Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.77 no.3 Johannesburg abr. 2022
http://dx.doi.org/10.17159/2519-0105/2022/v77no3a1
RESEARCH
The Induction of Bone Formation by the recombinant human transforming growth Factor-ß3: From preclinical studies in Papio ursinus to translational research in Homo sapiens
Ugo RipamontiI; Jakobus HoffmanII; Carlo FerrettiI, III
IMD, DDS, MDent, MFS, PhD (Med), FBSE, Bone Research Laboratory, School of Clinical Medicine - Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. ORCID Number: 0000-0002-6567-3594
IIMEng, South African Nuclear Energy Corporation (NECSA), P O Box 582, Pelindaba, Pretoria 0001, South Africa
IIIBDS, MDent, FCD(SA) MFOS, Bone Research Laboratory, School of Clinical Medicine - Internal Medicine, University of the Witwatersrand, Johannesburg, and 3Department of Maxillofacial Surgery, University of Pretoria, Pretoria, South Africa
ABSTRACT
AIM AND OBJECTIVES: Skeletal bone defects of the axial or the craniomaxillofacial skeletons still present formidable challenges to skeletal reconstructionists, tissue biologists and modern medicine. In systematic research experiments in the Chacma baboon Papio ursinus our laboratories have shown the previously unreported osteoinductive activity of the three mammalian transforming growth factor-ß (TGF-β) isoforms. This review discusses the induction of bone formation by the mammalian TGF-ßs with particular reference to the substantial and rapid induction of bone by the recombinant hTGF-ß3 from the laboratory benches, to pre-clinical studies in heterotopic and orthotopic mandibular sites of Papio ursinus to clinical translation in human patients.
DESIGN AND METHODS: A series of systematic research experiments in Papio ursinus using the hTGF-ß3 together with earlier experiments using the -ß1 and ß2 isoforms are reviewed and re-analyzed molecularly and morphologically to provide the basic research data for the reported clinical translation in human patients.
RESULTS: The three mammalian hTGF-ß isoforms and notably hTGF-ß3 induce rapid and substantial induction of heterotopic bone in intramuscular sites of Papio ursinus. Relatively low doses of hTGF-ß1 or hTGF-ß3 in binary application with hBMP-7 synergize to induce massive corticalized ossicles in the rectus abdominis muscle. In orthotopic mandibular sites, 125 and 250 doses of hTGF-ß3 induce bone formation across large mandibular defects in Papio ursinus with corticalized buccal and lingual plates by day 30, with modeling and maintenance of corticalized bone by 9 to 12 months after implantation of the 250 dose in 3 cm mandibular defects Papio ursinus
DISCUSSION: hTGF-ß3 significantly up-regulates RUNX-2 and Osteocalcin expression on day 15 controlling the differentiation of progenitor stem cells into the osteoblastic lineage. The induction of bone by the hTGF-ß3 is via the bone morphogenetic proteins pathway; hTGF-ß3 controls the induction of bone by regulating the expression of BMPs gene and gene products via Noggin expression, eliciting bone induction by up-regulating exogenous BMPs
Key words: Bone induction, bone morphogenetic proteins, transforming growth factors-ß proteins, transforming growth factors-ß3, redundancy, primates, human osteoinduction, inhibitors, translational clinical research
INTRODUCTION
In a previous communication to Frontiers1 addressing the regenerative frontiers of craniofacial reconstruction using the mammalian transforming growth factor-ß (hTGF-ß) isoforms, we bluntly addressed the grand challenges still facing cranio-maxillofacial and mandibular reconstruction in human patients. We would like to copy verbatim what we have then stated; many years later the surgical perspectives of mandibular regeneration in human patients have not yet regretfully changed:
"Restoring anatomical function of complex disfiguring craniofacial defects and anomalies remains a grand unsolved challenge. Those of us who have not suffered the outrage of facial deformity visited upon patients either as developmental misfortune or as the scourge of disease or violence can only imagine the effects thereof. Loss of facial features not only denies patients the most basic human functions but also rob them of a sense of identity with all associated mental anguish".1
The conundrum of regenerating large mandibular defects in clinical contexts remains a grand challenge in craniofacial tissue regeneration.2 This is in spite of the surgical advances together with outstanding discoveries in molecular, cellular and tissue biology. These fundamental developmental molecular and cell biology studies have significantly increased our molecular understandings of bone formation by induction in primates. This occurred after the explosion of mechanistic molecular studies at the end of last Century. Indeed, molecular biology techniques resolved the intimate knowledge of the cell and its several interactions with the surrounding extracellular matrix (ECM),3 including the cellular/ECM communications and the interactions of the ECM with single cellular topographical microenvironments. This novel information were proposed to be used to finely tune and control the induction of postnatal tissue morphogenesis.
The molecular dissection of the extracellular matrix of bone has Anally yielded the isolation of the osteogenic proteins of the transforming growth factor-ß (TGF-β) supergene family.4 Purification to homogeneity of crude extracts of demineralized bone matrices allowed amino acid sequence information of proteins chaotropically extracted with guanidinium-HCL.5,6 Molecular cloning followed, reporting the human recombinant proteins belonging to an entirely new family of proteins, the bone morphogenetic proteins (BMPs), members of the TGF-β supergene family.7-11
The later cloned recombinant human BMPs (hBMPs) were soon tested in a variety of animal models; these also included non-human primates' species for both appendicular and craniofacial skeletal regeneration.11-17 Experiments in pre-clinical surgical models proposed that the newly characterized and cloned molecular signals would regenerate bone across the skeleton, including cranio-mandibulo-facial reconstructions in human patients. This review on the osteoinductive capacity of hTGF-ßs in primates, from the Chacma baboon Papio ursinus to the human primate Homo sapiens describes with some details the biological significance of apparent redundancy of molecular signals endowed with the unique capacity to initiate bone formation in heterotopic extraskeletal sites,18 where there is no bone.16,17,19 All preclinical research experiments described in this manuscript have been approved by the Animal Research Ethics Committee (AREC) of the University, from the studies on the Selachian's' fishes Carcharinus obscurus to several experiments in the Chacma baboon Papio ursinus. The AREC no. and title of the study under which the experiment covers the implantation of the 250 dose of hTGF-ß3 in large full-thickness 3 cm mandibular defects in Papio ursinus is under waiver 201811-19-0 Translational approaches for bone constructs: their impact on facial bone reconstruction. The study in Papio ursinus was partially supported by Project No. AOCMF-19-03-R of AOCMF, Switzerland. Translational clinical research in human subjects was approved by the Human Research Ethics Committee (Medical) clearance certificated M170597 of the University of the Witwatersrand, Johannesburg.
Until 1993,20 either naturally-extracted and purified BMPs or hBMPs were the only described signals endowed with the unique prerogative to induce bone formation when implanted in heterotopic extraskeletal sites, i.e. subcutaneously, intramuscularly as well as after intra-parenchymatous implantation.11,19,21
The work of Sampath et al. 20 reported a comparatively high level of homology in decapentaplegic (dpp) and 60A genes in Drosophila melanogaster with BMP-2, BMP-4, and BMP-5 and BMP-6, respectively. The study highlighted the critical and developmental role of BMPs' amino acid sequence motifs for the evolutionary induction of the vertebrates.4,20 Drosophila and human secreted proteins retained and thus shared common developmental roles. Indeed, gene products of the fruit fly and Homo have been evolutionary conserved for a billion years and as such, recombinant human DPP and 60A proteins, when reconstituted with insoluble inactive collagenous bone matrix of chaotropically extracted rat bone, initiate the induction of bone in the subcutaneous rodent bioassay.20
These experiments have shown that phylogenetically ancestral signaling amino-acid motifs deployed in the fruit fly Drosophila melanogaster for dorso-ventral patterning are also operational to initiate the unique vertebrate trait of bone induction and development. The induction of bone crystallized the emergence of the skeleton, the vertebrate animals, the bipedal ancient hominids, the Australopithecines, speciation of Homo habilis and Homo erectus in Central and Southern Africa, soon followed by the explosion of the human clade across the planet.22-24
We reported that Nature has had a lesson to teach: 4,25,26 "Instead of evolving genes and gene products capable of initiating the induction of bone formation, Nature has rather usurped and recruited phylogenetically ancient gene products operating minor modifications in amino acid sequence motifs in the carboxy-terminal domains deployed for dorso-ventral patterning in the fruit fly to molecularly initiate the induction of bone formation, skeletogenesis, and the emergence of the vertebrates".21,23,24
Pleiotropy and Redundancy
Systematic research experiments in Papio ursinus showed that the induction of bone formation is not restricted to naturally derived or recombinantly produced hBMPs but extend to homologous yet molecularly different members of the TGF-β family.4,11 The three mammalian TGF-ß proteins induce substantial endochondral bone formation when implanted in intramuscular heterotopic sites of Papio ursinus. 4,19,24-27
In previous communications, we have asked the critical questions: "Which are the molecular signals that control the biological significance of apparent redundancy initiating the induction of bone formation?" 4,11,16,17 In marked contrast to rodents, lagomorphs and canine, the three mammalian TGF-β proteins initiate the substantial and rapid induction of bone formation in non-human primates. 4,19,26,27 The need for alternative to hBMPs to regenerate bone in man is now critical, after the published complications and performance failures of hBMP-2 and hOP-1, the latter protein also known as BMP-7. 17,28-30
At long last and finally so, the biotechnology industry has acknowledged that treatments by recombinant hBMPs require supra-physiological BMP concentrations, which are "associated with potential local and systemic adverse effects".31 To address the problem of high supra-physiological doses of the recombinant hBMPs to induce sub-optimal amounts of bone formation in humans, and thus to engineer and improve efficacy, a BMP/activing A chimera was constructed which showed superior activity to native BMPs at less concentrations than the currently FDA approved hBMP-2/ACS orthotopic device.31 It is mandatory to again quote a previous statement that "Reviews and perspectives on bone tissue engineering for alternative to hBMPs to regenerate bone in man is now critical, after the published complications and performance failures of hBMP-2 and hOP-1, the latter protein also known as BMP-7.17,28-30
As we have previously stated, "the conundrum of regenerative medicine and tissue engineering has been a newly developed research program which later morphed into the hyperbole of promised regenerative treatments based on published data in pre-clinical animal models, without any experimental evidence of translational research in clinical contexts".17
It is mandatory to again quote a previous statement that "Reviews and perspectives on bone tissue engineering report a series of successful novel procedures in animal models with the promise that the results obtained both in vitro and in vivo will eventually result in substantial differences in acute and chronic human disorders including but not limited to, myocardial infarction following transplantation of functional contractile myoblastic cells, liver, pancreas and kidney failure following transplantation of bioactive hepatocytes, healthy grown pancreatic islets as well as supra-assembling kidney tubular structures with filtering cells".32
Realistically however, none of the highlighted procedures above is actually routinely used in clinical contexts.33 Furthermore, "merely hypothesized yet published advanced in tissue engineering, have been published even in the awareness that the need of such functionalities is largely not substantiated by experimental data".34
The expression cloning of the BMPs, members of the transforming growth factor-ß (TGF-ß) supergene family 4,7-9,11 has however failed the translational research of the "Bone induction principle" 49 in clinical contexts.17 The next three decades of therapeutic use of single recombinant hBMPs, either hBMP-27,8 or hOP-19 showed that the translation of pre-clinical results to humans was all too unpredictable, often resulting in failure of bone regeneration.2 The observed limited translation of highly encouraging pre-clinical results to human osteoinduction indicates that must exist profound molecular differences regulating the induction of bone formation between not only genera but also species, including non-human vs. human primates. 2,11,17,21,63
Our current studies on the initiation of bone formation by the three mammalian hTGF-ßs has partly cast some insights into the induction of bone in primates vs. rodents, lagomorphs and canines. In the latter species, and for that matter in any other species but in non-human primates, and thus by extension to human primates, the three hTGF-ß isoforms fail to induce bone formation in heterotopic extraskeletal sites. 16,17,19,24 Systematic experiments in intramuscular sites of Papio ursinus by qRT-PCR have shown that the observed induction of bone is via several profiled bone morphogenetic proteins genes expressed upon the intramuscular implantation of doses of hTGF-ß3.17,35,36 The downstream expression of BMPs genes may escape the antagonist activity of Noggin, whereas on the other hand, direct implantation of high doses of recombinant hBMPs (several tens of mg) activate the Noggin antagonist pathway, limiting human osteoinduction in clinical contexts.17,35,36 Physiological expression of BMPs genes and gene products upon implantation of the hTGF-ß3 osteogenic device escapes the antagonist activity of Noggin, ultimately regulating the bone induction cascade.17,25,35,36
Experiments on day 1 535,36 showed RUNX-2 and Osteocalcin expression corresponding to the observed rapid induction of bone as seen morphologically on undecalcified histological sections. RUNX-2 was decreased in hNoggin pre-loaded macroporous bioreactors. RUNX-2 showed increased expression in hTGF-ß3/pre-loaded macroporous bioreactors, once again correlating to the induction of bone formation on day 30 after heterotopic intramuscular implantation of the super-activated macroporous bioreactors. 35,36
The substantial induction of bone formation by hTGF-ß3 in Papio ursinus shows TGF-βr TGF-e3 (but not TGF-6r), BMP-2, BMP-3, OP-1, RUNX-2 and Osteocalcin upregulation and expression. Morphologically, the reported genes' expression above is represented by pronounced osteoblastic with osteoid deposition together with marked capillary sprouting and angiogenesis. The significant rapid initiation of bone by 250 hTGF-ß3 when reconstituted with calcium phosphate-based bioreactors is the frontier for the novel molecular and morphological induction of bone formation in man.24
The use of a single recombinant hBMP delivered by collagenous substrata or similar organic matrices has failed to match autogenous bone grafts in human osteoinduction.2,17,31,35-38 A critical reappraisal of future osteoinductive strategies in human is required.17,29,38 Using the autologous bone graft as a sensible biological blueprint, molecular biologist and tissue engineers alike need now to resolve the molecular insights into the multiple molecular machinery that give the capacity to regenerate bone post transplantation of autogenous bone grafts (Fig. 1), and, at the same time, to critically re-appraise human osteoinduction.
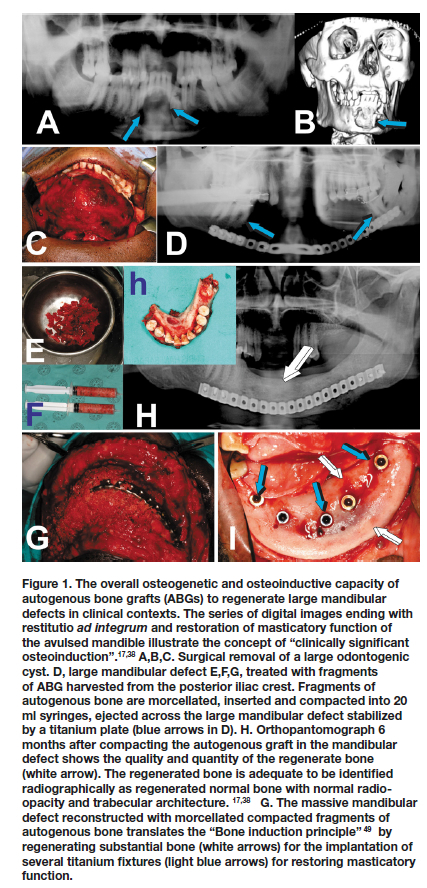
Craniofacial and mandibular regeneration in human patients using far too high doses of hBMPs has been the most severe operational and biological limitations of biotech companies' manufacturing recombinant hBMPs, i.e. hBMP-2 and hOP-1. We have also stated at few International Conferences of BMPs, that hBMPs treated human mandibular defects do not show often convincingly the induction of bone regeneration, with corticalization and remodeling of the newly formed ossicles. 39-41 Our Unit has highlighted the concept of "clinically significant osteoinduction", i.e. "the quality and quantity of regenerated bone adequate to be identified radiographically as normal bone, both in radio-opacity and trabecular architecture" (Fig. 1).17,38
The synergistic induction of bone formation or the induction of bone by single relatively high doses of hTGF-ß3 have shown that the recombinant morphogen induces bone following the expression of a variety of inductive morphogenetic proteins that result in the rapid induction of bone formation.24,37,42 Our molecular data thus show that bone induction as invocated by hTGF-ß3 recapitulates the synergistic induction of bone formation by low doses of hTGF-ß1 and hTGF-ß3 with a recombinant hBMP with a ratio by weight of 1:20.24,26,37
Molecularly, the synergistic induction of bone formation by binary applications of hOP-1 with hTGF-ß1 and hTGF-ß3, and particularly by hTGF-ß3 solo follows the up-regulation of Osteocalcin, RUNX-2, BMP-7, TGF-61 and hTGF-63.37
hTGF-ß3 generates multicellular bone organoids with the rapid induction of mineralized bone and osteoid covered by contiguous osteoblasts when implanted in heterotopic sites of the rectus abdominis muscle of Papio ursinus (Fig. 2).19,37,43 The morphological hallmarks of the synergistic induction of bone formation is the rapid induction of osteoid seams facing haemopoietic bone marrow that forms as early as day 15 after implantation in rectus abdominis sites.4,26,37,42
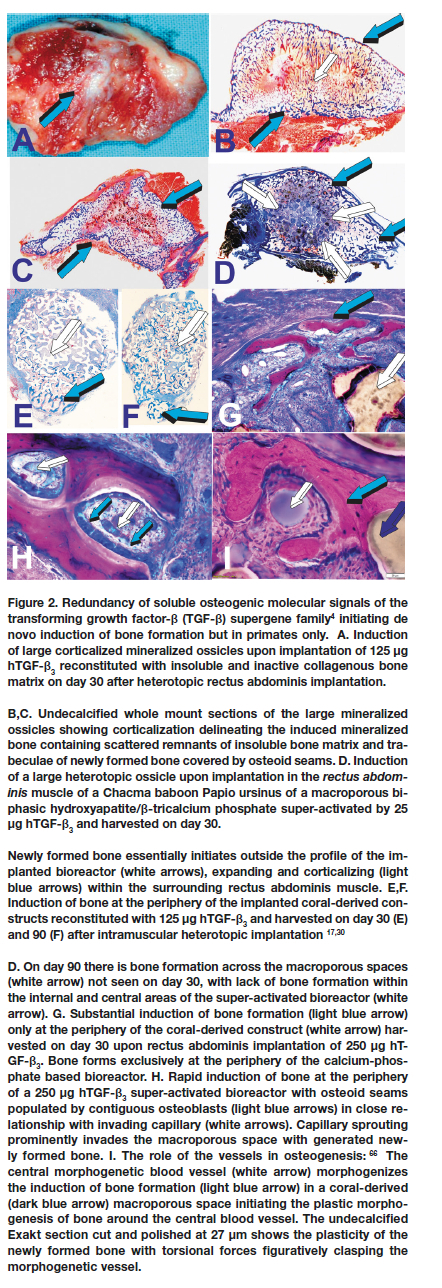
It is noteworthy that synergistic binary applications induce the morphogenesis of rudimentary embryonic growth plates, indicating that the "memory" of developmental events in embryo is re-deployed post-natally by the application of morphogen combinations.4,26,37
Importantly, our systematic studies on the hTGF-ß3 in Papio ursinus have shown that tissue induction and morphogenesis invocated by 250 of hTGF-ß3 solo if often higher than the synergistic induction of bone formation as shown by binary application of hTGF-ß3 with recombinant hOP-1.24,26,27,37
Mandibular tissue induction and regeneration by recombinant human transforming growth factor-ß3: Short-term morphological studies using 125 μg hTGF-ß3 solo and in synergistic binary application with hBMP-7 in the Chacma baboon Papio ursinus. The substantial induction of bone formation by the hTGF-ß3 isoform singly or in binary application with a recombinant human bone morphogenetic protein (hBMP-7)24,26,37,42 prompted us to design experiments to test the regenerative capacity of hTGF-ß3 in full-thickness segmental defects prepared in the Chacma baboon Papio ursinus.44
After extra-oral approach, the previously edentulized mandibles were exposed, and a 2.5 cm full thickness defect was prepared in each right mandible of three Chacma baboons Papio ursinus.44 Defects were stabilized with a titanium plate anchored on the distal and mesial remaining mandibular bone (Fig. 3). Defects were implanted with 125 hTGF-ß3 reconstituted with allogeneic insoluble collagenous bone matrix (ICBM). Animals were euthanized 30 days after mandibular implantation.Harvested tissues and prepared hemi-mandibles showed mineralization across the treated defects with regeneration of the buccal plates on day 30 with restoration of the mandibular profile (Fig. 3).44 Histological analyses on undecalcifled histological analyses (Figure 3, insets G, H) show the induction of mineralized bone within the defects (G).
There was induction of corticalized mineralized formed bone surrounding remnants of matrix carrier 30 days post implantation (Fig. 3). The significant induction of bone on day 30 by hTGF-ß3 solo in mandibular defects of Papio ursinus prompted us to clinically translate the rapid induction of bone by 125 hTGF-ß3 (Fig. 4). 43-46 Osteogenesis and restoration of a large mandibular defect in a human patient implanted with 125 hTGF-ß3 per gram pf matrix, was not comparable however to the rapid induction and mineralization of the newly formed bone as seen in our pre-clinical studies in Papio ursinus.44 Healing was however uneventful, maintaining masticatory function of the treated hemi-mandible more than 5 years after implantation (Fig. 4F).44,45
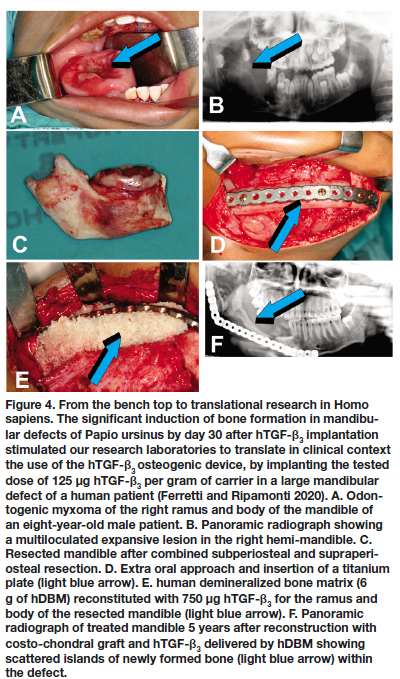
Because of the substantial bone forming activity by the binary application of relatively low doses of hTGF-ß1 and hTGF-ß3 with 25 osteogenic protein-1 (hOP-1, also known as BMP-7), additional animals were prepared with 2.5 cm full thickness mandibular defects and implanted with 2.5 mg hOP-1 and 125 hTGF-ß3 at a ratio of 20:1 by weight.26,37,42 Morphogens, recombined with allogeneic ICBM, induced substantial osteogenesis with expansion of the newly formed and mineralized buccal plates (Fig. 5).44
Recombinant human transforming growth factor-ß3: Long-term morphological studies using 250 Mg hTGF-ß3 in large mandibular defects of the Chacma baboon Papio ursinus
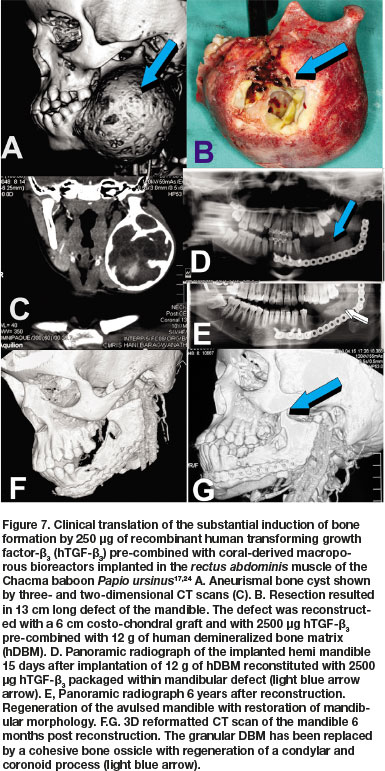
Concurrent studies in Papio ursinus to resolve the optimal dose for translation in clinical contexts, evaluated the reconstitution of 250 of hTGF-ß3 with coral-derived macroporous constructs. Intramuscularly implanted super-activated bioreactors were harvested on day 20 and processed for histological analyses.17,24 Histological analyses showed prominent and substantial bone formation outside the profile of the intramuscularly implanted super-activated bioreactors (Fig. 6A).17,24 The extensive induction of bone by 250 hTGF-ß3 albeit at the periphery of the macroporous bioreactors, proposed a novel dose for testing hTGF-ß3 in pre-clinical and clinical studies. Concurrent to the new non-human primate study described below, the 250 dose of hTGF-ß3 was translated in clinical contexts by implanting the recombinant morphogen in a massive mandibular defect in a human patient (Fig. 7).45,46 Induction of tissue morphogenesis was seen radiographically 6 months after transplantation (Fig. 7E), with regeneration of the ramus and body of the mandible with also regeneration of the surgically ablated coronoid process (Figs. 7F,G light blue arrow).
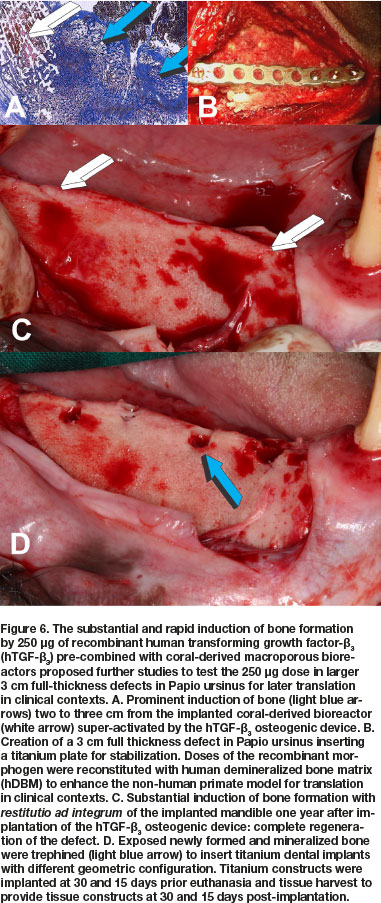
A cohort of Chacma baboons Papio ursinus was selected from the Wits Research Animal Facility (WRAF) directed by a veterinarian doctor, and continuously mentored and supervised by the Animal Ethics Control Committee (AECC) of the University.
The long-term study in Papio ursinus evaluated bone regeneration, remodeling and maintenance of regenerated mandibular defects (Animal Ethics ref.: 2018-11-19-0, study title: Translational approaches for bone constructs: Their impact on facial bone reconstruction [Waiver approved to amend study and extension of scope of the study using samples gathered by previous experimentation to finalize cutting of undecalcified sections on the Exakt technology from mandibular blocks to be embedded in Technovit]. Remodeling and maintenance of the newly formed bone was evaluated at 12 (4 animals), 10 (two animals), 9 (two animals) and 7 (two animals) months after the hTGF-ß3 implantation in large full thickness mandibular defects, three centimeters in length, surgically prepared in the right hemi-mandibles (Figs. 6B; 8A) in 10 Chacma baboon Papio ursinus. Implanted hemi-mandibles were re-exposed to analyze tissue regeneration and to trephine the newly generated bone by the recombinant morphogen to insert titanium implants with planar or geometrically generated surfaces.47 The latter prepared with a series of repetitive concavities along the titanium' construct (Figs. 8B; 9B;10B).47 Regenerated defects showed corticalization of the newly formed bone across the 3 cm defects with regeneration of the mandibular discontinuities (Figs. 6C;8B;10A).
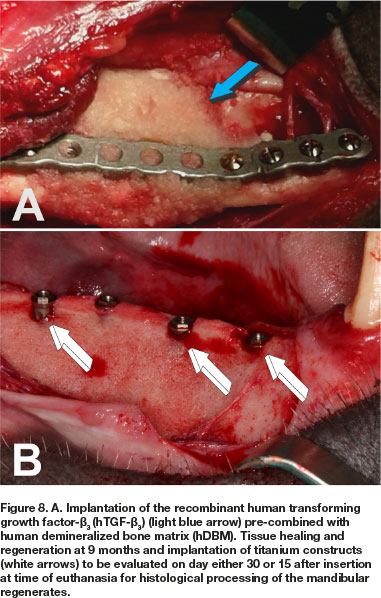
Harvested hemi-mandibles after bilateral carotid perfusion with 2 liters buffered saline and 2 liters buffered formalin were cleaned of adhesive muscular and connective tissues, processed and further fixed by immersion in 70% ethanol. Later, specimen blocks were embedded in Technovit 7200 VCL (Heraeus Kulzer Gmbh, Wehrheim, Germany). Undecalcified blocks were cut longitudinally on the Exakt diamond saw cutting and polishing technology. Specimens preparation, cutting and staining was performed by Morphisto AG, Germany).
Of note, the mean weight of the Chacma baboons at surgery and implantation, before the long-term study housing and euthanasia was 20.1 kg and, at tissue harvest and euthanasia after intra-carotideal perfusion by buffered saline and formalin perfusion, was 20.7 Kg.
These weight data are fundamental for our understanding of the adaptive capacity of Papio ursinus as well as the high standard of the WRAF of the University that provide functional non-human primate facilities and rooms with large cages highly ventilated with a positive pressure as well as diets that both maintain weight in long-term captive non-human primates' experimentation. The facilities of the University at the Medical School Faculty of Health Sciences provide thus highly acceptable standards and conditions as shown by weight at termination higher than at the beginning of the long-term study, with optimal fur' status at euthanasia more than one year after starting the study.
Mandibular blocks were analyzed by micro-focus X-ray computed tomography (μCT) scans (MIXRAD micro-focus X-ray laboratory, South African Nuclear Energy Corporation - NECSA, Pelindaba, Pretoria). An application was submitted and cleared by NECSA to scan and analyze the retrieved mandibular blocks.
All CT scans were conducted at 100kV and 100pA for optimal contrast with a Nikon XTH225ST micro-focus X-ray machine. The samples were mounted in a polystyrene sample holder to secure the sample during the scanning procedure. One thousand separate X-ray absorption images were obtained through a full 360 degree rotation with maximum magnification to ensure a resolution of 36 micrometer. All the images were then reconstructed to a 3D virtual volume using Nikon CTPro reconstruction software. The final 3D renderings were analyzed in Volume Graphics VGStudio Max software.
μCT scans 8 months after hTGF-ß3 implantation showed tissue induction across the full thickness defect, though the section across the middle of the regenerate shows nonunion, possibly reflecting the corticalization of the buccal and lingual cortices (Fig. 9A). High power view shows limited bone in contact with the concavities of the geometric titanium construct (Fig. 9B) 30 days after implantation harvested at euthanasia 8 months after hTGF-ß3 implantation. Histological analyses on undecalcified sections show bone formation across the surgically created full-thickness defects. There was the induction of corticalization of the newly formed mandibular ossicles (Figs. 9C,D). Remodeling and corticalization of the newly formed bone were evident on both 8 and 9 months after implantation (Figs. 9, 10), together with mineralized bone covered by osteoid seams as seen in 8 months specimens (Fig. 9E).
Inserted hydroxyapatite-coated implants (Figs. 9A,B) represent images 30 days after implantation in the newly regenerated mandibular construct. Inspection of the geometric profile of the titanium implants with concavities shows by day 30 still limited induction of bone within the profiled concavities (white arrow Fig. 9B).
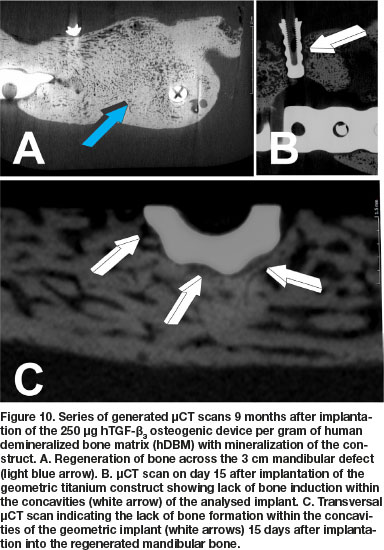
μCT scans 9 months after implantation of 250 doses of hTGF-ß3 showed substantial reconstruction of the large full-thickness mandibular defect surgically prepared in Papio ursinus (Fig. 10A). Of interest, the profile of the titanium implants harvested on day 15 from the 9 months mandibular regenerate show limited if any bone contact with the geometric configuration of the harvested implant (Fig. 10B). Lack of integration is also shown in a transversal μCT scan across a titanium construct that show limited bone formation against the concavities of the substratum (Fig. 10C white arrows).
Histological analyses on undecalcified Exakt polished and grounded sections showed remodeling and the induction of corticalization of the mandibular plate newly formed after implantation of the 250 doses of hTGF-ß3 (Fig. 11A). Histological detail of the titanium inserted in the 9 months mandibular regenerate and harvested on day 15 at euthanasia shows lack of bone formation into the concavities of the geometric construct (Fig. 11B white arrow). Limited bone deposition is thus shown in a corresponding histological section that correlate with the μCT scan on day 15, reporting minimal if any bone differentiation within the concavities of the geometric constructs (Fig. 11B, undecalcified Exakt section; μCT scans Fig. 10B). There is remodeling and corticalization of the newly formed mineralized bone at 9 months after implantation of the hTGF-ß3 osteogenic device. Remodeling and maintenance of the newly formed bone is maintained up to 12 months after implantation of the recombinant morphogen, keeping the corticalized profile of the newly formed hemi-mandible (Fig. 11D).
DISCUSSION
Restoring normal function and appearance of complex disfiguring craniofacial defects and/or anomalies in human patients still remains a grand unsolved challenge. Systematic studies in the rectus abdominis muscle, the calvarium and the mandible, respectively, showed that the cellular and molecular machineries of non-human primate tissues are differently activated when compared to rodents, lagomorphs and canine tissues when challenged with the soluble osteogenic molecular signals of the TGF-ß superfamily. 4,11,17,19,24,36
The molecular machinery of primate tissues and cells is endowed with transmembrane receptor' ligands that phosphorylate and respond to the three mammalian TGF-β proteins. In the non-human primate Papio ursinus, the three mammalian proteins, and prominently the TGF-ß3 morphogen, induce the substantial formation of endochondral bone. Research so far has shown that the TGF-ß proteins initiate endochondral bone formation in primates only.17,21,24 Results obtained in full thickness mandibular defects of Papio ursinus have shown the remarkable inductive capacity of hTGF-ß3 in craniofacial defects of non-human primate species with regeneration as early as 30 days post implantation, and with corticalization of the outer cortices.43,44
The substantial bone initiated in both heterotopic intramuscular and mandibular orthotopic sites prepared in Papio ursinus17,19,24,36 proposed the clinical translation of the newly developed hTGF-ß3 osteogenic device in a human patient affected by a large mandibular odontogenic myxoma. The multiloculated expanding lesion in the right ramus and body of mandible was ablated via combined subperiosteal and supraperiosteal resections. Panoramic radiograph 5 years post-reconstruction with costo-chondral graft and 125 hTGF-ß3 per gram of hDBM shows the induction of bone within the defect.
Though healing was uneventful, later pre-clinical studies showed the substantial induction of bone by 250 hTGF-ß3 when implanted intramuscularly in Papio ursinus pre-combined with coral-derived macroporous bioreactors.17,24,30 The 250 hTGF-ß3 dose was then implanted in larger 3 cm full thickness mandibular defects in Papio ursinus, and the reported study in this communication was partially supported by Project No. AOCMF-19-03-R of AOCMF, Switzerland.The pre-clinical study showed mandibular regeneration by the selected dose of the recombinant morphogen when recombined with human demineralized bone matrix (hDBM). The use of hDBM in Papio ursinus further translated the clinical potential of the pre-clinical study in Papio ursinus.
μCT scans and histological analyses showed regeneration across the mandibular defects with maintenance and remodeling if the newly formed and mineralized bone up to 9 and 12 months after implantation of the recombinant morphogen. Of interest, osteoid seams were seen over newly formed mineralized bone up to 9 months after healing, highlighting the substantial osteogenic capacity of the hTGF-ß3 soluble molecular signal.
μCT scans showed the induction of mineralized newly formed bone Alling the treated defects. Undecalcified histological sections prepared by the Exakt diamond saw technology confirmed mineralization and corticalization of newly induced bone across the defects. Long-term studies in non-human primates are critical to study remodeling and maintenance of the newly formed bone. The presented undecalcified sections show maintenance and continuous remodeling of the newly induced bone with regions of osteoid synthesis up to 9 and 12 months after implantation of the hTGF-ß3 osteogenic device.
In his Editorial Comment "The reality of a Nebulous Enigmatic Myth" 48 Marshall Urist stated that research on the bone induction principle "are bound to dispel the myth and appreciate the reality of bone induction for the benefit of patients with crippling diseases of the bone and joints". More than fifty years later, the Bone Research Laboratory not in Los Angeles but in Johannesburg still strongly perceives "The reality of a Nebulous Enigmatic Myth" when reading that a disproportionate number of milligrams of now available hBMPs are needed to induce limited bone volumes in human patients.
The promise of therapeutic osteoinduction has been recognized during last Century research, and pre-clinical studies including non-human primate experimentation have suggested a primary role for hBMPs in human osteoinduction. Tissue and molecular biologists together with skeletal reconstructionists alike have learned that human osteoinduction is a totally different biological topic when compared to results obtained in pre-clinical animal models. It is also possible that preclinical animal studies may or may not adequately translate and reproduce morphogen-related therapeutic responses in clinical contexts.63
The study reported here evaluates the 250 dose of hTGF-ß3 to test in non-human primates a possibly more incisive formation of bone not only in Papio ursinus but in human patients too. The latter showed viable regenerates after mandibular reconstruction with 250 hTGF-ß3 several years after human implantation.45
The paradigm of bone tissue engineering is epitomized by the remarkable work of AH Reddi and his School at the National Institutes of Health (NIH), Bone Cell Biology Section, where it was found that molecular combinations of soluble and insoluble signals or substrata initiate the bone induction cascade.10 As Reddi reported in a classic title by now: "Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials"10 , the reconstitution of a soluble morphogenetic signals with an insoluble signal or substratum triggers the induction of bone morphogenesis, even if the signals are implanted in heterotopic sites of animal models, where there is no bone.
Of note, our laboratories have reported a modified paradigm in which the insoluble signal or substratum initiates resorption via a downstream of molecular and cellular cascades sculpting resorption pits and lacunae in the shape of concavities within the implanted bioceramic bioreactor.51 Lacunae, pits and concavities are the biological continuum of the induction of bone formation. 50-55 This has resulted in the substantial induction of bone formation in biomimetic bioreactors almost completely resorbed and replaced by bone 365 days after calvarial implantation in Papio ursinus.50
To further study the role of the concavity initiating the induction of bone formation, the reported long-term study of the 250 hTGF-ß3also compared different geometric configurations of titanium implants, i.e. planar surface implants vs. modified surfaces with a series of concavities along the titanium surfaces.47 The presented images showed lack of bone formation within concavities on day 15 with some bone forming in the concavities by day 30.
In previous studies,47 we have reported as a first that titanium implants with geometric configurations in the form of repetitive concavities along the titanium coated by highly crystalline hydroxyapatite were shown to be intrinsically osteoinductive.
In our studies, we have asked the critical question: can bone spontaneously initiates by uncoated titanium substrata with or without geometric configurations, that is, a series of repetitive concavities along the planar surfaces? Our previous experimentation in Papio ursinus showed that titanium concavities coated by crystalline hydroxyapatites per se initiate the induction of bone formation even when implanted in heterotopic intramuscular sites, where there is no bone.47,52,54,55
A review of the literature shows that the only available experiments reporting the intrinsic osteoinductivity by titanium' substrata is a study where titania' constructs were implanted heterotopically in the dorsal musculature of canines.56 The reported data have highlighted that the implanted titania were macroporous titanium constructs with a superior in vitro and in vivo apatite forming capacity, bonding directly to living bone in vivo.56 This in vivo apatite-forming could have possibly initiated the formation of bone as reported by Fujibayashi et al.56 Of note, macroporous constructs were also chemically and thermally treated, including alkali and heat treatment with sodium removal.57
Though the presented titania' bioreactors short implantation study does not cast as yet any mechanistic insights into the allegedly proposed spontaneous inductivity by titanium substrata, the limited bone formation against the bioreactors implanted into newly formed bone further indicate that titanium metal is not osteoinductive per se, and that the reported in vivo osteoinductivity 56,57 is due to its in vivo apatite-forming ability after chemical and thermal treatments. Indeed macroporous blocks were acid- and heat-treated to form apatite layers on the titanium surfaces.58-62 The most convincing results that pure titanium is not intrinsically osteoinductive is that titanium' bioreactors without alkali and heat treatments lack osteoinductive capacity when heterotopically implanted in identical canines models.56,61,62
The hTGF-ß3 reported in our studies in non-human and human primates has shown once again that the translation of the "bone induction principle" 49 is still a difficult if not an impossible target when compared to the results obtained in non-human primate species that showed substantial bone formation by the newly developed hTGF-ß3/based osteogenic device.
The cellular and molecular basis responsible for the reported substantial differences in regenerative patterns amongst mammals and particular in non-human vs. human primates need now to be evaluated, and basic research should be devoted to analyse genetically the mammalian wound healing traits controlling tissue induction and morphogenesis beyond morphogens and stem cells.
Animal experimentation and the use of different animal models are also critical challenges for translational research in human patients. As Brubaker and Lauffenburger sate, "Direct translation of observations in rodents or nonhuman primates to humans frequently disappoints, for reasons including discrepancies in complexity and regulation between species".63 Mechanistically, the molecular machinery of rodents vs. non-human and human primates is fundamentally different, at least when responding to the osteogenic proteins of the TGF-ß supergene family.4
In his contribution to the physiological functions of TGF-ß, Sporn and colleagues describe that TGF-ß induces the rapid induction of fibrosis and angiogenesis in vivo, together with stimulation of collagen formation in vitro.64 In marked contrast, the three mammalian TGF-ß isoforms, and notably the hTGF-ß3 protein are inducers of substantial and prominent induction of bone formation in heterotopic extraskeletal sites, where there is no bone, thus showing a molecularly significant response to selected proteins and ligands at the receptor levels, with phosphorylation and induction of bone via the Smad' related pathway.
To end, research into translational regenerative induction of bone formation will require further studies particularly highlighting the reasons and why the three mammalian TGF-ß isoforms induce bone in primates but not in mice, rodents, lagomorphs and canines and how to boost the substantial induction of bone formation in heterotopic sites vs. orthotopic mandibular sites. Blueprints for translational regenerative medicine are offering pathways that may help to better define regenerative molecules and morphogens.65
ACKNOWLEDGMENTS
The experiments describe above report research data spanning decades of research experimentation at the Bone Research Laboratory (BRL) since its inception in 1994 at the Medical School of the University. The merging of the BRL with the School of Clinical Medicine enhanced strategic research collaboration with the Laboratories of Molecular and Cellular Biology of Internal Medicine headed by Raquel Duarte PhD. The molecular and morphological connubium jointly resolved the molecular insights into the induction of bone formation by the recombinant human transforming growth factor-ß3 and the spontaneous and/or intrinsic induction of bone formation by calcium phosphate-based macroporous bioreactors in non-human primates. The incisive molecular insights of Raquel Duarte and of her team are greatly acknowledged. The described studies were continuously supported by the University of the Witwatersrand, Johannesburg, the South African National Research Foundation and by ad hoc grants to the BRL. We thank the University for the continuous privilege of using non-human primates in experimentation. Novartis AG, Zurich, Switzerland, is greatly acknowledged for the gift of the recombinant hTGF- β3. We would like to thank Southern Implants of Graham Blackbeard for the kind gift of the titania' constructs.
The described studies using the 250 hTGF-ß3 dose implanted in larger three cm full thickness mandibular defects in Papio ursinus were partially supported by Project No. AOCMF-19-03-R of AOCMF, Switzerland. The presented work would not have been possible without the continuous expertise of Barbara van den Heever and Ruqayya Parak, who brought undecalcified bone histology to a superior art. Ruqayya added her unique expertise of processing undecalcified sections cut on the Exakt diamond saw donated to the BRL by a Wellcome Trust grant in 1997/98. To her, special recognition is offered. The senior author gratefully thanks Angela De Gouveia for providing continuous IT connection outside her M&B shop at Broederstroom that provided capacity at any day and time to write, revise and continuously polishing the manuscript. A final thank to the Chacma baboon Papio ursinus for continuously providing optimal microenvironments for the induction of bone formation. To the senior author, the pleiotropic multi-faceted niches of the induction of bone formation by the osteogenic soluble molecular signals in Papio ursinus still provide an exhilarating ride across tissue induction, morphogenesis, differentiation and dedifferentiation, stemness, from chondrogenesis in the shark Carcharinus obscurus to osteogenesis in non-human and human primates.
REFERENCES
1. Ripamonti U, Klar RM. Regenerative frontiers in craniofacial reconstruction: grand challenges and opportunities for the mammalian transforming growth factor-β proteins. Front Physiol. 2010;1:143. Published 2010 Nov 11. doi:10.3389/fphys.2010.00143. [ Links ]
2. Ferretti C, Ripamonti U. The Conundrum of human osteoinduction: Is the bone induction principle failing the clinical translation? J Craniofac Surg. 2021;32(4):1287-89. [ Links ]
3. The power of one. Introduction. Cell 2014; 157:3 [ Links ]
4. Ripamonti U. Osteogenic proteins of the transforming growth factor-ß superfamily. In: HL Henry and AW Norman (Eds.), Encyclopedia of Hormones. Academic Press, Elsevier Inc., 2003; pp 80-86. [ Links ]
5. Wang EA, Rosen V Cordes P, et al: Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci U S A 1988;85(24):9484 [ Links ]
6. Luyten FP, Cunningham NS, Ma S, et al. Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J Biol Chem. 1989;264(23):13377-13380. [ Links ]
7. Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: Molecular clones and activities. Science 1988; 242:1528-34. [ Links ]
8. Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, Wozney JM. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9843-7. doi: 10.1073/pnas.87.24.9843. [ Links ]
9. Özkaynak E, Rueger DC, Drier EA, Corbett C, Ridge RJ, Sampath TK, Oppermann H. OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J 1990; Jul;9(7):2085-93. [ Links ]
10. Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: Inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000; 6(4): 351-359. [ Links ]
11. Ripamonti U. Soluble osteogenic molecular signals and the induction of bone formation. Biomaterials. 2006 Feb;27(6):807-22. doi:10.1016/j.biomaterials.2005.09.021 [ Links ]
12. Cook JD, Wolfe MW, Salkeld JL, Rueger DC. Effects of recombinant human osteogenic protein-1 on healing of segmental defects in non-human primates. J Bone Joint Surg. 1995;77A: 734-750. [ Links ]
13. Ripamonti U, van den Heever B, Sampath TK, Tucker MM, Rueger DC, Reddi AH. Complete regeneration of bone in the baboon by recombinant human osteogenic protein-1 (hOP-1, bone morphogenetic protein-7). Growth Factors. 1996;13(3-4). doi:10.3109/08977199609003228 [ Links ]
14. Ripamonti U, van den Heever B, Crooks J, Tucker MM, Sampath TK, Rueger DC, Reddi AH. Long-term evaluation of bone formation by osteogenic protein-1 in the baboon and relative efficiency of bone-derived morphogenetic proteins delivered by irradiated xenogeneic collagenous matrices. J Bone Miner Res. 2000; 15(9):1798-09. doi: 10.1359/jbmr.2000.15.9.1798. [ Links ]
15. Ripamonti U. Bone induction by recombinant human osteogenic protein-1 (hOP-1, hBMP-7) in the primate Papio ursinus with expression of mRNA of gene products of the TGF-β superfamily. J Cell Mol Med. 2005;9:911-28. [ Links ]
16. Ripamonti U, Ramoshebi LN, Matsaba T, Tasker J, Crooks J, Teare J. Bone induction by BMPs/OPs and related family members. The critical role of deliverey systems. J Bone Joint Surg Am. 2001;83-A Suppl1 (Pt2):S116-27.PMID. [ Links ]
17. Ripamonti U, Duarte R, Ferretti C. Re-evaluating the induction of bone formation in primates. Biomaterials. 2014 Nov;35(35):9407-22. doi: 10.1016/j. biomaterials.2014.07.053. Epub 2014 Aug 23. [ Links ]
18. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899. doi:10.1126/science.150.3698.893 [ Links ]
19. Ripamonti U, Ramoshebi LN, Teare J, Renton L, Ferretti C. The induction of endochondral bone formation by transforming growth factor-ß3: Experimental studies in the non-human primate Papio ursinus. J Cell Mol Med. 2008; 12:1029-1048. [ Links ]
20. Sampath TK, Raska KE, Doctor JS, Tucker RF, Hoffmann FM. Drosophila transforming growth factor beta superfamily proteins induce endochondral bone formation in mammals. Proc Natl Acad Sci USA, 1993; 90(13): 6004-08. https://doi.10.1073/ pnas.90.13.6004. [ Links ]
21. Ripamonti U, Duarte R, Ferretti C, Reddi AH. Osteogenic competence and potency of the bone induction principle: Inductive substrates that initiate "Bone: Formation by Autoinduction". J Craniofac Surg. 2021; in press. [ Links ]
22. Ripamonti U. Recapitulating development: a template for periodontal tissue engineering. Tissue Eng. 2007 Jan;13(1):51-71. doi: 10.1089/ten.2006.0167. PMID: 17518581. [ Links ]
23. Ripamonti U. Biomimetism, biomimetic matrices and the induction of bone formation. J Cell Mol Med. 2009;13(9B):2953-2972. doi:10.1111/j.1582-4934.2008.00562.x [ Links ]
24. Ripamonti, U. Induction of Bone Formation in Primates. The Transforming Growth Factor beta 3, CRC Press, Taylor & Francis Group, Boca Raton, USA, 2016. [ Links ]
25. Ripamonti U, Ferretti C, Teare J, Blann L. Transforming growth factor-beta isoforms and the induction of bone formation: implications for reconstructive craniofacial surgery. J Craniofac Surg. 2009;20(5):1544-1555. doi:10.1097/SCS.0b013e3181b09ca6 [ Links ]
26. Ripamonti U, Duneas N, van den Heever B, Bosch C, Crooks J. Recombinant transforming growth factor-beta1 induces endochondral bone in the baboon and synergizes with recombinant osteogenic protein-1 (bone morphogenetic protein-7) to initiate rapid bone formation. J Bone Miner Res. 1997;12(10):1584-1595. doi:10.1359/jbmr.1997.12.10.1584 [ Links ]
27. Ripamonti U, Crooks J, Matsaba T , Tasker J. Induction of endochondral bone formation by recombinant human transforming growth factor-B2 in the baboon (Papio ursinus). Growth Factors. 2000;17(4):269-285. doi:10.3109/08977190009028971 [ Links ]
28. Ripamonti U, Ferretti C, Heliotis M. Soluble and insoluble signals and the induction of bone formation: molecular therapeutics recapitulating development. J Anat. 2006;209(4):447-68. doi: 10.1111/J.1469-7580.2006.00635.x. [ Links ]
29. Ripamonti U, Heliotis M, Ferretti C. Bone morphogenetic proteins and the induction of bone formation: from laboratory to patients. Oral Maxillofac Surg Clin North Am. 2007;19(4):575-vii. doi:10.1016/j.coms.2007.07.006 [ Links ]
30. Ripamonti U, Teare J, Ferretti C. A Macroporous Bioreactor Super Activated by the Recombinant Human Transforming Growth Factor-ß(3). Front Physiol. 2012;3:172. Published 2012 Jun 7. doi:10.3389/ fphys.2012.00172 [ Links ]
31. Seeherman HJ, Berasi SP, Brown CT, et al. A BMP/ activin A chimera is superior to native BMPs and induces bone repair in nonhuman primates when delivered in a composite matrix. Sci Transl Med. 2019;11(489):eaar4953. doi:10.1126/scitranslmed. aar4953 [ Links ]
32. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920-926. doi:10.1126/science.8493529 [ Links ]
33. Williams D. F. Tissue engineering: the multidisciplinary epitome of hope and despair, in Studies in Multidisciplinarity eds Paton R., McNamara L. (Amsterdam: Elsevier BV; ) 2006, 483-524. [ Links ]
34. Martin I. Engineered tissues as customized organ germs. Tissue Eng Part A. 2014;20(7-8):1132-1133. doi:10.1089/ten.tea.2013.0772 [ Links ]
35. Klar RM, Duarte R, Dix-Peek T, Ripamonti U. The induction of bone formation by the recombinant human transforming growth factor-ß3. Biomaterials. 2014;35(9):2773-2788. doi:10.1016/j.biomaterials.2013.12.062 [ Links ]
36. Ripamonti U, Dix-Peek T, Parak R, Milner B, Duarte R. Profiling bone morphogenetic proteins and transforming growth factor-ßs by hTGF-ß3 pre-treated coral-derived macroporous bioreactors: the power of one. Biomaterials. 2015;49:90-102. doi:10.1016/j. biomaterials.2015.01.058 [ Links ]
37. 37 Ripamonti U, Parak R, Klar RM, Dickens C, Dix-Peek T, Duarte R. The synergistic induction of bone formation by the osteogenic proteins of the TGF-ß supergene family. Biomaterials. 2016;104:279-296. doi:10.1016/j.biomaterials.2016.07.018 [ Links ]
38. Ferretti C, Ripamonti U, Tsiridis E, Kerawala CJ, Mantalaris A, Heliotis M. Osteoinduction: translating preclinical promise into clinical reality. Br J Oral Maxillofac Surg. 2010;48(7):536-539. doi:10.1016/j.bjoms.2009.08.043. [ Links ]
39. Ferretti C, Teare J, Renton L, Ripamonti U. Mandibular reconstruction with TGF-ß superfamily proteins: Preclinical and clinical results. 7th International Conference on Bone Morphogenetic Proteins, Lake Tahoe, USA, July 2008. [ Links ]
40. Ripamonti U, Ferretti C. The induction of bone formation by bone morphogenetic proteins in non-human and human primates. 8th International Conference on Bone Morphogenetic Proteins, Leuven, Belgium, September 2010. [ Links ]
41. Ripamonti U. BMPs/TGF-ß superfamily: Challenges for clinical translation. 9th International Conference on Bone Morphogenetic Proteins, Lake Tahoe, USA, June 19-23, 2012. [ Links ]
42. Ripamonti U, Klar RM, Renton LF, Ferretti C. Synergistic induction of bone formation by hOP-1, hTGF-beta3 and inhibition by zoledronate in macroporous coral-derived hydroxyapatites. Biomaterials. 2010;31(25):6400-6410. doi:10.1016/j.biomaterials.2010.04.037 [ Links ]
43. Ripamonti U. The induction of bone formation: From bone morphogenetic proteins to the transforming growth factor-ß3 protein - redundancy, pleiotropy and the induction of cementogenesis. SADJ. 2021; 76(6):331-356. [ Links ]
44. Ripamonti U, Ferretti C. Regeneration of Mandibular Defects in non-Human Primates by the Transforming Growth Factor-ß3 and Translational Research in Clinical Contexts. In: Ripamonti U (ed.) CRC Press Taylor & Francis, Boca Raton USA, Induction of Bone Formation in Primates. The Transforming Growth Factor-beta3; 2016, Chapter 5, 105-121. [ Links ]
45. Ferretti C, Ripamonti U. Long Term Follow-Up of Pediatric Mandibular Reconstruction With Human Transforming Growth Factor-ß3. J Craniofac Surg. 2020;31(5):1424-1429. doi:10.1097/SCS.0000000000006568 [ Links ]
46. Ripamonti U, Feretti C. Grand challenges for craniomandibulofacial reconstruction by human recombinant transforming growth factor-ß3. In: Tuan RS, Guilak F, Atala A, editors, Keystone Symposia on regenerative tissue engineering, Breckenridge CO, USA; 2012. http://www.keystonesymposia.org. [ Links ]
47. Ripamonti U, Roden LC, Renton LF. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials. 2012;33(15):3813-3823. doi:10.1016/j.biomaterials.2012.01.050 [ Links ]
48. Urist MR. The reality of a nebulous, enigmatic myth. Clin Orthop Rel Res. 1968; 59:3-6. [ Links ]
49. Urist MR, Silverman BF, Büring K, Dubuc FL, Rosenberg JM. The bone induction principle. Clin Orthop Relat Res. 1967;53:243-283. [ Links ]
50. Ripamonti U, Richter PW, Nilen RW, Renton L. The induction of bone formation by smart biphasic hydroxyapatite tricalcium phosphate biomimetic matrices in the non-human primate Papio ursinus. J Cell Mol Med. 2008;12(6B):2609-2621. doi:10.1111/j.1582-4934.2008.00312.x [ Links ]
51. Ripamonti U. Biomimetic functionalized surfaces and the Induction of bone formation. Tissue Eng Part A. 2017;23(21-22):1197-1209. doi:10.1089/ten.tea.2017.0321. [ Links ]
52. Ripamonti U, Crooks J, Kirkbride AN. Sintered porous hydroxyapatites with intrinsic osteoinductive activity: geometric induction of bone formation. S Afr J Sci 1999;95:335-343. [ Links ]
53. Ripamonti U. Soluble, insoluble and geometric signals sculpt the architecture of mineralized tissues. J Cell Mol Med. 2004;8(2):169-180. doi:10.1111/j.1582-4934.2004.tb00272.x [ Links ]
54. Ripamonti U. The concavity: The "Shape of Life" and the control of bone differentiation. 2012 Feature Paper - Science in Africa, May 2012. [ Links ]
55. Ripamonti U. Functionalized surface geometries induce: "Bone: Formation by Autoinduction". Front Physiol. 2018;8:1084. Published 2018 Feb 6. doi:10.3389/ fphys.2017.01084 [ Links ]
56. Fujibayashi S, Neo M, Kim HM, Kokubo T, Nakamura T. Osteoinduction of porous bioactive titanium metal. Biomaterials. 2004;25(3):443-450. doi:10.1016/s0142-9612(03)00551-9 [ Links ]
57. Fujibayashi S, Nakamura T, Nishiguchi S, Tamura J, Uchida M, Kim H-M, Kobuko T. Bioactive titanium prepared by alkali and heat treatment. J Biomed Mat Res. 2001; 56(4):562-570. [ Links ]
58. Kobuko T, Miyaji F, Kim H-M. Spontaneous formation of apatite layer on chemically treated titanium metals. J Am Ceram Soc. 1996; 79(4): 1127-1129. [ Links ]
59. Nishiguchi S, Kat H, Fujita H, Kim H-M, Miyaji F, Kobuko T, Nakamura T. Enhancement of bone-bonding strengths of titanium alloy implants by alkali and heat treatments. J Biomed Mater Res (Appl Biomater), 1999;48(5):689-699. [ Links ]
60. Uchida M, Kim H-M, Kobuko T, Fujibayashi S, Nakamura T. Effect of water treatment on the apatite-forming activity of NaOH-treated titanium metal. J Biomed Mater Res (Appl Biomater), 2002;63(5):522-530. [ Links ]
61. Takemoto M, Fujibayashi S, Neo M, Suzuki J, Matsushita T, Kobuko T, Nakamura T. osteoinductive porous titanium implants: Effects of sodium removal by dilute HCl treatment. Biomaterials. 2006;27(13):2682-2691. [ Links ]
62. Kawai T, Takemoto M, Fujibayashi S, Akiyama H, Tanaka M, Yamaguchi S, Pattanayak DK, Doi K, Matsushita T, Nakamura T, Kobuko T, Matsuba. Osteoinduction on acid and heat treated porous Ti metal samples in canine muscle. PLos One. 2014; 10;9(2):e88366. Doi: 10.1371/ journal.pne.0088366. eCollection 2014.PMID: 24520375. [ Links ]
63. Brubaker DK, Lauffenburger DA. Translating preclinical models to humans. Science. 2020;367(6479):742-743. doi:10.1126/science.aay8086 [ Links ]
64. Roberts AB, Sporn MB, Assoian RK, Smith JM,Roche NS, Wakefield LM et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83(12):4167-4171. doi:10.1073/ pnas.83.12.4167 [ Links ]
65. Armstrong JPK, Keane TJ, Roques AC, Patrick PS, Mooney CM, Kuan W-L, Pisupati V, Oreffo ROC et al. Sci Transl Med 2020;12,eaaz2253. [ Links ]
66. Trueta J. The role of the vessels in osteogenesis. J Bone joint Surg. 1963; 45B, 402-18. [ Links ]
 Correspondence:
Correspondence:
Ugo Ripamonti
E-mail: ugo.ripamonti@wits.ac.za
Author contributions:
1 . Ugo Ripamonti - Contribution 60%
2 . Jakobus Hoffman - Contribution 20%
3 . Carlo Ferretti - Contribution 20%














