitably across the globe - yet hoarding, misuse, intense competition between and within countries, price gouging, export blocks and corruption in the acquisition thereof became the norm19,23-26.
Reports vary on the prevalence of infection with the SARS-Cov-2 virus among hospital staff27,28. It was reported in August 2020 that 27,360 South African hospital workers, which included doctors, nurses, porters and other hospital staff, had contracted COVID-19 since the start of the outbreak29. Health professionals who work in close physical proximity to patients are considered to be at a higher risk of SARS-CoV-2 infection7,30,31, with oral health professionals being among those32,33. Studies reporting on the rate of infection among dental professionals revealed a prevalence of 0.9% in the United States of America, 1.9% in France and 10.8% in Italy34-36. A study on infection among staff in an Argentine dental training facility reported a prevalence of 4%37. No published information exists on the prevalence of COVID-19 infection among South African, and to our knowledge, African oral health workers (OHWs). To effectively support OHWs, this study was conducted to gain insights into the demographic profile and clinical experience of OHWs at one of the largest public hospitals in the country, namely the Charlotte Maxeke hospital, Johannesburg, Gauteng Province, in which the Wits Oral Health Centre (WOHC) is located.
METHODS
A cross-sectional, analytic study was conducted, utilising a hand delivered, self-administered questionnaire among the OHWs employed at the WOHC who tested positive for COVID-19 between 1 May and 31 December 2020. Participation was voluntary, with anonymity assured in the reporting of results. The study received ethical clearance from the Human Research Ethics Committee of the Faculty of Health Sciences, University of Witwatersrand (M2010114). Testing was performed by real-time reverse transcription-PCR (rRT-PCR) testing on a naso-pharyngeal swab. Demographic data such as age, marital status, job category, mode of transport to and from work and isolation were collected. Other questions pertained to training in the use and availability of PPE, testing for the virus and posttest counselling, as well as comorbidities and COVID-19 symptoms experienced. Data was captured in Microsoft Excel, coded and imported into Stata (StataCorp, USA) for analysis.
RESULTS
Socio-demographic characteristics
Forty-nine members (22.4%) of a total staff establishment of 219 at the WOHC were infected with the SARS-CoV -2 virus between 1 May and 31 December 2020 according to data obtained from the Health and Safety Committee of the hospital. Of those, 46 consented to be part of the study, yielding a 94% response rate and included clinicians, human resources staff, procurement and support staff.
From Table I it is evident that approximately 80% of staff members who participated were in the 31 to 50 year age group. Respondents who were directly involved in clinical service rendering included dentists, dental assistants, oral hygienists, dental therapists, registrars and constituted 47,8% of infected staff. Support staff, (26%) included cleaners who worked in areas where clinical services were rendered, with financial and human resource administrators comprising an equal proportion (26.1%).
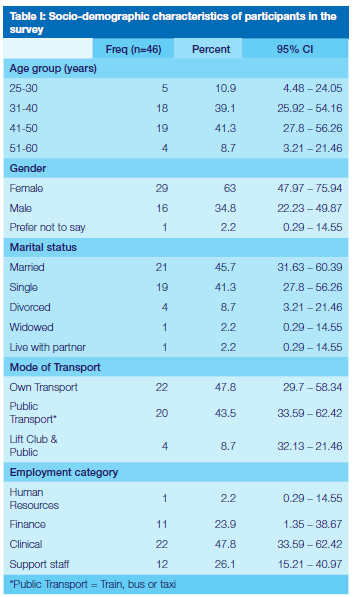
Many participants (76.1%) believed that they contracted the virus at work (Table II) and more than half reported sharing an office with 5 or more colleagues. Only 24 (52.2%) reported to have received post-test counselling, of whom 58% were counselled by someone not connected to the workplace. Most staff members (72%) were tested for COVID-19 at a government health facility and the method of testing was a nasopharyngeal swab and subsequent RT-PCR.
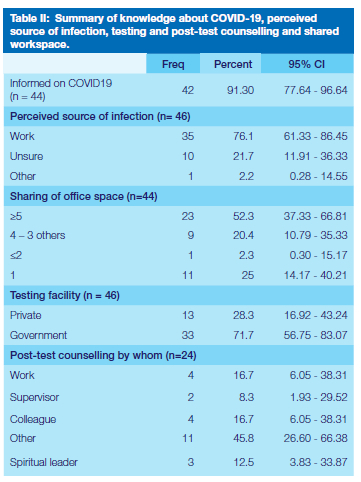
Testing for COVID-19
Figure I depicts the number of participants testing positive for COVID-19 during the 8 months of the study, with the highest number of staff (37%) testing positive during July. This constituted a window period after the first hard lockdown (Level 5) in South Africa was lifted and another level 4 lockdown introduced shortly thereafter38.
Personal protective equipment
Ninety one percent of staff reported having received training on the use of PPE. Members of staff directly involved in patient treatment were dentists, dental specialists, dental therapists, oral hygienists and dental assistants, including cleaners/support staff working in the clinical area. PPE that was available to clinical staff included N95 mask (intermittently), plastic aprons, surgical masks, surgical gown, face shield and or goggles and shoe covers. All participants who worked in clinical areas reported having access to all aforementioned PPE, except N95 masks which at times were in short supply. Administrative staff had access to surgical masks which were issued in the workplace or otherwise their own cloth masks. Hand and surface disinfectants were also freely available in the workplace.
Comorbidities and symptoms experienced
A high percentage (68.8%) of staff were healthy, with no underlying chronic illnesses. There was no statistically significant association between individuals who reported existing co-morbidities and the number of symptoms experienced before and during COVID-19 infection.
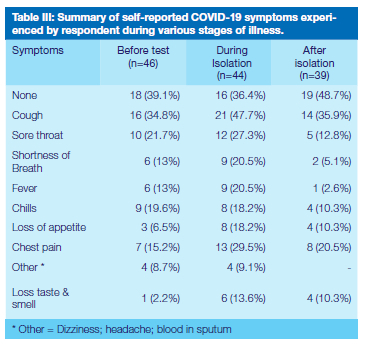
Self-reported symptoms are summarised in Table III with respondents who reported not experiencing any symptoms before testing, during isolation and after isolation being 18 (39.1%), 16 (36.4%) and 19 (48.7%) respectively. Significantly more females reported not to have experienced any or few symptoms during isolation than their male counterparts (Pearson's chi2 p=0.01). Cough (34.8%) and sore throat (21.7%) were the most common symptoms reported before testing and during isolation, however, 14 (35.9%) participants reported cough and lingering chest pain (20.5%) after isolation. Eleven respondents (23.9%) reported loss of taste and smell at various stages of the infection, of which one experienced it as the only symptom before testing and during and after isolation.
DISCUSSION
The current study evaluating the profile and clinical experiences 46 OHWs infected with SARS-CoV-2 virus at the WOHC, Johannesburg, is to our knowledge the first published information on OHWs in South Africa and Africa. The African continent, like other countries in the developing world, were expected to be particularly hard hit by the COVID-19 pandemic, citing the numerous socioeconomic impediments, including inadequate health care resource, as well as the short supply of PPE5,16,32. The WOHC is situated in the Charlotte Maxeke hospital which is a large tertiary hospital in the centre of Johannesburg, the largest city in the country. Dentistry is regarded as one of the three professions with the highest occupational risk of infection32,33. Patients treated at government health facilities are mostly from the low socio-economic sector of the population and who are at greater risk of being infected and thus pose an increased risk of infection to staff employed in government hospitals and clinics.
The method of testing utilised during the time that study participants were identified with COVID-19 was rRT-PCR, and laborious as it is, it was the only method employed at the time with no rapid diagnostic tests available. The infection prevalence of 22.4% among staff in this study is 5 times higher compared to the 4% reported for oral health workers at an academic institution in Argentina37, a country that is 11th on the global list of COVID-19 infections and that has 70 600 per 1 million infections compared to South Africa with 26 700 per 1 million of the general population1.
Seventy six percent of staff in this study believed that they contracted the infection at work27,28. During a pandemic it is not always possible to determine the exact source of infection and infected colleagues, community exposure and the workplace may pose an equal risk to become infected39,40. In addition, approximately 50% of respondents make use of some form of public transport, e.g. train, bus or taxi, which are notoriously overcrowded41-43. With 73% of staff sharing office space with 3 or more others, social distancing requirements cannot be met and warrants the implementation of mitigating measures to reduce office occupancy and improve ventilation. This exceptionally high prevalence of COVID-19 is nevertheless of concern and all aspects of infection prevention should receive serious consideration.
The vast majority of those infected were in the 31 - 50 year age bracket (80%), with females constituting (63%) of infected staff, which corresponds with the gender (females 60%) and age (32 year mean age) related findings of other studies44,45, however, this finding might be related to the staff composition at the WOHC which is mostly female. The high number of staff who reported no existing comorbidities (69%) is similar to a study comparing HCWs and non-HCWs in terms of severity of disease which reported HCWs to be more healthy and less likely to be hospitalised and had less severe symptoms46,47. The proportion of staff (31.2%) reporting co-morbidities correlates with reported comorbidities among the general South African population, namely diabetes and or high blood pressure48,49.
Reported numbers of HCWs who tested positive for COVID-19 but remained asymptomatic vary considerably (1.3 to 50%)50,51, with the 40% of staff members in our study who remained asymptomatic falling within this wide range. Asymptomatic participants in this study chose to be tested based on a perceived high risk of exposure either to infected patients or co-workers. The possibility exists that the prevalence of infected but asymptomatic workers could be higher with staff who experienced no symptoms not going for testing. The danger of cross-infection through asymptomatic health care workers raises the question of regular testing of clinical staff, particularly in a high-risk profession such as dentistry where patients, clinicians and dental assistants all work in close proximity and despite avoiding elective procedures, aerosol generating procedures cannot be completely excluded. In an academic or training institution students are also present in this relatively small space, further increasing the risk of cross-infection44,52.
The most frequently self-reported COVID-19 symptoms during isolation were cough (47.7%) and sore throat (27.3%), with coughing reported by 35.9% of participants to have lingered after isolation. The prevalence of symptoms reported in the literature covers a wide range and this may be due to some being self-reported, as in this study, while in other instances they were actual diagnoses by health professionals53,54. Only 13.6% of participants reported loss of taste and smell during isolation, and again, the published prevalence thereof varies widely, from as low as 4% to 83.9%55,56.
While infected staff hailed from all categories of employment, less than half (47.8%) were directly involved in clinical service rendering, which corresponds with other studies57. Except for N95 masks that were intermittently available, clinical staff reported to have had access to all recommended PPE33. The 52% of infected staff who were not directly involved in clinical service rendering, namely cleaners in the clinical areas and administrative staff, necessitate that all categories of employees be included in regular training on COVID-19 prevention. Although there can be no certainty as to the source of infection, similar to what is suggested in other studies, the actual source of infection among workers in a health care facility may well be through exposure to asymptomatic colleagues39.
The high number of staff who tested positive for the virus in July 2020, coincided with a brief period when stringent lockdown measures were lifted and various restrictions on movement and social gathering again being instituted38. Unpublished, routinely collected patient treatment data showed that patients refrained from attending hospitals and clinics during Level 5 lockdown and returned for treatment with the easing of restriction. This may correlate with the high number of staff becoming infected during July when higher numbers of patients again visited dental facilities to seek emergency dental treatment.
Working in a public hospital, it was convenient for the staff who suspected they were infected with SARS-CoV-2 virus to be tested there (72%). Another important finding of this investigation is that, although most participants reported to have been informed on COVID-19, more than half did not receive counselling after testing positive for COVID-19. In light of the widely published negative psychological impact of the pandemic on health care workers58,59, it is important that counselling be made available in the workplace for all staff who may be in need thereof.
CONCLUSION
The exceptionally high prevalence of OHWs in this study who tested positive across the various categories of employment, emphasises the importance that OHWs be included when hospitals report COVID-19 positivity among health care staff. Another important aspect highlighted by this study is that almost equal numbers of clinical and administrative staff tested positive for COVID-19, which necessitates the inclusion of all categories of staff in training and awareness campaigns on infection prevention. Equally important is that measures be instituted to reduce the infection risk in overcrowded office/working spaces. While the report indicated adequate access to PPE for those directly involved in clinical service rendering, the high percentage of staff who remained asymptomatic warrant consideration of regular rapid screening of clinical staff. In light of the significant psychological burden that the pandemic places on health care workers, it is important that all workers who might require counselling service have access to it.
Acknowledgment
The contribution of all members of staff of the Wits Oral Health Center is gratefully acknowledged. Specifically the facilitation of Matron Sr Mquqo and Ms S Hassan during data collection stage is greatly acknowledged.
The authors report no conflict of interest and this research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author contributions:
1. SM Nemutandani: 30%
2. Y Malele-Kolisa: 40%
3. E Blignaut: 30%
REFERENCES
1. Worldodometer. COVID-19 CORONAVIRUS OUTBREAK. 2020. [cited 2021 May 22] Available from: https://www.worldometers.info/coronavirus [ Links ]
2. South African Government News Agency. President Ramaphosa announces a nationwide lockdown. 2020. [cited 2020 June 21] Available from: https://www.sanews.gov.za/south-africa/president-ramaphosa-announces-nationwide-lockdown/ [ Links ]
3. South African Government. DISASTER MANAGEMENT ACT, 2002 DECLARATION OF A NATIONAL STATE OF DISASTER. Pretoria: Government Printer, Pretoria; 2020. [cited 2020 June 20] Available from: http://www.gpwonline.co.za/Gazettes/Gazettes/43096_15-3_CoOperativeGovTradAff.pdf [ Links ]
4. Hatefi S, Smith F, Abou-El-Hossein K, et al. COVID-19 in South Africa: lockdown strategy and its effects on public health and other contagious diseases. Public Health 2020;185:159-60. [ Links ]
5. Chersich MF, Gray G, Fairlie L, et al. COVID-19 in Africa: care and protection for frontline healthcare workers. Global Health 2020;16:46. [ Links ]
6. Chang D, Xu HJ, Rebaza A,et al. Protecting healthcare workers from subclinical coronavirus infection. Lancet Respir Med. 2020. doi: 10.1016/ S2213-2600(20)30066-7 [ Links ]
7. Iacobucci G. Covid-19: GP surgeries close for two weeks after staff test positive. BMJ 2020;368:m936. [ Links ]
8. Soriano V, Barreiro P. Impact of New Coronavirus Epidemics on HIV-Infected Patients. AIDS Rev 2020;22:57-8. [ Links ]
9. Zhu F, Cao Y, Xu S, Zhou M.et al. Co-infection of SARS-CoV-2 and HIV in a patient in Wuhan city, China. J Med Virol 2020;92:529-30. [ Links ]
10. Grobler L, Mehtar S, Dheda K, et al. The epidemiology of tuberculosis in health care workers in South Africa: a systematic review. BMC Health Serv Res 2016;16:416. [ Links ]
11. Huang Z, Zhuang D, Xiong B, et al. Occupational exposure to SARS-CoV-2 in burns treatment during the COVID-19 epidemic: Specific diagnosis and treatment protocol. Biomed Pharmacother 2020;127:110176. [ Links ]
12. Kang L, Li Y, Hu S, et al. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry 2020;7:e14. [ Links ]
13. Rose C. Am I Part of the Cure or Am I Part of the Disease? Keeping Coronavirus Out When a Doctor Comes Home. N Engl J Med 2020;382:1684-5. [ Links ]
14. McMahon DE, Peters GA, Ivers LC, et al. Global resource shortages during COVID-19: Bad news for low-income countries. PLoS Negl Trop Dis 2020;14:e0008412. [ Links ]
15. Xiao H, Zhang Y, Kong D, et al. The Effects of Social Support on Sleep Quality of Medical Staff Treating Patients with Coronavirus Disease 2019 (COVID-19) in January and February 2020 in China. Med Sci Monit 2020;26:e923549. [ Links ]
16. O'Sullivan ED. PPE guidance for covid-19: be honest about resource shortages. BMJ 2020;369:m1507. [ Links ]
17. Rimmer A. Covid-19: GPs call for same personal protective equipment as hospital doctors. BMJ 2020;368:m1055. [ Links ]
18. Moberly T. Chinese premier rallies medics in coronavirus fight. BMJ 2020;368:m343. [ Links ]
19. Kapata N, Ihekweazu C, Ntoumi F, et al. Is Africa prepared for tackling the COVID-19 (SARS-CoV-2) epidemic. Lessons from past outbreaks, ongoing pan-African public health efforts, and implications for the future. Int J Infect Dis 2020;93:233-6. [ Links ]
20. Nkengasong JN, Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. Lancet 2020;395:841-2. [ Links ]
21. Makoni M. Africa prepares for coronavirus. Lancet 2020;395:483. [ Links ]
22. Koh D. Occupational risks for COVID-19 infection. Occup Med (Lond) 2020;70:3-5. [ Links ]
23. Gilbert M, Pullano G, Pinotti F, et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet 2020;395:871-7. [ Links ]
24. Phan LT, Maita D, Mortiz DC, et al. Personal protective equipment dotting practices of healthcare workers. J Occup Environ Hyg 2019;16:575-81. [ Links ]
25. Mahase E. Covid-19: hoarding and misuse of protective gear is jeopardising the response, WHO warns. BMJ 2020;368:m869. [ Links ]
26. Phagane T. 2020 Headlines: Corruption in procurement of PPE. SABC News; 2020. [cited 2021 April 20] Available from:https://www.sabcnews.com/sabcnews/2020-headlines-corruption-in-procurement-of-ppe/ [ Links ]
27. Kluytmans-van den Bergh MFQ, Buiting AGM, Pas SD, et al. Prevalence and Clinical Presentation of Health CareWorkers With Symptoms of Coronavirus Disease. JAMA Netw Open 2020;3(5):e209673. [ Links ]
28. Lai X, Wang M, Qin C, et al. Coronavirus Disease 2019 (COVID-2019) Infection Among Health CareWorkers and Implications for Prevention Measures in a Tertiary Hospital inWuhan, China. JAMA Netw Open 2020;3(5): e209666. [ Links ]
29. Shange N. Coronavirus infection rate among health workers in SA is 5% - below global average (timeslive.co.za). Times Live. Johannesburg, South Africa: Sunday Times; 2020. [cited 2021 February 5] Available from:https://www.timeslive.co.za/news/south-africa/2020-08-13-coronavirus-infection-rate-among-health-workers-in-sa-is-5-below-global-average [ Links ]
30. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet 2020;395:e39. [ Links ]
31. Lai THT, Tang EWH, Chau SKY, et al. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: an experience from Hong Kong. Graefes Arch Clin Exp Ophthalmol 2020. doi: 10.1001/jamanetworkopen.2020.9666 [ Links ]
32. Gamio L. The Workers Who Face the Greatest Coronavirus Risk. New York Times. New York; 2020. [cited 2020 August 15] Available from: https://www.nytimes.com/interactive/2020/03/15/business/economy/coronavirus-worker-risk.html. [ Links ]
33. Kowalski LP, Sanabria A, Ridge JA, et al. COVID-19 pandemic: Effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head Neck 2020;42:1259-67. [ Links ]
34. Estrich CG, Mikkelsen M, Morrissey R, et al. Estimating COVID-19 prevalence and infection control practices among US dentists. J Am Dent Assoc 2020;151:815-24. [ Links ]
35. Gallus S, Paroni L, Re D, et al. SARS-CoV-2 Infection among the Dental Staff from Lombardy Region, Italy. Int J Environ Res Public Health 2021;18. doi: 10.3390/ijerph18073711 [ Links ]
36. Jungo S, Moreau N, Mazevet ME, et al. Prevalence and risk indicators of first-wave COVID-19 among oral health-care workers: A French epidemiological survey. PLoS One 2021;16:e0246586. [ Links ]
37. Sebastian P, Jorge P, Ariel G, et al. Assesment of SARS-CoV-2 infection-in dentists and supporting staff at a university dental hospital in Argentina. J Oral Biol Craniofac Res 2021;11:169-73. [ Links ]
38. Turner KJ, Le Grange K, Nkgadima R. TIMELINE : 10 months of Covid-19 in SA. IOL; 2021. [cited 2021 May 21] Available from: https://www.iol.co.za/news/south-africa/western-cape/timeline-10-months-of-covid-19-in-sa-7120954d-e536-4f0e-a7b7-7883b026bada [ Links ]
39. Mandic-Rajcevic S, Masci F, Crespi E, et al. Source and symptoms of COVID-19 among hospital workers in Milan. Occup Med (Lond) 2020;70:672-9. [ Links ]
40. Talbot LR, Romeiser JL, Spitzer ED, et al. Prevalence of IgM and IgG antibodies to SARS-CoV-2 in health care workers at a tertiary care New York hospital during the Spring COVID-19 surge. Perioper Med (Lond) 2021;10:7. [ Links ]
41. Schraer R. Coronavirus: What's the risk on transport? Unite Kingdom: BBC News; 2020. [cited January 10] Available from: https://www.bbc.com/news/health-51736185 [ Links ]
42. Nakweya G. Public transport could stifle Africa's COVID-19 control. Nairobi, Kenya: SciDevNet; 2020. [Cited 2021 March 2] Available from: https://www.scidev.net/sub-saharan-africa/news/public-transport-could-stifle-africa-s-covid-19-control/ [ Links ]
43. Morawska L, Tang JW, Bahnfleth W, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int 2020;142:105832. [ Links ]
44. Alshahrani MS, Alnimr A, Alnassri S, et al. Prevalence of the SARS-CoV-2 Infection Among Post-Quarantine Healthcare Workers. J Multidiscip Healthc 2020;13:1927-36. [ Links ]
45. Abohamr SI, Aldossari MA, Alaklobi FA, et al. Clinical characteristics and in-hospital outcome of medical staffinfected with COVID-19 in Saudi Arabia. A retrospective single-center study. Saudi Med J 2020;41:1336-43. [ Links ]
46. Kim R, Nachman S, Fernandes R, et al. Comparison of COVID-19 infections among healthcare workers and non-healthcare workers. PLoS One 2020;15:e0241956. [ Links ]
47. Huete-Perez JA, Cabezas-Robelo C, Paiz-Medina L, et al. First report on prevalence of SARS-CoV-2 infection among health-care workers in Nicaragua. PLoS One 2021;16:e0246084. [ Links ]
48. Hunter-Adams J, Battersby J. Health care providers' perspectives of diet-related non-communicable disease in South Africa. BMC Public Health 2020;20:262. [ Links ]
49. van Zyl S, van Rooyen FC, Joubert G, et al. A Comparison of the Socio-Behavioral-Metabolic Risk Profiles and Associated Factors for Chronic Diseases of Lifestyle in Urban and Rural Communities in Central South Africa. Front Public Health 2020;8:570676. [ Links ]
50. Mohr NM, Harland KK, Krishnadasan A, et al. Diagnosed and Undiagnosed COVID-19 in US Emergency Department Health Care Personnel: A Cross-sectional Analysis. Ann Emerg Med 2020. doi: 10.1016/j.annemergmed.2020.12.007 [ Links ]
51. Mukhtar A, Afishawy M, Alkhatib E, et al. Asymptomatic SARS-CoV-2 infection among healthcare workers in a non-COVID-19 Teaching University Hospital. J Public Health Res 2021. doi: 10.4081/jphr.2021.2102 [ Links ]
52. Al-Qahtani AA. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Emergence, History, Basic and Clinical Aspects. Saudi J Biol Sci. 2020/04/28 edn; 2020. doi: 10.1016/j.sjbs.2020.04.033 [ Links ]
53. Gholami M, Fawad I, Shadan S, et al. COVID-19 and healthcare workers: A systematic review and metaanalysis. Int J Infect Dis 2021;104:335-46. [ Links ]
54. Nicolas D, Camos-Carreras A, Spencer F, et al. A Prospective Cohort of SARS-CoV-2-Infected Health Care Workers: Clinical Characteristics, Outcomes, and Follow-up Strategy. Open Forum Infect Dis 2021;8:ofaa592. [ Links ]
55. Kandakure VT, Valvi HR, Khokle P, et al. Prevalence and Recovery from Newly Onset Anosmia and Ageusia in Covid 19 Patients at our Teritary Care Centre. Indian J Otolaryngol Head Neck Surg 2021:1-8. [ Links ]
56. Sbrana MF, Fornazieri MA, Bruni-Cardoso A, et al. Olfactory Dysfunction in Frontline Health Care Professionals During COVID-19 Pandemic in Brazil. Front Physiol 2021;12:622987. [ Links ]
57. Persoon IF, Volgenant CMC, van der Veen MH, et al. [Impact of the coronavirus on Dutch oral health care and practice]. Ned Tijdschr Tandheelkd 2021;128:211-20.[In Dutch] [ Links ]
58. Temsah MH, Al-Sohime F, Alamro N, et al. The psychological impact of COVID-19 pandemic on health care workers in a MERS-CoV endemic country. J Infect Public Health 2020;13:877-82. [ Links ]
59. Xiao X, Zhu X, Fu S, et al. Psychological impact of healthcare workers in China during COVID-19 pneumonia epidemic: A multi-center cross-sectional survey investigation. J Affect Disord 2020;274:405-10. [ Links ]
 Correspondence:
Correspondence:
Yolanda Malele-Kolisa
Head of Clinical Unit, Department of Community Dentistry, Wits School of Oral Health Sciences, University of the Witwatersrand
Johannesburg, South Africa. E-mail: Yolanda.Kolisa@wits.ac.za














