Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.73 no.7 Johannesburg Ago. 2018
http://dx.doi.org/10.17159/2519-0105/2018/v73no7a2
RESEARCH
Debris contamination of endodontic hand files in dental practice
GD BuchananI; N WarrenII; MY GamieldienIII
IBChD, PDD (Endo), MSc (Dent). Department of Odontology, School of Dentistry, University of Pretoria, Pretoria, South Africa
IIBChD, PGDipDent (Endo), MSc (Odont). Department of Odontology, School of Dentistry, University of Pretoria, Pretoria, South Africa
IIIBChD, PGDipDent (Oral Surg), MSc (Anat). Department of Maxillo-facial and Oral Surgery, School of Dentistry, University of Pretoria, Pretoria, South Africa
ABSTRACT
INTRODUCTION: The risk of cross-contamination validates the need to assess how adequately dental instruments can be cleaned and sterilised
AIMS AND OBJECTIVES: This study aimed to evaluate residual debris contamination of endodontic hand files collected from private practice following routine reprocessing procedures
DESIGN: A cross-sectional observational study was conducted
METHODS: Clinically used and reprocessed endodontic hand files were collected from 27 dental practices. Information regarding the routine decontamination procedures of each practice was also submitted. The endodontic files were assessed by two previously calibrated examiners using a stereomicroscope and were scored for the presence or absence of remnant debris using a modified scoring system. Statistical evaluation of the data estimated the frequency and proportions of debris in each scoring position. Cohen's Kappa statistic assessed inter-examiner agreement and groups were compared using Fisher's Exact test
RESULTS: In total, 401 endodontic hand files were examined. Debris was found on 94% of files. Inter-examiner agreement was fair to moderate over the entire dataset. Group B was found to contain significantly less debris than the other groups
CONCLUSION: Routine decontamination methods used in general dental practice do not effectively remove debris from endodontic hand files
Key words: Cross-contamination, debris, decontamination, endodontics
INTRODUCTION
Endodontic hand files are instruments used to prepare the root canals of teeth.1 Historically, the re-use of these instruments, after cleaning and sterilisation, to treat multiple patients has been regarded as safe clinical practice. Although some regions, such as the United Kingdom, have made the single-use of endodontic files obligatory,2 economic pressures in many countries may dictate a high rate of the re-use of these instruments.
Contaminated dental instruments must be both cleaned and sterilised prior to re-use.1,3,4 This is necessary to prevent cross infection between patients and to avoid introducing additional foreign microorganisms into the root canal system, which potentially may compromise treatment outcomes.1
The risk of transmission of both infectious diseases5,6 as well as of prion disease2 validates the need to assess the cleanliness of all dental instruments, including endodontic hand files, prior to reuse. The presence of biological debris, such as blood, dentin, dental pulp tissue and microorganisms on endodontic files may hinder the sterilisation of these instruments.7,8 Evidence contrary to this possibility, does however, exist.9-11 A 2004 study showed no bacterial growth after steam sterilisation, irrespective of the cleaning method used.10
Previous studies have demonstrated the inadequacy of routine decontamination procedures in rendering dental instruments free of biological debris.2,12-14 However, these studies were conducted elsewhere and no information regarding the cleaning of endodontic files in South Africa could be found. A cleaning protocol for endodontic files was developed by an Australian research group in 2004 that consistently resulted in rotary nickel-titanium endodontic files which were completely free of biological debris.15 Whilst complete decontamination of endodontic files was shown to be possible under the conditions of that study, it is questionable whether this reflects the situation found in everyday clinical practice.
The present study aimed to determine the extent of residual debris contamination of endodontic hand files following the application of routine reprocessing procedures in private general dental practice in Pretoria, South Africa. Details regarding the decontamination methods used by the participants were also recorded.
METHOD AND MATERIALS
Ethical clearance was obtained from the Ethics Committee of the Faculty of Health Sciences, University of Pretoria, prior to the commencement of the investigation. A cross-sectional, observational study was designed and conducted.
Twenty-seven general dental practices in Pretoria, South Africa, were contacted and invited to participate on a voluntary basis. Convenience sampling had been used to select practices known to provide endodontic services. Each participant was requested to submit 15 endodontic hand files which had undergone the decontamination processes following clinical use which was routinely used by their practice. Hand files of the K-type and Hedstrom files were included. Barbed broaches, rotary files and other hand instruments (e.g. finger spreaders) were excluded. The decontamination methods employed by each practice were also recorded. The number of previous uses of each file was not recorded. An independent moderator assigned the group of files from each practice (n=27) a unique randomised identifier to de-identify the samples from each participant. A calibration group, comprising 15 stainless steel hand files (M-Access, Dentsply Maillefer, Baillegues, Switzerland), taken directly from the manufacturers' packaging, was included to facilitate calibration of the two examiners and for direct comparison with the collected files of each group.
Assessing the files
Two independent examiners assessed each file under light microscopy at 40X magnification using a stereomicroscope (Olympus, SZ-CTV, Japan). An endodontic ruler was mounted alongside the endodontic file. Each quarter of the cutting blade of the sample was allocated a number: position: 1, 2, 3, and 4, consecutively from the tip of the file to the end of the file's cutting blade (Figure 1). Each possible scoring position on a file was scored based on the presence or absence of debris - a score of 1 denoted the presence of debris and a score of 0 denoted its absence. A novel scoring system, based on earlier research by Smith et al.12 but modified for use in the present study, was devised (Table 1). Following microscopic evaluation, the scores of the examiners were combined to provide a single reading per scoring position. If a discrepancy in scoring arose between examiners, a score of 1 (positive score) was assigned to that scoring position. This was done to eliminate the possibility of half scores (i.e. 0.5) as a score of 0.5 was considered to represent the presence rather than the absence of debris. The data was captured in Microsoft Excel 2016.


Frequency and distribution of debris in each group at each scoring position was determined. Cohen's Kappa statistic assessed inter-rater agreement. Groups were compared with each other using Fisher's Exact test. All statistical analyses were performed using the SAS software suite, Release 9.4 (SAS Institute Inc., North Carolina, USA).
RESULTS
A total of 401 endodontic hand files were analysed in this study. Seventeen participants provided the requested number of files, five provided more and five provided fewer. The calibration group consisted of 15 individual endodontic files removed directly from the manufacturer's packaging.
Twenty-four of the 401 files were found to be completely free of debris following routine reprocessing (6% of the total number of files). The rest of the files (94%) all displayed some debris contamination.
Seven of the 27 groups contained some individual files that scored zero in all four positions by both examiners (i.e. completely clean files). These groups and the cleaning methods they employed are displayed in bold in Table 2. Of the seven participants that produced "clean" (debris-free) files, five reported using the same method of cleaning: namely a combination of manual and ultrasonic cleaning.

Group B was an outlier and displayed the greatest number of completely clean files of all groups (n = 11/15). When compared with the remaining groups using Fisher's Exact test, group B contained significantly more clean files than group V, which contained the second highest number of "clean" files (n = 4/15). By extension, group B was significantly less debris-contaminated than all other groups in the dataset.
Table 3 represents the distribution of debris as a percentage found in each assigned scoring position. Inter-examiner agreement was found to be fair in scoring position 2 and moderate over the other scoring positions, as assessed using a simple Kappa test (0.41, 0.39, 0.41, and 0.52 for positions 1 to 4, respectively).
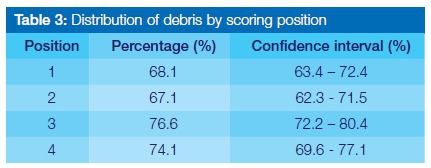
Figure 2 provides a graphical representation of the total number of clean and debris-contaminated scoring positions per group.

DISCUSSION
The present study found 94% of endodontic files to be debris-contaminated following routine reprocessing. This finding is in agreement with other studies which found 98% and 96% of the samples analysed to remain contaminated with debris.13,24 Some authors have also found in their studies that a significant portion of evaluated endodontic files display visual evidence of debris contamination following routine decontamination procedures.12-14 Two of these investigations assessed more than 20 dental practices and included over 200 files.13,14
The present study revealed a variation in residual debris contamination of endodontic files between groups. This is likely the result of the differences between practices in the methods employed for endodontic file decontamination. There is no standardised cleaning method for reprocessing endodontic files in South Africa, as evidenced in Table 2. This is in agreement with the findings of previous research which found the cleaning procedures of dental instruments from different practices to be inconsistent and poorly controlled.16
In this study, it was demonstrated that the files with the least amount of debris contamination were collected from practices where a combination of manual and ultrasonic cleaning methods were used for reprocessing. This is in agreement with previous research15,21 which recommended the use of manual cleaning and pre-soaking of endodontic files in an enzymatic agent prior to ultrasonification.
This must however be interpreted with caution, despite the finding that Group B contained statistically more clean files than any other group. The cleaning method used by this practice (Group B), namely manual cleaning with ultrasonication, was also used by several other practices, which produced no clean files. It therefore appears that correlations cannot be drawn between cleaning method and file cleanliness, given this particular dataset.
The number of uses of endodontic files was not recorded in this particular study and it is unknown whether a correlation exists between the level of debris contamination and the number of uses/sterilisation cycles of files. Investigation of this aspect may enhance future studies.
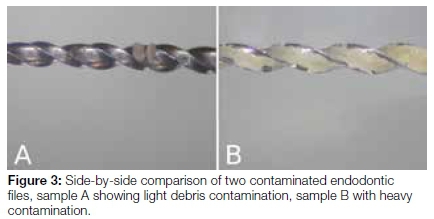
The quantity of debris visualised on individual files also varied greatly. Some samples displayed heavy contamination whilst others revealed only light debris contamination (Figure 3). Due to the nature of the scoring system used, this did not affect the specific score assigned to a file. The modification of the scoring system also allowed debris to be scored separately at the apical and coronal positions of a file, which was not possible when the original scoring system was applied.12
Despite the high level of debris contamination following routine decontamination procedures, as found in both the present and previous studies, the success rate of endodontic treatment remains high.17 Low endodontic treatment failure rates may be due to the sterilisation processes which endodontic files undergo rather than the efficiency of debris removal prior to sterilisation.
There is no consensus regarding the potential detrimental effects caused by debris found on dental instruments.7,9,18
Disagreement exists on whether or not residual debris on clinically used endodontic files can be sterilised.8,9,11,18 There is evidence in support of,9,11,18 and against,8 both these possibilities that residual debris may lead to failure of the sterilisation process. Despite this ongoing debate surrounding the ability to sterilise residual debris, the American Dental Association (ADA) continues to advise the removal of bioburden from dental instruments, including endodontic files, prior to sterilisation.4
Dead cells and foreign particles can trigger inflammation, even if they are sterile.19 It is possible to transfer sterile debris from endodontic files between patients when files are clinically reused.9 Whether or not this debris will lead to a clinically significant inflammatory response and/or failure of endodontic treatment requires further investigation. The presence of debris on endodontic files has however not been linked to the spread of infectious disease.20
In 2004, Parashos, Linsuwanont and Messer recommended a cleaning protocol which rendered rotary endodontic files 100% clean of biological debris.15 This protocol included manual cleaning in consisting of ten vigorous strokes a scouring sponge soaked in 0.2% chlorhexidine, pre-soaking for 30 minutes in an enzymatic agent, followed by 15 minutes of ultrasonic cleaning prior to sterilisation. If endodontic files are to be reused and re-processed between cases it may be prudent to implement this protocol, as it has been proven to be effective.
It has been suggested that time constraints of a busy dental practice may contribute to a reduction in the quality of cleaning of dental instruments.22 Furthermore, it has been postulated that inconsistencies in infection control in less developed countries may be the result of these procedures being performed by inadequately trained staff members.23
Regardless of the reasons for a lack of proper decontamination of dental instruments, it is the ethical responsibility of dental practitioners and auxiliary dental staff to remain up to date with currently prescribed infection control and decontamination guidelines to minimise the potential risk of spreading disease during dental treatment.23
The relative risk of the spread of prion disease and the inability to adequately clean endodontic files led to the UK moving toward a single-use policy regarding these instruments.2 Whilst no data on the occurrence of prion disease could be found in South Africa,25 the results of this study could prompt guidelines to be established regarding the use and reprocessing of endodontic files.
Manufacturers of endodontic instruments currently produce endodontic hand files packaged specifically for single-use,26 27 with some citing as a reason the inability to adequately clean debris from these instruments.26
CONCLUSION
The present study demonstrates the inadequacy of decontamination methods routinely employed in general dental practice for the removal of debris from endodontic hand files. Ninety-four percent of files in this study still contained debris following reprocessing. The highest possible standards of infection control should be maintained by employing proven methods of instrument reprocessing if endodontic files are to be re-used. If the available cleaning methods prove too difficult or unreliable to implement in clinical practice, dental practitioners may consider abandoning the reuse of endodontic files and adopting the alternative of single-use. Guidelines regarding endodontic file reprocessing should be established for South Africa.
Acknowledgements
The authors thank Prof. H.S. Schoeman for providing statistical support.
References
1. Carrotte P. Endodontics: Part 5 Basic instruments and materials for root canal treatment. Br Dent J 2004; 197(8): 455-64. [ Links ]
2. Walker JT, Dickinson J, Sutton JM, Raven NDH, Marsh, PD. Cleanability of dental instruments-implications of residual protein and risks from Creutzfeldt-Jakob disease. Br Dent J 2007; 203(7): 395-401. [ Links ]
3. Segall RO, Carlos E, Brady JM, Ayer, WA. Evaluation of debridement techniques for endodontic instruments. Oral Surg Oral Med Oral Pathol 1977; 44(5): 786-91. [ Links ]
4. American Dental Association. Sterilisation and Disinfection of Dental Instruments [Internet]. 2009. Chicago. [cited 2017 August]. Available from: https://www.google.co.za/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=r ja&uact=8&ved=0ahUKEwiK8MWhxOjLAhUFtRQKHbS6DRYQFggaMAA&url=http%3 A%2F%2Fwww.ada.org%2F%2Fmedia%2FADA%2FMember%2 520Center%2FFIles%2Fcdc_sterilisation.ashx&usg=AFQjCNFjn4HyMGokJZXCw jOeRYol15JRPw&sig2=OzMEBW1hz1LTu3DMsmZtSg&bvm=bv.117868183,d.ZWU [ Links ]
5. Messer H, Parashos P, Moule A. Should endodontic files be single-use only? Aust Endod J 2003; 29(3): 143-5. [ Links ]
6. Laheij AMGA, Kistler JO, Belibasakis GN, Välimaa, H, De Soet JJ' European Oral Microbiology Workshop (EOMW) 2011. Healthcare-associated viral and bacterial infections in Dentistry. J Oral Microbiol 2012; 4(1): 17659. [ Links ]
7. Reams Gj, Baumgartner JC, Kulid Jc. Practical application of infection control in endodontics. J Endod 1995; 21(5): 281-4. [ Links ]
8. Morrison A, Conrod S. Dental burs and endodontic files: are routine sterilisation procedures effective? J Can Dent Assoc 2009; 75(1): 39. [ Links ]
9. Johnson MA, Primack PD, Loushine RJ, Craft DW. Cleaning of endodontic files, Part I: The effect of bioburden on the sterilisation of endodontic files. J Endod 1997; 23(1): 32-4. [ Links ]
10. Van Eldik DA, Zilm PS, Rogers AH, Marin PD. A SEM evaluation of debris removal from endodontic files after cleaning and steam sterilisation procedures. Aust Dent J 2004; 49(3): 128-35. [ Links ]
11. Souza MA, Menin MLF, Montagner F, Cecchin D, Farina AP. SEM and microbiological analysis of dirt of endodontic files after clinical use and your influence on sterilisation process. Dent Press Endod 2011; 1(1): 82-6. [ Links ]
12. Smith A, Dickson M, Aitken J, Bagg J. Contaminated dental instruments. J Hosp Infect 2002; 51(3): 233-35. [ Links ]
13. Smith A, Lange A, Perrett D, McHugh S, Bagg J. Residual protein levels on reprocessed dental instruments. J Hosp Infect 2005; 61(3): 237-41. [ Links ]
14. Letters S, Smith AJ, McHugh S, Bagg J. A study of visual and blood contamination on reprocessed endodontic files from general dental practice. Br Dent J 2005; 199(8): 522-5. [ Links ]
15. Parashos P, Linsuwanont P, Messer HH. A cleaning protocol for rotary nickel-titanium endodontic instruments. Aust Dent J 2004; 49(1): 20-7. [ Links ]
16. Bagg J, Smith AJ, Hurrell D, McHugh S, Irvine G. Pre-sterilisation cleaning of re-usable instruments in general dental practice. Br Dent J 2007; 202(9): E22. [ Links ]
17. Burry JC, Stover S, Eichmiller F, Bhagavatula P. Outcomes of primary endodontic therapy provided by endodontic specialists compared with other providers. J Endod 2016; 42(5): 702-5. [ Links ]
18. Van Eldik DA, Zilm PS, Rogers AH, Marin PD. Microbiological evaluation of endodontic files after cleaning and steam sterilisation procedures. Aust Dent J 2004; 49(3): 122-7. [ Links ]
19. Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol 2009; 28: 321-42. [ Links ]
20. Hartwell G, Bowles W, Peters O, Peikoff M, Torneck C. Joint AAE/CAE special committee on single-use of endodontic instruments [Internet]. 2011. Available from: http://www.endoexperience.com/documents/CAEAAESUIstatement.PDF [accessed 8 August 2015]. [ Links ]
21. Guandalini B, Vendramini I, Leonardi DP, Tomazinho FSF, Tomazinho PH. Comparative analysis of four cleaning methods of endodontic files. Rev Sul-bras Odontol 2014; 11(2): 154-8. [ Links ]
22. Burkhart NW, Crawford J. Critical steps in instrument cleaning: removing debris after sonication. J Am Dent Assoc 1997; 128(4): 456-63. [ Links ]
23. Scarlett MI, Grant LE. Ethical oral health care and infection control. J Dent Educ 2015; 79(Suppl 5): S45-7. [ Links ]
24. Popovic J, Gasic J, Zivkovic S, Petrovic A, Radicevic G. Evaluation of biological debris on endodontic instruments after cleaning and sterilisation procedures. Int Endod J 2010; 43(4): 336-41. [ Links ]
25. Oosthuysen J, Potgieter E, Blignaut E. Compliance with infection control recommendations in South African dental practices: a review of studies published between 1990 and 2007. Int Dent J 2010; 60(3): 181-9. [ Links ]
26. Ready-steel [Internet]. DentsplyMaillefer; [cited 2018 Apr 9]. Available from: http://www.maillefer.com/ready-steel/ [ Links ]
27. Glide path [Internet]. FKG Dentaire; [cited 2018 Apr 9]. Available from: http://www.fkg.ch/products/endodontics/glide-path [ Links ]
 Correspondence:
Correspondence:
Glynn Dale Buchanan
Oral and Dental Hospital, 31 Bophelo Road
Prinshof Campus, Riviera, Pretoria, 0002
South Africa
Tel: 012 319 2214.
E-mail: glynn.buchanan@up.ac.za














