Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.77 no.2 Johannesburg Mar. 2022
http://dx.doi.org/10.17159/2519-0105/2022/v77no2a1
RESEARCH
Comparison of capsule-mixed versus hand-mixed glass ionomer cements. Part 1: compressive strength and surface hardness
S ArnoldI; N WarrenII; G D BuchananIII; R LombardIV
IBChD, PGDip (Endo), MSc (Dent). Department of Odontology, School of Dentistry, Faculty of Health Sciences, University of Pretoria, 31 Bophelo Road, Prinshof Campus, Riviera, Pretoria, 0002, South Africa. ORCID 0000-0002-7518-9560
IIBChD, PGDip (Endo), MSc (Dent). Department of Odontology, School of Dentistry, Faculty of Health Sciences, University of Pretoria, 31 Bophelo Road, Prinshof Campus, Riviera, Pretoria, 0002, South Africa. ORCID 0000-0003-1006-9565
IIIBChD, PGDip (Endo), MSc (Dent). Department of Odontology, School of Dentistry, Faculty of Health Sciences, University of Pretoria, 31 Bophelo Road, Prinshof Campus, Riviera, Pretoria, 0002, South Africa. ORCID 0000-0003-2957-166X
IVBChD, MSC (Dent). Department of Odontology, School of Dentistry, Faculty of Health Sciences, University of Pretoria, 31 Bophelo Road, Prinshof Campus, Riviera, Pretoria, 0002, South Africa. ORCID 0000-0001-8150-5446
ABSTRACT
INTRODUCTION: Dental restorative glass ionomer cements (GIC) are available as hand-mixed or capsulated products. Capsulation facilitates uniform ratios of powder to liquid, that should result in an optimal end-product. If this is evident, the degree to which capsulated GIC are mechanically stronger will aid in deciding when to use them instead of the hand-mixed variety.
OBJECTIVES: The compressive strength and surface hardness of hand-mixed GIC were compared to capsule-mixed equivalents.
METHODS: Eighty samples were manufactured from hand-mixed GIC: Riva Self Cure; Fuji IX GP; Ketac Universal, Ketac Molar Easymix, and equivalent capsule-mixed GIC: Riva Self Cure; Fuji IX GP; Ketac Universal Aplicap and Ketac Molar Aplicap
Compressive fracture strength was tested using a universal testing apparatus. Surface hardness was measured with a Vickers digital micro-hardness tester.
RESULTS: Significant differences were found between the compressive strength of RSCH and RSCC (P = 0.027), and, between KMH and KMC (P < 0.001). Significant differences in surface hardness were found between FIXH and FIXC (P = 0.031), KUH and KUC (P < 0.001), as well as KMH and KMC (P = 0.006).
CONCLUSION: Three capsulated forms of GIC (RSCC, KUC, KMC) demonstrated superior mechanical properties. Capsulated GIC offer advantages which may translate to clinical application.
Key words: Capsule-mix, Compressive strength, Glass ionomer cement, Hand-mix, Surface hardness.
ABBREVIATIONS FOR ARTICLE
• GIC - Glass Ionomer cement
• FIXC - GC Fuji IX GP capsule-mix
• FIXH - GC Fuji IX GP hand-mix
• RSCC - Riva Self Cure capsule-mix
• RSCH - Riva Self Cure hand-mix
• KUC - Ketac Unversal Aplicap capsule-mix
• KUH - Ketac Universal hand-mix
• KMC - Ketac Molar Aplicap capsule-mix
• KMH - Ketac Molar Easymix hand-mix
• °C - degrees Celsius
• % - percentage
• MPa - mega-Pascal/s
• VHN - Vickers Hardness Number
• rpm - revolutions per minute
• SD - Standard deviation
• IQR - interquartile range
INTRODUCTION
Dental materials are constantly evolving to offer therapeutic applications for the restoration of teeth, both the primary and permanent dentition.1 Glass ionomer cements (GIC) are routinely applied for dental restorations as they demonstrate a unique ability to bond chemically to tooth structure.2 This results in excellent marginal adaptation and a sound seal between the set cement and tooth structure, preventing micro-leakage.3,4 Bonding systems are not needed for glass ionomers, rendering these materials less technique sensitive 5 and more cost effective than resin composites, within the limitations of GIC clinical applications. As GIC absorb and release fluoride they can inhibit secondary caries formation.6,7 As GIC are self-curing, they do not undergo polymerization shrinkage and can be placed in bulk.7,8 The co-efficient of thermal expansion of these materials are similar to tooth structure, decreasing internal tension in the tooth structure adjacent to the restoration.7,9 These materials are low in toxicity and biocompatible with both the dental pulp and surrounding soft tissues.7,9
GIC are commercially available for clinical use in two distinctive forms. Firstly, there is a two-bottle system consisting of a glass powder with a separate polyalkenoic acid liquid, which is hand-mixed.10,11 Secondly there is a pre-packaged, capsulated formulation, containing a blend of glass powder and vacuum-dried polyalkenoic acid in one compartment,10,11 with a second, separate compartment containing either distilled/deionised water or a solution of tartaric acid and water.10,12 The capsulated form is mixed using mechanical mixing devices.11
Capsulated GIC have the advantages of a pre-proportioned, set powder to liquid ratio, standardised mixing techniques and standardised mixing times.10,13 It can be argued that capsulated products could be more user-friendly and time efficient as compared to hand-mixed products.14,15 Once mixed, the GIC can be injected into a prepared cavity directly from the capsule.13
The vibratory action of conventional mechanical mixing machines has been reported to lead to increased porosity in some capsulated GIC as compared to their hand-mixed equivalents.13,16 Porosity within the cement acts as a source of concentrated stress, which negatively affects the set material's strength and homogeneity.9,13,16
Hand-mixed GIC, however, also have disadvantages. Operator variability is the leading cause of inconsistency of hand-mixed glass ionomer materials.11,17,18 Both the density of the glass powder and mixing technique of the operator play a role in the actual volume of powder that is dispensed using the measuring scoop.18 The volume of the liquid component is difficult to calibrate due to inconsistencies in the angle at which the bottle is held and the pressure exerted on the bottle by the operator when dispensing the liquid.11,17,19 Air bubbles present in the liquid can influence the volume of liquid dispensed.18 Both the mixing time and manipulation technique can contribute to operator-induced variability.18 The environmental humidity and temperature have also been shown to influence the cement's consistency.18
In the clinical setting, the aforementioned problems are exacerbated as oftentimes the scoop and dropper bottle systems supplied by the manufacturer are not used, and the products are mixed according to the operators' desired consistency.10,19,20 All these factors may result in a mixture not having the ideal characteristics when mixed according to the manufacturer's instructions.18,19,21 The resulting material will subsequently be weaker, with altered chemical, mechanical and physical properties,18,19 and inconsistent setting times.18 Scientific literature reports conflicting evidence regarding whether capsulated GIC or hand-mixed GIC offer superior performance.13,22 Dowling and Flemming10 advocated the use of capsule-mixed glass ionomer restoratives due to the superior mechanical properties of these products. A study done by Kaushik et al.,16proved the contrary, and promoted the use of hand-mixed glass ionomer restoratives. White et al.,23 reported that capsule-mixed glass ionomer luting cements have inferior mechanical properties to their hand-mixed equivalents. The findings of the study by Mitchell et al.,24 however, showed that capsule-mixed glass ionomer luting cements were superior.
OBJECTIVES
The aim of this present study was to compare the compressive strength and surface hardness of four commercially available GIC in both their hand-mixed and capsule-mixed formulations.
METHODS
Ethical approval for this comparative in vitro study was obtained from the Research Ethics Committee of the Faculty of Health Science of the University of Pretoria (Protocol number: 206/2017). The materials tested in the present study included eight glass ionomers: Riva Self Cure Hand-mix (RSCH, SDI Ltd., Victoria, Australia); Fuji IX GP Hand-mix (FIXH, GC, Tokyo, Japan); Ketac Universal Hand-mix (KUH, 3M, St. Paul, Minnesota, USA); and Ketac Molar Easymix Hand-mix (KMH, 3M, St. Paul, Minnesota, USA), Riva Self Cure Capsules (RSCC, SDI Ltd., Victoria, Australia); GC Fuji IX GP
Capsules (FIXC, GC, Tokyo, Japan); Ketac-Universal Aplicap Capsules (KUC, 3M, St. Paul, Minnesota) and Ketac-Molar Aplicap Capsules (KMC, 3M, St. Paul, Minnesota).
According to the information in the manufacturer's brochures for each product, these dental cements have similar compressive strength, surface hardness and wear resistance.15,25,26
This research was performed in a controlled environment meeting the manufacturer's recommendations of a temperature of 23 +/- 1°C and relative humidity of 50 +/-5%.27,28 The test materials were mixed and dispensed into polytetrafluoroethylene (PTFE) cylinders with the following internal dimensions: six millimetres (mm) in height and four mm in diameter, in accordance with previously described methodology.10,18,28,29,30 The moulds were constructed from PTFE tubing, supported by a custom-made Perspex® matrix.13 Specimens were prepared by two operators with the same level of training in order to simulate operator variability.19,29 Both dental operators had over > 15 years of clinical experience and experience in Academic teaching and training (Dentistry) for > eight years. The calibration of the two operators was ensured by both carrying out all preparation precisely as per the manufactures' instructions and all research procedures carried out strictly according to the research protocol.
The measuring scoops and liquid dropper bottles provided for each respective hand-mixed material were used to measure the exact quantities as prescribed by each manufacturer. The powder and liquid quantities of the hand-mixed materials were intentionally not weighed to simulate the setting in clinical practice.
Four groups of ten cylindrical specimens were manufactured for each of the four hand-mixed and capsule-mixed glass ionomers for each test that would be performed. Following the manufacturer's instructions, the GC Fuji IX GP capsules were shaken to loosen the powder before activation.31
Following the manufacturer's recommendations, each capsule was activated for two seconds to break the membrane separating the powder and liquid compartments.10,27,31 The capsules were then immediately placed into an applicable mechanical mixing machine.
The 3M ESPE capsules were mixed in the RotomixTM (3M ESPE, United Kingdom) as follows: eight seconds vibratory action; three seconds centrifuging action, at a frequency of 2950 rpm (as recommended by the manufacturer).10,13,27 Following the manufacturer's instructions, the other capsules were mixed by vibratory action, with an amalgammixer (Amalgamator SYG 200, SMACO, Switzerland) for 10 seconds.10,27 Immediately after mixing, each capsule was placed in an appropriate applicator to facilitate the extrusion of the material.10,27
Following the respective manufacturer's instructions, each of the hand-mixed GIC, was manually mixed on a waxed-paper mixing pad using the scoop and dropper system provided.19
The cylindrical moulds were placed on a polyester sheet in the custom Perspex® matrix. The mixed cement for each sample of all groups was dispensed into the moulds within 60 seconds of mixing.13,18,29 To minimise the incorporation of air bubbles the capsulated glass ionomers were extruded slowly which provided laminar flow with the nozzle positioned along one side of the mould.10,29,32 The hand-mixed glass ionomers were dispensed into the moulds using a stainless steel spatula and were allowed to passively flow into each mould to minimise the incorporation of air bubbles.10,18 A polyester sheet was placed over the filled moulds and the samples were compressed by applying slight pressure with a glass slab weighing 60 grams,33 to extrude the excess material and flatten the surface.9,33,34
The Ketac Molar specimens were covered with petroleum jelly and the Ketac Universal specimens were not covered with any coating, according to manufacturer's instructions.15,26 The specimens of the remaining test groups were covered with their respective coatings as recommended by the manufacturers.35 An LED curing light (Valo, Ultradent Products Inc., South Jordan, USA), with a light-intensity of 450nm was used to cure the coatings for ten seconds. All specimens were placed into glass containers of distilled water at a consistent temperature (37+/- 1°C) in an incubator (Binder ED23, Tuttlingen, Germany) for one hour.13,18,29 After this surplus cement was removed from the top and bottom of the moulds with silicon carbide paper (880 grit), under running water.13 Each specimen was carefully removed from its mould and stored in distilled water at 37 °C for 23 hours and testing took place 24 hours after production.18,29,36 Any specimen with visible defects such as bubbles or cracks was discarded and replaced to achieve optimal samples for the final number of samples.18,29
Compressive fracture strength
A universal testing apparatus (MTS Criterion Model C45.305, MTS Systems Corporation, MN 55344-2290, USA) was used to measure the compressive fracture strength of each specimen. The flat, circular ends of each cylindrical specimen were placed between the plates of the testing apparatus.55 Moist Alter paper was placed on each circular surface to prevent dehydration before testing.37 A compressive load, which gradually increased at a rate of 1 mm/min, was applied along the long axis of each specimen, until the sample fractured.10'18'27'29,30 The resulting data was analysed using the tester software -MTS Testsuite (TW Elite software, MN 55344-2290 USA). The load to fracture was recorded.
The following equation was used to calculate the compressive fracture strength*, P (MPa):
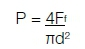
*where: Ff represents the load at fracture (N); π constant for Pi is 3.14 and d the mean diameter of the specimen in millimetres (mm).10,17,29,37
Surface hardness
The micro-hardness of the surface of each sample was digitally measured with a Vickers pyramid square diamond indenter (Future-Tech FV 700, Kanagawa, Japan). Five points were designated on one of the circular surfaces of each specimen before the surface hardness test was undertaken. The first allocated point was set in the midline of the long axis of the sample. The other four points were evenly spaced one millimetre apart, lateral (left and right) to the first point.38 The indenter of the Vickers' hardness machine was pressed vertically into the surface of each sample with an opening angle of 136 degrees, at these five points of each specimen.34 A load of 500 micro Newtons (mN) was applied by the indenter,15,37 at each of the five pre-designated points on each specimen,39 with a dwell time of five seconds.34,40 The Vickers hardness number (VHN), was calculated by the instrument for each indentation according to the selected diagonals.37 The mean VHN in N/mm2 for each specimen was determined.40
Statistical analysis
Statistical analysis was performed using SAS (SAS Institute Inc, Carey, NC, USA), release 9.4, running on Microsoft windows for personal computer. The applied statistical tests, two-sided and P values less than 0.05, were considered significant. Mean values for compressive strength and surface hardness were compared using the two-sample t-test. Thus any significant differences between the means of the paired test groups could be determined. The non-parametric Wilcoxon Rank-Sum test was used to compare the median values of the paired groups.
The null hypothesis was that there would be no difference in either the compressive strength nor the surface hardness, between the hand-mixed glass ionomers and each material's equivalent capsule-mixed glass ionomer. Data was examined for normality by evaluating the data distribution and the Shapiro-Wilk test. The data showed normal distribution, despite a few exceptions which were determined to be chance outcomes.
RESULTS
The results of the compressive strength tests are reflected in Table I. A statistically significant difference (P = 0.027) was found between the mean compressive strengths of the RSCH and RSCC paired groups, at 24.04 MPa. The difference between medians of these two materials was also statistically significant (P = 0.045), differing by 21.35 MPa. The mean compressive strength of KMH and KMC differed by 49.11 MPa. This difference was also statistically significant (P < 0.001), and the median values of these two groups differed by 56 MPa (P < 0.001). No significant differences were observed in the compressive strength of samples of the FIXH- and FIXC- groups (P > 0.05). Similarly, no significant differences were observed between the compressive strength of the samples of the KUH- and KUC- groups (P > 0.05).
The results of the surface hardness testing are represented in Table II. The surface hardness data for the FIXH and FIXC paired group exhibited a mean difference of 11.5 N/mm2 in VHN values for the two groups, which was statistically significant (P = 0.031). In addition, the median VHN for the two groups differed by 15.36 N/mm2, which was also statistically significant (P = 0.034).
The surface hardness between the KUH and KUC paired group was significantly different: the mean VHN for the two groups differed by 43.96 N/mm2 (P < 0.001) and the median VHN for the two groups differed by 47.03 N/mm2 (P < 0.001).
The KMH and KMC paired group presented with mean VHN values that differed by 12.95 N/mm2 (P = 0.006). The median VHN values differed for the two groups by 18.54 N/mm2 (P = 0.019). There was no significant difference in the surface hardness of RSCH and RSCC, neither in the mean (P = 0.124), nor the median (P = 0.290).
DISCUSSION
Dental materials and the clinical application thereof is continuously evolving to try and produce dental restorations that are biologically compatible, have optimal mechanical properties, as well as the best possible aesthetics.41,42 Testing the compressive strength and surface hardness of dental materials, gives a close indication to the potential longevity and wear resistance of these materials.41 The compressive strength of a dental material can measure both the durability,42 and the brittleness of a material.40
The present study indicates that there may be an advantage in the use of RSCC with respect to the compressive strength of this material. Significant differences were found between the mean and median compressive strengths of the RSCH and RSCC paired group specimens. Previous studies by Dionysopoulos et al.38and Mulder and Mohamed,37 reported similar compressive strength values for RSCC to those found in the present study.
The present study showed that there was no significant difference in the compressive strength of the FIXH and FIXC specimens tested. Fleming and Zala,22 previously found FIXH to have a lower compressive strength as compared to FIXC. Dowling and Fleming,17 however, reported that FIXH had significantly higher compressive strength values than those of FIXC,43 suggesting that the compressive strength of the hand-mixed version is superior for this material-paired group.17 The compressive strength values from the present study were slightly lower as compared to the manufacturer's in-house research.43 No definitive recommendations could therefore be made with regards to this material.
No significant differences in compressive strength were observed between the KUH and KUC groups in the present study. Similar compressive strength values for these materials were reported by Dionysopoulos et al.44 Compressive strength values for this material, published by the manufacturer as in-house research demonstrated higher compressive strength compared to those of the present study.15,26 The study conducted by Mulder and Mohamed,37 reported slightly lower compressive strength values for KUC when compared to this present study.
In this present study, the compressive strength of KMC was significantly higher when compared to KMH. This finding suggests that the capsule-mix may be advantageous for clinical use, specifically where high compressive strength is important. This finding is in agreement with the findings of Nomoto and McCabe.13 Dowling and Fleming,17 however, found no significant difference between the mean compressive strength of KMH and KMC in their investigation. The compressive strength values for KMC in the present study correlate well with the findings of Fleming et al.,29regarding this encapsulated glass ionomer.
Determining correlations between mixing methods and mechanical properties of glass ionomer cements may be complicated.13,17 The chemical composition and setting phase progression are the two most critical factors that regulate the mechanical properties of GIC.13 The powder to liquid ratio can influence the mechanical properties of this material, as this influences the concentration of reinforced glass fillers particles in the set cement.13
The more powder added to a constant volume of liquid, the higher the concentration of reinforced glass fillers and the more resistant the product will be to compressive forces.13 A reduced powder to liquid volume will reduce the reinforced glass Aller content of the set cement, and reduce the material's ability to resist crack proliferation under compressive forces.10 Billington et al.,^9reported that the powder content of hand-mixed glass ionomers used in clinical practice was only 37% of that recommended by the manufacturer. Dowling and Fleming,10 published similar findings, reporting a powder content below 50% of manufacturer's recommendations.
For RSC and FIX materials, the powder to liquid ratios for the hand-mix versions are slightly higher than those of the equivalent capsule-mix products. KUC also has a slightly higher powder to liquid ratio than KUH. The powder to liquid ratio of KMH is substantially higher than that of KMC. The reason for this high powder to liquid ratio in KMH (the hand-mixed glass ionomer), could be attributed to the highly granular nature of the powder, making it less dense, more flowable and more absorbent.45 These differences in powder to liquid ratios between hand-mixed and equivalent capsule-mixed products may influence research findings when comparing mixing methods and mechanical properties. In striving to produce glass ionomers with ideal properties in either hand-mixed or capsule-mixed preparations, the challenge lies in finding an optimal balance between powder to liquid ratio, polyacid concentration and the molecular weight of the polyacid.46
Voids in the set cement could either be the result of insufficient wetting of the powder by the liquid, or the inadvertent inclusion of air during the mixing procedure. The presence of voids and the concentration of reinforced glass filler particles have a notable impact on the mechanical properties of GIC.13 The larger and the more voids present, the greater the probability of fracture at low levels of stress.13 Xie et al.,19 suggested higher compressive strength values to be related to more dense surface textures with fewer, smaller voids and tightly packed glass Aller particles. Material failure has been attributed to surface irregularities and cracks in conjunction with internal and surface porosity.22
Surface hardness is defined as the ability of a material to resist permanent indentation or piercing when a force is applied to the material.40 The harder the surface, the higher the Vickers hardness measurement (VHN) will be.40 Xie et al.,9concluded that higher surface hardness values of glass ionomers are attributed to three factors: a variety of different shapes and sizes of glass particles, a highly fused particle-polymer matrix and a dense surface texture.
The present study found no significant difference in the VHN of RSCH and RSCC, which shows that neither of these two products offer an advantage over the other with respect to the property of surface hardness. Two previous studies reported similar values for surface hardness of RSCC with the values obtained in the present study.37,38
The present study identified significant differences in surface hardness between the FIX, KU, and KM paired groups. The capsule-mixed specimens of FIX, KU and KM exhibited significantly higher surface hardness numbers (VHN) in comparison to their respective, hand-mixed equivalents. In this present study, the surface hardness of KUC correlates well with the findings of both Alrahlah,39 and those of the manufacturer.15 Surface hardness values for KUC in the present study were higher than those reported by both Dionysopoulos et al.,38and Mulder and Mohamed.37 The surface hardness values of FIXC from this present study were similar to those of the manufacturer (GC, Tokyo, Japan).43
The durability, strength, working and setting times of glass ionomer cements are dependent upon the use of the correct ratio of powder to liquid.18,37 Studies have been conducted to determine the mechanical properties of the set glass ionomer cement when lower powder/liquid ratios are used than recommended.22,37 Mulder and Mohamed,37 evaluated and compared the actual powder/liquid ratios of several different commercially available capsulated glass ionomers. The capsules were disassembled, and the powder and liquid components were individually weighed. This study concluded that neither the compressive strength nor the surface hardness would be adversely affected by the findings.37
Manufacturers place precise values in grams for powder and liquid weights in product brochures and packaging. Furthermore, manufacturer's in-house research is based on these precise values.37 A higher volume of powder to liquid ratio leads to shorter working and setting times, and higher compressive strength.37 Final restorations mixed with a decreased powder to liquid ratio are more susceptible to acid erosion.37,47 Capsulated GIC have been reported to be more costly per application in comparison to their hand-mixed equivalents.18 Dental practitioners may thus elect to use hand-mixed glass ionomers instead of the capsulated equivalents of these materials. Prentice et al.,36experimented with the mixing times of GIC. It was found that optimal strength and handling properties required a capsule-mixing time of between eight to ten seconds.36 Decreasing the mixing time was shown to prolong the working time and the setting time.36 Increasing the mixing time led to increased viscosity of the material and reduced both the working and setting time.36 The specimens that were mixed for 12 seconds showed an increase in modulus of elasticity and compressive strength.36 If the mixing time was increase to 14 seconds, gelation occurred too quickly, rendering the dispensing and placement of the material very difficult.36 This increased mixing time also decreased the modulus of elasticity and compressive.36 In busy private practices, dentists may attempt to take advantage of the increased viscosity and decreased working and setting times of longer mixed capsulated GIC, however it is cautioned that this may negatively affect the mechanical properties of the final cement.36
The fluoride release profile of GIC could be influenced by the mixing method of these materials. De Moor et al.,48 reported that mechanical mixing of capsulated GIC lead to a more predictable fluoride release than hand-mixed GIC.48 Fluoride release is dependent on the acid-base and setting reactions.47 This finding indirectly implies that both reactions are more consistent in capsule-mixed GIC.48
CONCLUSION
Within the limitations of the present study, it is evident that the correct mixing technique of glass ionomer cements is crucial to achieve the optimal mechanical properties of the final set material. The properties tested in this study showed that glass ionomer materials displayed variations that directly link the mixing technique to the resulting mechanical properties of the end-product.
The compressive strength of the capsule-mixed RSC was superior to hand-mixed RSC, indicating that the capsule-mix version of this material is advantageous for clinical use where occlusal forces are higher. Although FIXC performed better in surface hardness tests, no conclusion can be made about the superiority in compressive strength of the capsule-mix versus that of the hand-mixed product. Although not statistically significant, both the surface hardness and compressive strength of KUC were slightly better than those of KUH. Both the compressive strength and the surface hardness values of KMC were significantly higher than those of KMH.
Overall, the findings of the present study suggest that capsulated glass ionomer cements are superior to their hand-mixed counterparts. Therefore, these materials should be advocated for clinical use. The clinician is reminded that when using glass ionomer materials, optimal clinical results can only be accomplished if the correct mixing method/ technique, mixing time and powder to liquid ratio are applied. This applies for both hand-mixed and capsulated variants of this dental material.
Acknowledgements
The South African Division of the IADR contributed to the research related to this article, by awarding funds from the Cornelius Pameijer Fellowship. Funds were provided by the University of Pretoria, Faculty of Health Sciences to purchase the 3M ESPE glass ionomers. GC South Africa donated the GC glass ionomers included in this study. Wright-Millners, South Africa donated the SDI glass ionomers included in this study. The authors declare that the dental materials tested in the experiments for this study were provided by each one of the sponsors, without, any prerequisites, limitations and/or terms, and/or conditions in the testing of and/or recording or publication of any results, for each of the dental materials that were assessed. Thus, all reported findings of this current research study are objective and free of bias or influence from the sponsors.
Conflict of interest
The authors declare that they have no conflict of interest related to any aspect of this research project.
REFERENCES
1. Savin C, Petcu A, Gavrilӑ L, Mӑrțu-Ştefanache M-A, Bӑlan A. Dental materials for coronary obturation utilized in pedodontics. Int J Med Dent. 2016; 20(3):171-6. [ Links ]
2. Pitel ML. Reconsidering glass-ionomer cements for direct restorations. Compend Contin Educ Dent. 2014; 35(1):26-31. [ Links ]
3. Sidhu SK, Nicholson JW. A review of glass-ionomer cements for clinical dentistry. J Funct Biomater. 2016; 7(3):1-15. [ Links ]
4. Ilie N, Hickel R. Mechanical behavior of glass ionomer cements as a function of loading condition and mixing procedure. Dent Mater J. 2007; 26(4):526-33. [ Links ]
5. Van Meerbeek B, De Munck J, Yoshida Y, et al. Adhesion to enamel and dentin: Current status and future challenges. Oper Dent. 2003; 28(3):215-35. [ Links ]
6. Forsten L. Fluoride release and uptake by glass ionomers. Scand J Dent Res. 1991; 99(3):241-5. [ Links ]
7. Croll TP, Nicholson J. Glass ionomer cements in pediatric dentistry: Review of the literature. Pediatr Dent. 2002; 24(5):423-9. [ Links ]
8. Knight GM. The benefits and limitations of glass-ionomer cements and their use in contemporary dentistry. In: Glass-ionomers in dentistry: Springer; 2016: 57-79. [ Links ]
9. Xie D, Brantley W, Culbertson B, Wang G. Mechanical properties and microstructures of glass-ionomer cements. Dent Mater. 2000; 16(2):129-38. [ Links ]
10. Dowling AH, Fleming GJ. Are encapsulated anterior glass-ionomer restoratives better than their hand-mixed equivalents? J Dent. 2009; 37(2):133-40. [ Links ]
11. Wilson AD, Nicholson JW. Acid-base cements: Their biomedical and industrial applications: Cambridge University Press; 2005. [ Links ]
12. Baig MS, Fleming GJ. Conventional glass-ionomer materials: A review of the developments in glass powder, polyacid liquid and the strategies of reinforcement. J Dent. 2015; 43(8):897-912. [ Links ]
13. Nomoto R, McCabe JF. Effect of mixing methods on the compressive strength of glass ionomer cements. J Dent. 2001; 29(3):205-10. [ Links ]
14. 3M ESPE Canada. Product specification for Ketac Molar Quick Aplicap. 3M ESPE Canada Multimedia Online Resources I [updated 19 Jan 2012; cited 2019 15 August] Available from: https://multimedia.3m.com/mws/media/296110O/3m-ketac-molar-quick-aplicap-glass-ionomer-filling-material.pdf. [ Links ]
15. 3M ESPE Deutschland. Product specification for Ketac Universal Aplicap. [updated 16 Aug 2016; cited 2019 24 July]. Available from: http://multimedia.3m.com/mws/media/1090408O/ketac-universal-aplicap-technical-product-profile-pdf.pdf [ Links ]
16. Kaushik M, Sharma R, Reddy P, Pathak P, Udameshi P, Jayabal NV. Comparative evaluation of voids present in conventional and capsulated glass ionomer cements using two different conditioners: An in vitro study. Int J Biomater. 2014; 2014:1-5. [ Links ]
17. Dowling AH, Fleming GJ. Is encapsulation of posterior glass-ionomer restoratives the solution to clinically induced variability introduced on mixing? Dent Mater. 2008; 24(7):957-66. [ Links ]
18. Fleming GJ, Farooq AA, Barralet JE. Influence of powder/ liquid mixing ratio on the performance of a restorative glass-ionomer dental cement. Biomater. 2003; 24(23):4173-9. [ Links ]
19. Billington R, Williams J, Pearson G. Variation in powder/ liquid ratio of a restorative glass-ionomer cement used in dental practice. Br Dent J. 1990; 169(6):164-7. [ Links ]
20. Fleming G, Marquis PM, Shortall A. The influence of clinically induced variability on the distribution of compressive fracture strengths of a hand-mixed zinc phosphate dental cement. Dent Mater. 1999; 15(2):87-97. [ Links ]
21. Mount GJ, Hume WR, Ngo HC, Wolff MS. Preservation and restoration of tooth structure: John Wiley & Sons; 2016. [ Links ]
22. Fleming G, Zala D. An assessment of encapsulated versus hand-mixed glass ionomer restoratives. Oper Dent. 2003; 28(2):168-77. [ Links ]
23. White SN, Yu Z. Compressive and diametral tensile strengths of current adhesive luting agents. Journal Prosthet Dent. 1993; 69(6):568-72. [ Links ]
24. Mitchell C, Douglas W. Comparison of the porosity of hand-mixed and capsulated glass-ionomer luting cements. Biomater. 1997; 18(16):1127-31. [ Links ]
25. SDI Australia. Product specification for Riva Self cure HV. [updated 25 September 2017; cited 2019 24 July]. Available from: http://www.sdi.com.au/WPENGINE/wpcontent/uploads/brochure/brochures_EN/RIVA_SC_ Bro_EN.pdf [ Links ]
26. 3M ESPE Deutschland. Product specifications for Ketac Universal Powder and Liquid. [updated 25 Nov 2016; cited 2019 24 July]. Available from: http://multimedia.3m.com/mws/_media/1090406O/_3m-ketac-universal-handmix-technical-product-profile-ltr-global.pdf [ Links ]
27. Fleming GJ, Kenny SM, Barralet JE. The optimisation of the initial viscosity of an encapsulated glass-ionomer restorative following different mechanical mixing regimes. J Dent. 2006; 34(2):155-63. [ Links ]
28. McKinney J, Antonucci J, Rupp N. Wear and microhardness of glass-ionomer cements. J Dent Res. 1987; 66(6):1134-9. [ Links ]
29. Fleming GJ, Dowling AH, Addison O. The crushing truth about glass ionomer restoratives: Exposing the standard of the standard. J Dent. 2012; 40(3):181-8. [ Links ]
30. Baig MS, Dowling AH, Cao X, Fleming GJ. A discriminatory mechanical testing performance indicator protocol for hand-mixed glass-ionomer restoratives. Dent Mater. 2015; 31(3):273-83. [ Links ]
31. GC America. Operatory instructions for GC Fuji IX GP capsules. [updated 5 Apr 2019; cited 2019 16 September]. Available from: http://www.gcamerica.com/products/operatory/GC_Fuji_ IX_GP/325282-GCFujiIXGP-IFU4L. pdf [ Links ]
32. Baig MS, Dowling AH, Fleming GJ. Hertzian indentation testing of glass-ionomer restoratives: A reliable and clinically relevant testing approach. J Dent. 2013; 41(11):968-73. [ Links ]
33. Coldebella CR, Santos-Pinto L, Zuanon ACC. Effect of ultrasonic excitation on the porosity of glass ionomer cement: A scanning electron microscope evaluation. Microsc Res Tech. 2011; 74(1):54-7. [ Links ]
34. Menne-Happ U, Ilie N. Effect of heat application on the mechanical behaviour of glass ionomer cements. Clin Oral Investig. 2014; 18(2):643-50. [ Links ]
35. Zoergiebel J, Ilie N. Evaluation of a conventional glass ionomer cement with new zinc formulation: Effect of coating, aging and storage agents. Clin Oral Investig. 2013; 17(2):619-26. [ Links ]
36. Prentice LH, Tyas MJ, Burrow MF. The effect of mixing time on the handling and compressive strength of an encapsulated glass-ionomer cement. Dent Mater. 2005; 21(8):704-8. [ Links ]
37. 37. Mulder R, Mohamed N. Variation of powder/liquid ratios of capsulated glass-ionomer materials. NZ Dent J. 2019; 115(2):47-56. [ Links ]
38. Dionysopoulos D, Tolidis K, Strakas D, Gerasimou P, Sfeikos T, Gutknecht N. Effect of radiant heat on conventional glass ionomer cements during setting by using a blue light diode laser system (445 nm). Lasers Med Sci. 2017; 32(3):703-9. [ Links ]
39. Alrahlah A. Diametral tensile strength, flexural strength, and surface microhardness of bioactive bulk All restorative. J Contemp Dent Pract. 2018; 19(1):13-9. [ Links ]
40. Yap A, Cheang P, Chay P. Mechanical properties of two restorative reinforced glass-ionomer cements. J Oral Rehabil. 2002; 29(7):682-8. [ Links ]
41. Hamid DMA, Mahmoud GM, El-Sharkawy FM, Auf EAA. Effect of surface protection, staining beverages and aging on the color stability and hardness of recently introduced uncoated glass ionomer restorative material. Fut Dent J. 2018; 4(2):288-96. [ Links ]
42. Šalinović I, Stunja M, Schauperl Z, Verzak Ž, Malčić AI, Rajić VB. Mechanical properties of high viscosity glass ionomer and glass hybrid restorative materials. Act Stomato Croa. 2019; 53(2):125-31. [ Links ]
43. GC Europe. Operatory instructions for Fugi IX GP. [updated 18 January 2018; cited 2019 1 August]. Available at: https://cdn.gceurope.com/v1/PID/fuji9gp/leaflet/LFL_Fuji_IX_GP_(FAST)_ en.pdf [ Links ]
44. Dionysopoulos D, Tolidis K, Tortopidis D, Gerasimou P, Sfeikos T. Effect of a calcium chloride solution treatment on physical and mechanical properties of glass ionomer cements. Odontology. 2018; 106(4):429-38. [ Links ]
45. 3M ESPE Canada. Product brochure for Ketac Molar Easymix. [updated 19 March 2004; cited 2019 26 September]. Available at: http://multimedia.3m.com/mws/media/273485O/ketac-molar-easymix-brochure.pdf. [ Links ]
46. Guggenberger R, May R, Stefan K. New trends in glassionomer chemistry. Biomaterials. 1998; 19(6):479-83. [ Links ]
47. Bueno LS, Silva RM, Magalhães APR, et al. Positive correlation between fluoride release and acid erosion of restorative glass-ionomer cements. Dent Mater. 2019; 35(1):135-43. [ Links ]
48. De Moor RJ, Verbeeck RM, De Maeyer EA. Fluoride release profiles of restorative glass ionomer formulations. Dent Mater. 1996; 12(2):88-95. [ Links ]
 Correspondence:
Correspondence:
Samantha Arnold
E-mail: samantha.arnold@up.ac.za
Telephone: +27 12 319 2559 / 084 521 2429
Author contributions:
1 . Samantha Arnold - 40%
2 . Nichola Warren - 25%
3 . Glynn D. Buchanan - 20%
4 . Riaan Lombard - 15%














