Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Dental Journal
On-line version ISSN 0375-1562
Print version ISSN 0011-8516
S. Afr. dent. j. vol.77 n.1 Johannesburg Feb. 2022
http://dx.doi.org/10.17159/2519-0105/2022/v77no1a3
RESEARCH
Frozen sections in head and neck surgery and the impact of intraoperative analysis on final resection margins: An institutional study
T KungoaneI; L RobinsonII; T K MadibaIII
IBDS, MSc (Dent) Oral Pathology, FC Path (SA) Oral, M Dent (Oral Pathology), Oral Pathologist/Head Clinical Unit/ Senior lecturer, Department of Oral Pathology and Oral Biology, School of Dentistry, University of Pretoria, ORCID ID:0000-0003-4312-7418
IIBChD, PDD (MaxillofacialRadiology), PDD (Forensic Odontology). Registrar (Oral Pathology), Department of Oral Pathology and Oral Biology, School of Dentistry, University of Pretoria, ORCID ID: 0000-0002-0549-7824
IIIB Dent Ther, BDS, DHSM, MChD (Community Dentistry). Head Clinical Unit/ Adjunct Professor, Department of Community Dentistry, School of Dentistry, University of Pretoria, ORCID ID:0000-0002-0171-0595
ABSTRACT
BACKGROUND: Frozen section (FS) analysis is an indispensable tool for intraoperative patient management.
AIMS: To assess the utilisation of head and neck FS analysis, with a particular focus on the concordance rate between the intraoperative FS margin analysis and the final FFPE results. Additionally, to determine whether FS analysis had any impact on intraoperative patient management. Lastly, to determine the impact of the FS analysis on the final margin status of resection specimens.
MATERIALS AND METHODS: Histopathology reports from January 2015 to December 2018 were reviewed at Pretoria Oral and Dental Hospital to analyse all FS requests involving the head and neck region. Captured data was analysed to determine the concordance rate, discordance rate, and FS deferral rates, with correlations performed using the Chi-square test.
RESULTS: Eighty-two frozen section cases were reviewed with a total of 312 FS tissue sections performed. The majority (73%) of the FS requests were from the Maxillofacial and Oral Surgery (MFOS) department for the assessment of surgical margins. The FS-FFPE concordance and discordance rates were at 97.5% and 2.4% respectively, with a deferral rate of 1.2%. Additional surgical margins were only received in 16 of the 26 cases with positive margins on intraoperative FS analysis. There was no statistically significant correlation between intraoperative FS positive margin status and advanced pathological T staging.
CONCLUSIONS: The concordance rate between intraoperative FS margin analysis and final FFPE results were within an acceptable range. In a significant number of cases, the intraoperative FS margin analysis did not influence further surgical management.
Keywords: Head and Neck Pathology, Frozen Section Analysis, Resection Margins
INTRODUCTION
Frozen section (FS) analysis is an invaluable adjunct in surgical pathology. The procedure is done for immediate intraoperative diagnosis to guide intraoperative patient management.1 The reasons for intraoperative FS requests include the evaluation of surgical margins, confirmation of malignancy, tumour classification, assessment of tissue viability for organ transplant, and the evaluation of lymph nodes for sentinel metastasis.2 Due to the constraints of the head and neck tissue spaces, FSs are most commonly requested to assess surgical margins.3-7 In an ideal clinical setting, an intraoperative FS analysis confirming involved margins by tumour should propel surgeons to alter the surgical management and submit additional margins.7-8
Several head and neck studies exist in the literature evaluating the concordance rates between intraoperative FS diagnosis and the final formalin-fixed paraffin-embedded (FFPE) tissue diagnosis. In these studies, the reported concordance ranges between 90-97%.2,9-11 Literature is sparse on the concordance rate between intraoperative FS margin analysis and final FFPE results, and the impact of FS analysis on the final resection margin status.11,12 The current study aims to determine the utilisation of head and neck FS analysis at our institution, with a particular focus on the concordance rate between the intraoperative FS margin analysis and the final FFPE results. Additionally, to determine whether FS analysis had any impact on intraoperative patient management. Lastly, the study aims to determine the impact of the FS analysis on the final margin status of resection specimens.
MATERIALS AND METHODS
A four-year retrospective study evaluating pathology reports at Pretoria Oral and Dental Hospital, Gauteng was undertaken. This included patients operated at the Steve Biko Academic Hospital, located within the vicinity of the histopathology laboratory. All FS requests involving the head and neck region performed from 1 January 2015 to 31 December 2018 were included in the study. Reports were accessed from the electronic pathology database of the Department of Oral Pathology and Oral Biology.
These reports were given study numbers to ensure anonymity. The information collected included: surgical departments requesting the FS, reasons for the request, initial histological diagnosis if known, number of tissue fragments frozen per surgical procedure, intraoperative FS margin analysis (positive or negative for tumour), final FFPE results (positive or negative for tumour), resection specimen margin status (positive for tumour, tumour <5mm from margin or negative for tumour), lymph node status and the final pathological diagnosis. Discrepancies between the intraoperative FS analysis and final FFPE results were documented as yes or no, and the reasons, if available, were recorded. Errors, whereby the FS analysis did not correlate with the final margin status, were recorded as processing errors (gross sampling, histological sampling and surgical sampling) or interpretation errors (false positive and false negative results) based on the final pathology reports. Tumour misclassifications, whereby the intraoperative FS tumour diagnosis did not correlate with the final FFPE diagnosis, were not assessed in this study.
Statistical analysis
Statistical analysis was performed using SPSS (IBM, version 25) software. Captured data was analysed to determine the concordance rate, discordance rate, and FS deferral rates. The concordance rate was determined by comparing the agreement between the intraoperative FS analysis and the final FFPE findings. Correlation of the FS analysis and the final FFPE results were performed as well as a calculation of sensitivity and specificity of the FS analysis compared to the final FFPE results. FS analysis was correlated with the final margin status and the pathological staging. Correlations were performed using the Chi-square test, where a p-value of less than 0.05 was considered statistically significant.
RESULTS
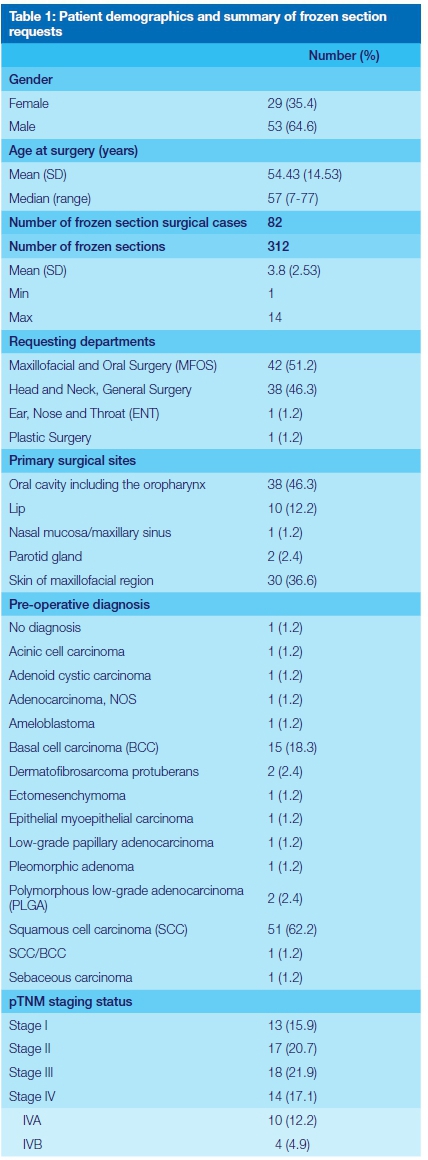
A total of 82 cases, whereby FS analysis was requested, were reviewed. In total 312 FS tissue sections were performed, with an average of 3.16 FS tissue sections performed per surgical case. Most of the surgical FS requests were from the Maxillofacial and Oral Surgery (MFOS) department (51%), with the majority (73%) of the requests to determine surgical margin status. Most tissues were submitted from the oral cavity (46.3%), mainly from the tongue (13.4%). None of the patients received prior chemotherapy and or radiation. These results are summarised in Table 1.
Concordance rates between the intraoperative frozen section margin analysis and the final FFPE results
The concordance rates were calculated on 79 of 82 cases as shown in Table 2. In these 79 cases, only two cases were discordant, resulting in a FS-FFPE concordance rate of 97.5%. Only in a single surgical case was the diagnosis deferred, with a deferral rate of 1.2%.
Impact of the intraoperative frozen section margin analysis on the final margin status
Intraoperative FS analysis was performed to determine margin status in 60 of 82 cases. In these 60 cases, 26 were diagnosed with positive margins involved by tumour and a single case with atypical cells. Additional surgical margins were only submitted in 15 (55.5%) of these 27 cases.
Thirty-three (55%) FS cases were diagnosed with negative margins. In these cases, 15 had a final negative surgical margin status, 9 with close margins (<5mm) and 9 with positive margins. These results are summarised in Table 3.
Intraoperative frozen section margin analysis, final margin status and pathological staging
An intraoperative positive margin status was recorded in 27 FS cases (26 positive margins and a single case showing atypical cells). This was correlated with the final pathological TNM staging (UICC, TNM Classification of Malignant Tumours, 7th Ed.)13 as depicted in Table 1. Of these, only a single surgical case was not staged. In those with a pathological TNM staging, the majority of the cases were pT2 (42%), followed by pT1 (34%), pT3 (15.3%) and pT4 (7.9%). There was no statistically significant correlation between an intraoperative FS positive margin status and an advanced pT staging (p=0.42). However, a statistically significant correlation existed between advanced tumour stage (III-IV) and a final positive margin status (p=0.001).
DISCUSSION
Frozen section analysis forms an integral part of surgical pathology with the main objective of effective and timely intraoperative patient management.13 Essential information required before performing the FS procedure should include the date and time of the surgery, reasons for the FS request and preoperative diagnosis, if available.14 Not all FS requests in cases where a preoperative diagnosis is unknown will yield an immediate diagnosis, and if any doubt, the diagnosis may be deferred. The practice of intraoperative FS analysis places pathologists directly within the patient management decision-making team, hence the results of the FS should be documented in the patients' operative notes. FS requests should aid patient care and caution must be exercised to avoid inappropriate FS requests.15 Frozen section should not be requested if the results have no bearing on intraoperative patient management.
Intraoperative FS artefacts may hinder histological assessment and diagnosis. These include tissue shrinkage, folds and tears, and bubbles under the coverslip amongst others.16 Cautery artefact produced from electrocautery during surgery may also hinder accurate histological assessment, both intraoperatively and on the final resection specimen. Pathologists may request tissue from the surgical bed which was not cauterised to improve assessment. In addition, discordance between the intraoperative FS margin analysis and the final FFPE results may occur.9 These discrepancies may result from pre-analytical errors in gross sampling, histologic sampling or surgical sampling. Gross sampling errors occur when the lesional tissue is present in the specimen, but was not sampled during the FS. Lesional tissue present within the tissue frozen, but not on the FS slide accounts for histologic sampling error. In contrast, surgical sampling errors are surgeon dependent, and occur when non-lesional tissue is sampled by the surgical team for FS analysis and subsequently lesional tissue submitted as a separate specimen for final analysis.9 Post-analytical interpretation errors include false positive and false negative results, as well as tumour misclassifications whereby the intraoperative FS tumour diagnosis does not correlate with the final FFPE diagnosis.9 Not all tissue is suitable for intraoperative FS analysis. Adipose tissue is difficult to freeze, whilst cartilage is difficult to keep on the slide as it often washes off in alcohol. Bone is difficult to cut with a cryostat and often requires decalcifying. FS analysis on tissue from the marrow cavity of bony margins is however possible, and may be useful in assessing the presence of tumour in the bony margin intraoperatively.
Clear margins are crucial in determining the need for additional surgical resection and/or neoadjuvant therapy, and for overall patient prognosis.17,18 The head and neck region is a confined space with continuous tissue compartments, posing challenges in obtaining tumour-free margins. Numerous studies have evaluated the use of FS analysis in the head and neck region to assess surgical margins, with correlation of intraoperative FS findings and final FFPE results.4,5911,12,19 The adequacy of intraoperative FS margin analysis for head and neck squamous cell carcinomas has been reported at approximately 97%, with 83% sensitivity and 98% specificity.11 While figures are variable in reported studies, it is nonetheless consistently above 90%, which compares favourably with FS margin status evaluation at other body sites.20
The discordance rate between FS findings and the final FFPE diagnosis in the current study was 2.4%, with a sensitivity and specificity of 96.9% and 97.8% respectively. This is in line with current literature, where discordance rates in the head and neck region ranged from 1.4% to 11.8%, with a mean of approximately 3.2%.11 The two cases in our study which contributed to this discordance rate culminated from sampling errors and misinterpretation respectively. Sampling errors occur when the FS analysis is tumour-free and the final FFPE diagnosis shows the presence of tumour. A study by Gandour-Edwards et al.,21found that 83.8% of errors resulted from sampling rather than an interpretive error. Intraoperative tissue sampling techniques for margin assessment is a contentious issue. Thomas-Robbins et al.,3reviewed literature comparing two tissue harvesting techniques, the tumour-directed (from the resected specimen) sampling approach and the patient-directed (from the tumour bed) sampling approach. Their study found the tumour-directed specimen approach to be superior in assessing margins. Interpretation errors occur when the results of the FS analysis are not confirmed on the final FFPE permanent sections.22 When in doubt, the pathologist may defer the diagnosis, which prevents interpretive error. In this study the deferral rate was 1.2%, which is within ranges reported in the head and neck region in the literature.8
It was interesting to note that intraoperatively, additional surgical margins were only received in 16 of the 26 cases with positive margins on intraoperative FS analysis. This finding shows that in 10 cases, the results of the FS analysis did not have any influence on further intraoperative patient management. The reasons why additional margins were not submitted were not documented in the surgical notes. A variety of speculative reasons exist why additional margins were not submitted, including limited operative time and anatomical constraints. Hence, proper preoperative surgical planning is crucial to ensure adequate resectability of tumours.
Nine cases with negative intraoperative FS margin analysis showed positive margins on the final resection specimen. This was attributed to an intraoperative gross sampling error by either the surgeon or the pathologist. A 2006 pathological survey found that most surgeons submit small tissue fragments to pathologists for intraoperative margin assessment.10 Although this approach has its advantages, it may also underestimate the real status of resection margins, particularly in complex tumour resections. Hinni et al.,23recommended that intraoperative margin surveillance should be specimen dependent, with surgeons and pathologists participating in specimen mapping. This approach allows for effective intraoperative communication between surgeons and pathologists, ensuring that true margins are adequately sampled and assessed.
The current study noted several cases with positive final margins even after additional surgical margins were taken. To enable accurate additional resection, a study by van Lanschot et al.,24advocated a paired tagging of the tumour bed and the resection specimen to allow for easy review in cases of positive margins. In general, tissue under tension will contract following resection. Studies addressing margin shrinkage in patients with head and neck cancer found mucosal contraction in the order of 20% to 25%.8,21 This is in contrast to Chen et al.,25who reported average shrinkage in length, width, and depth at 4.40%, 6.18%, and 4.10% respectively.
This study also evaluated the correlation between the FS margin status and the final resection margin status. Two out of 60 cases (3.3%) showed discordant results between the FS analysis and the final resection margin status, with one case having a positive final margin status. This figure is well below the rates reported by Ord and Aisner in which 7 patients (14.5%) had final positive margins not detected on FS analysis.6 The current results on final margin status should be viewed with caution, as a clear margin on FS analysis is dependent on whether the whole margin was examined histologically and if the margin submitted intraoperatively is the same margin sampled on the resection specimen.4
This study did not And any significant correlation between intraoperative positive margin status and pathological T stage. However, a statistically significant correlation was noted between final positive margin status and pTNM staging. Tumours with an advanced tumour stage (III-IV) were more likely to show positive margins on the final resection specimen. These results are similar to a study by Gerber et al.,26 in which positive final surgical margins increased by a factor of Ave in pT4-stage tumours compared to pT1 tumours. These findings are likely due to the extent of disease in T4 tumours with infiltration into the surrounding soft tissue and bone affecting the ability of surgeons in acquiring tumour-free margins.
Finally, it should be noted that achieving negative surgical margins does not guarantee local disease control. Many researchers have suggested other methods to stratify patient risk for local disease recurrence, regardless of clear surgical margins intraoperatively. For example, a commonly used method proposed by Brandwein-Gensler et al.,17suggests using a histologic risk assessment based on the worst pattern of invasion to differentiate indolent from more aggressive tumours.
The limitations of this study centers around its retrospective nature, based solely on pathological reports within a small sample size. In addition, there was no information as to why additional margins were not submitted in cases with positive intraoperative margins. When assessing margins intraoperatively, dysplasia at margins was not documented in most cases, and surgical margins were usually recorded as positive or negative for tumour. A 5 mm cut-off was used to indicate a close margin on the final resection specimen, however, this criteria was not applied during intraoperative FS analysis. These limitations may have influenced the final resection margin status.
CONCLUSION
Although the concordance rate between intraoperative FS margin analysis and the final FFPE results in this study is within an acceptable range, there is room for improvement. Preoperative surgical planning, including advanced diagnostic imaging, is important to avoid unnecessary FS requests that have no impact on intraoperative surgical management. This was illustrated in the current study by the lack of additional margins in cases with positive intraoperative FS margins. It is vital that adequate sampling is done intraoperatively to avoid false negative results. A negative FS margin status may not correlate with a tumour-free margin on the resection specimens as this is dependent on tissue sampling.
DECLARATIONS
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the University of Pretoria, Faculty of Health Sciences Research Ethics Committee (Reference no.: 531/2018). All procedures followed the ethical standards of the Helsinki Declaration of 1975, as revised in 2008.
References
1. Gal AA. The centennial anniversary of the frozen section technique at the Mayo Clinic. Arch Pathol Lab Med. 2005;129(12):1532-1535. [ Links ]
2. Sawady J, Berner JJ, Siegler EE. Accuracy of and reasons for frozen sections: a correlative, retrospective study. Hum Pathol. 1988;19(9):1019-1023. [ Links ]
3. Thomas Robbins K, Triantafyllou A, Suárez C, et al. Surgical margins in head and neck cancer: intra- and postoperative considerations. Auris Nasus Larynx. 2019;46(1):10-17. [ Links ]
4. Olson SM, Hussaini M, Lewis Jr JS. Frozen section analysis of margins for head and neck tumor resections: reduction of sampling errors with a third histologic level. Original Article. Mod Pathol. 2011;24:665. [ Links ]
5. DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. The Laryngoscope. 2000;110(10):1773-1776. [ Links ]
6. Ord RA, Aisner S. Accuracy of frozen sections in assessing margins in oral cancer resection. J Oral Maxillofac Surg. 1997;55(7):663-669. [ Links ]
7. Mohiyuddin SMA, Padiyar BV, Suresh TN, et al. Clinicopathological study of surgical margins in squamous cell carcinoma of buccal mucosa. World Journal of Otorhinolaryngology-Head and Neck Surgery. 2016;2(1):17-21. [ Links ]
8. Mistry RC, Qureshi SS, Kumaran C. Post-resection mucosal margin shrinkage in oral cancer: Quantification and significance. J Surg Oncol. 2005;91(2):131-133. [ Links ]
9. Sams SB, Wisell JA. Discordance between intraoperative consultation by frozen section and final diagnosis: a classification model to guide quality improvement. Int J Surg Pathol. 2017;25(1):41-50. [ Links ]
10. Raab SS, Tworek JA, Souers R, Zarbo RJ. The Value of Monitoring Frozen Section-Permanent Section Correlation Data Over Time. Arch Pathol Lab Med. 2006;130(3):337-342. [ Links ]
11. Layfield EM, Schmidt RL, Esebua M, Layfield LJ. Frozen section evaluation of margin status in primary squamous cell carcinomas of the head and neck: a correlation study of frozen section and final diagnoses. journal article. Head Neck Pathol. 2018;12(2):175-180. [ Links ]
12. Du E, Ow TJ, Lo YT, et al. Refining the utility and role of frozen section in head and neck squamous cell carcinoma resection. The Laryngoscope. 2016;126(8):1768-1775. [ Links ]
13. Roy S, Parwani AV, Dhir R, Yousem SA, Kelly SM, Pantanowitz L. Frozen section diagnosis: is there discordance between what pathologists say and what surgeons hear? Am J Clin Pathol. 2013;140(3):363-369. [ Links ]
14. Taxy JB. Frozen section and the surgical pathologist: a point of view. Arch Pathol Lab Med. 2009;133(7):1135-1138. [ Links ]
15. McIntosh ER, Harada S, Drwiega J, Brandwein-Gensler MS, Gordetsky J. Frozen section: guiding the hands of surgeons? Ann Diagn Pathol. 2015;19(5):326-329. [ Links ]
16. Desciak EB, Maloney ME. Artifacts in frozen section preparation. Dermatol Surg. 2000;26(5):500-504. [ Links ]
17. Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. The American journal of surgical pathology. 2005;29(2):167-178. [ Links ]
18. Upile T, Fisher C, Jerjes W, et al. The uncertainty of the surgical margin in the treatment of head and neck cancer. Oral Oncol. 2007;43(4):321-326. [ Links ]
19. Melancon CC, Clinger JD. The use of frozen section in the early diagnosis of acute invasive fungal sinusitis. Otolaryngology-Head and Neck Surgery. 2017;157(2):314-319. [ Links ]
20. Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129(12):1536-1543. [ Links ]
21. Gandour-Edwards RF, Donald PJ, Wiese DA. Accuracy of intraoperative frozen section diagnosis in head and neck surgery: experience at a university medical center. Head Neck. 1993;15(1):33-38. [ Links ]
22. Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice. The results of an International American Head and Neck Society member survey. Head Neck. 2005;27(11):952-958. [ Links ]
23. Hinni ML, Ferlito A, BrandweinBGensler MS, et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35(9):1362-1370. [ Links ]
24. van Lanschot CG, Mast H, Hardillo JA, et al. Relocation of inadequate resection margins in the wound bed during oral cavity oncological surgery: A feasibility study. Head Neck. 2019:1-8. [ Links ]
25. Chen C-H, Hsu M-Y, Jiang R-S, Wu S-H, Chen F-J, Liu S-A. Shrinkage of head and neck cancer specimens after formalin fixation. J Chin Med Assoc. 2012;75(3):109-113. [ Links ]
26. Gerber S, Gengler C, Grätz KW, Kruse AL. The impact of frozen sections on final surgical margins in squamous cell carcinoma of the oral cavity and lips: a retrospective analysis over an 11 years period. Head Neck Oncol. 2011;3(1):56. [ Links ]
 Correspondence:
Correspondence:
Prof TK Madiba
Email: thommy.madiba@gmail.com
Tel: (012) 319-2417 Cell: 0845036175
Author contributions:
1 . Dr T Kungoane: 36%
2 . Dr LM Robinson: 34%
3 . Prof TK Madiba: 30%














