Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.73 no.4 Johannesburg may. 2018
CASE REPORT
Oral Medicine Case Book: Epidermolysis bullosa acquisita
M NegiI; S Mulder-van StadenII; A JefthaIII; H HolmesIV
IBChD, MSc. Division of Oral Medicine and Periodontics, Faculty of Dentistry, University of the Western Cape
IIBChD, MChD. Division of Oral Medicine and Periodontics, Faculty of Dentistry, University of the Western Cape
IIIBChD, MChD. Division of Oral Medicine and Periodontics, Faculty of Dentistry, University of the Western Cape
IVBChD, MSc, MChD. Division of Oral Medicine and Periodontics, Faculty of Dentistry, University of the Western Cape
CASE REPORT
A 49-year-old female was referred from the Dermatology department to the Oral Medicine Department at the University of the Western Cape (UWC), Oral Health Centre, Tygerberg campus. The patient complained of a sore mouth and difficulty in brushing her teeth. She had been diagnosed with epidermolysis bullosa acquisita (classical type) 12 years ago and was being managed by her Dermatologist with topical and systemic steroids (Dovate® ointment and 10mg prednisone daily).
The extra oral examination revealed extensive sloughing of the skin of the hands, chest and back. The hands showed atrophic scarring, skin fragility and nail loss on numerous fingers, which also demonstrated restricted movement (Figures 1, 2).
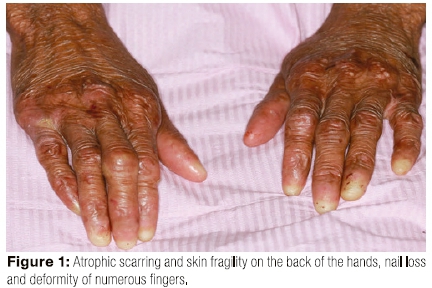
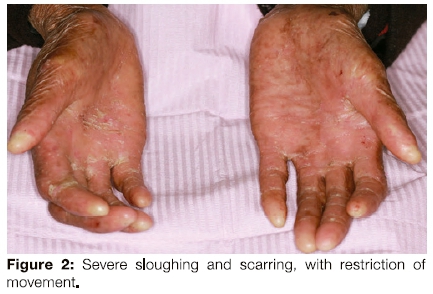
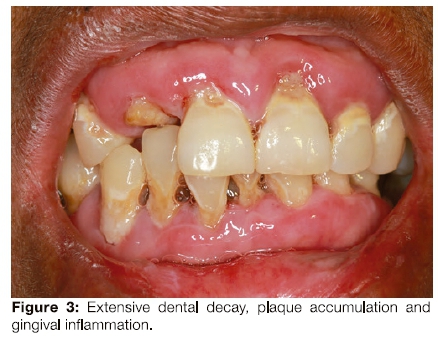
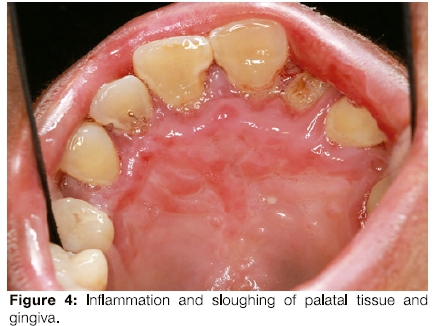
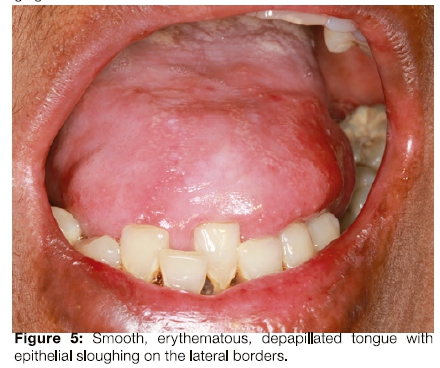
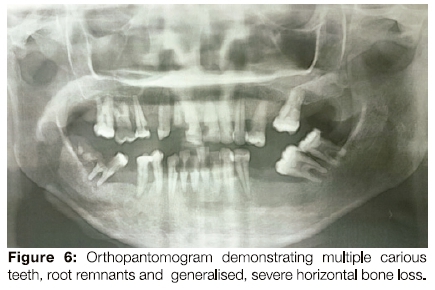
The patient had limited mouth opening because of scarring related to repeated episodes of ulceration (Figure 3) and poor oral hygiene. Her gingiva was inflamed (Figure 4). The middle to anterior dorsal surface of her tongue was atrophic, smooth and erythematous, while the posterior dorsal tongue had white yellowish plaques (Figure 5). The orthopantomogram demonstrated multiple carious teeth with generalised, severe horizontal boneloss (Figure 6).
An appointment was scheduled for a scaling as well as extraction of root remnants and teeth with a hopeless prognosis. The patient was booked for a follow-up at the Oral Medicine and Periodontology Department two weeks later, but failed to return for her appointment,
DISCUSSION
Epidermolysis bullosa (EB) is a group of inherited blistering diseases, which can be present from birth, or be acquired and manifest in adult life.1 The first description of a patient with a bullous disease reminiscent of EB (with no associated familial history) was reported by Elliot in 1895.2 In the early 1970s, Roenigk et al. proposed the first diagnostic criteria for EBA.2
The diagnostic criteria included:
1. Spontaneous or trauma-induced blisters resembling hereditary dystrophic EB
2. Adult onset of the disease
3. No associated family history for EB
4. Exclusion of all other bullous diseases2
EBAisarare, autoimmune, sporadic, subepithelial, mucocutaneous blistering disease, which usually occurs In adulthood.1 Skin fragility, non-Inflammatory tense bullae, milla and extensive scarring typically characterize EBA.1 Otherwise, EBA may manifest as an inflammatory bullous eruption reminiscent of bullous pemphigoid or another sub-epithelial autoimmune blistering disorder.1'2
Limited numbers of paediatric cases of EBA have been reported, but it is adults who are most commonly affected. Increased risks for EBA development based upon gender, ethnicity or geographic location have not been definitively established In the literature.1, 3
Pathogenesis
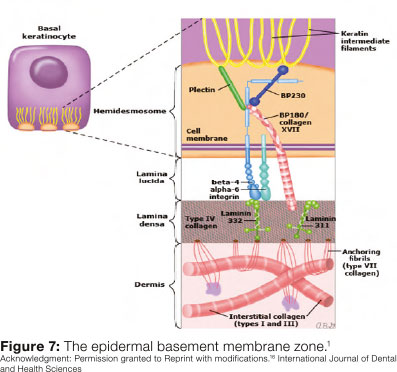
Although the pathogenesis of EBA is not clearly defined, evidence reveals that it involves an autoimmune process (type III hypersensitivity) with the production and deposition of antibodies against type VII collagen, which Is a major constituent of the anchoring fibrils (adhesion structures In the dermal-epithelial junction - DEJ)1, 4(Figure 7).
This theory concept is based upon several observations1:
-
Direct immunofluorescence (DIF) microscopy of peri-lesional skin from patients with EBA showed antibody deposition at the basement membrane zone (BMZ).
-
Indirect Immunofluorescence (IF) revealed antibodies against type VII collagen In the serum of patients with EBA.
-
Immuno-electron microscopy detected antibody deposition in the lamina densa area of the BMZ, a site consistent with the location of anchoring fibrils.
-
Declining numbers of normal anchoring fibrils were present in the DEJ in EBA, a finding consistent with epidermal blistering and skin fragility.
-
Animal models indicate that passive transfer of antibodies against type VII collagen can induce clinical features consistent with EBA.
-
Serum levels of type VII collagen antibodies identified by ELISA correlate with the severity of skin lesions.
-
Patients with bullous systemic lupus erythematosus (SLE), which is also accompanying with antibodies against type VII collagen, develop sub-epidermal blistering.
CLINICAL VARIANTS
Classical EBA manifestations were firstly defined as non-Inflammatory, mucocutaneous bullae with accompanying skin fragility.1 EBA may also present as an inflammatory blistering disease characterized by descriptions that resemble bullous pemphigoid, linear IgA bullous dermatosis, mucous membrane (cicatricial) pemphigoid, or Brunsting-Perry pemphigoid.1 The classical and bullous pemphigoid-like subtypes are the most common presentations of EBA.
Classical (non-inflammatory) EBA
Patients with classical EBA present with skin fragility and noninflammatory tense vesicles and bullae, which rupture quickly and develop erosions. The parts that are frequently subject to minor trauma are the most common locations for lesion progression, such as the hands, feet, knees, elbows, and lower back. These blisters usually heal with scarring and milia (small epidermal inclusion cysts).1,5
Mucosal involvement is common in classical and also in inflammatory EBA. It can be subclinical with associated pain or itching, or may present as adhesions or erosions on the oral, nasal, pharyngeal, laryngeal, esophageal, ocular or ano-genital mucosa.6
Nail loss, alopecia, fibrosis of fingers and hands and esophageal stenosis may manifest in severe cases.1,5
Inflammatory EBA
These subtypes of EBA are similar to other autoimmune subepithelial blistering disorders. Compared to classical EBA, skin fragility is not a characteristic feature.1
- Bullous pemphigoid-like EBA
This disorder shares the clinical manifestation with bullous pemphigoid that is the most common autoimmune subepithelial blistering disease.1,5 Unlike the non-inflammatory lesions of classic EBA, the condition manifests with widespread tense bullae with inflamed or urticarial skin. The lesions are common in the trunk, extremities and skin folds similar to bullous pemphigoid. Scarring and milia are not obvious findings.5
-
Mucous membrane pemphigoid-like EBA
This may appear as a mucosal-predominant disorder with clinical manifestations that are similar to mucous membrane pemphigoid.1,5 The initial disease findings are erosions and scarring on the mucosal surfaces of the mouth, upper oesophagus, conjunctiva, anus or vagina.5
- IgA bullous dermatosis-like EBA
This has clinical, histologic, and direct immunofluorescence (DIF) manifestations that resemble IgA-mediated bullous dermatoses.1 The clinical manifestations may present with the annular distribution of vesicles and bullae characteristic of linear IgA bullous dermatosis. Associated mucosal Involvement is common.1,5
- Brunsting-Perry pemphigoid-like EBA
The disorder resembles Brunsting-Perry pemphigoid, which Is a rare sub- epithelial blistering disorder.1 It presents as vesiculobullous eruptions, primarily appearing on the head and neck and heals with scarring.1,5
DIAGNOSIS
EBA resembles other sub-epithelial blistering disorders in some clinical, pathologic and immunohistologic features. Establishing a diagnosis can be challenging. Once the initial assessment suggests the presence of an autoimmune sub-epithelial blistering disorder and reveals findings consistent with EBA, additional Investigations may be utilized to verify the diagnosis.1
Initial patient evaluation
The evaluation should include the following1:
-
A full patient history and complete skin examination which Involves an evaluation of the morphology and distribution of skin lesions
This assessment assists in limiting the differential diagnosis. For example, a diagnosis of classical EBA must be considered in adults who present with consistent clinical manifestations. These Include skin fragility and trauma-induced tense bullae, which result in milia and scars, with no family history of any hereditary blistering disorder. Because of the numerous EBA's morphologies, the probability of EBA should still be considered when clinical descriptions indicate any other sub-epithelial blistering disease.
-
A biopsy for routine histopathology and direct immunofluorescence (DIF)
A tissue biopsy of affected skin or mucosa must be taken for routine histologic examination to define the level of blistering. A peri-lesional skin or mucosal biopsy for DIF should also be obtained to identify autoantibody deposition. A punch biopsy of 4mm is classically used to retrieve tissue specimens.
-
Immunofluorescence at basement membrane zone (BMZ)-split skin
Once the clinical, histologic and DIF results are reliable with an autoimmune sub-epithelial blistering disease, immunofluorescence on skin artificially split within the BMZ is helpful to rule out bullous pemphigoid and linear IgA bullous dermatosis, thus narrowing the differential diagnosis to EBA and a few other uncommon disorders.
Indirect immunofluorescence (IDIF) microscopy on salt-split skin is a simple and reliable tool compared with DIF, which helps to sub classify subepidermal autoimmune bullous diseases into 'roof and 'floor' binding conditions.7 EBA is a standard floor-binding sub-epidermal autoimmune bullous disease. On the other hand, the floor binding of salt-split skin is not only revealed in EBA but also in anti-laminin 332 mucous membrane pemphigoid and anti-p200 pemphigoid.7
EBA shares clinical manifestation with other multiple disorders. Some of these disorders, frequently mistaken with EBA, are listed below:1,5
-
Bullous pemphigoid
-
Linear IgA bullous dermatosis
-
Porphyria cutanea
-
Bullous systemic lupus
-
Recessive dystrophic epidermolysis bullosa
Management
Treatment strategies are determined by the clinical presentation. However, a multidisciplinary approach is necessary, including a nutritionist, dermatologist, hematologist, plastic surgeon, ophthalmologist, cardiologist, gastroenterologist, dentist, nurse and an occupational therapist.8
The first line of treatment in most of EBA patients is colchicine because of its efficiency and less associated side effects.1,5 Colchicine is a familiar microtubule inhibitor, which also plays a role in regulating autoimmunity by inhibiting antigen presentation to Τ cells.9 Colchicines in high doses have been reported to be effective for both classical and inflammatory EBA patients.10 Diarrhoea is an adverse effect which limits its use in EBA patients with inflammatory bowel disease (IBD).1
Rituximab is a monoclonal antibody, which targets CD20 on both mature and immature Β cells.1,5 As a result of destroying Β cells, the circulating antibodies and Β cells are reduced, leading to an Increase in immunosuppression.5
Cyclosporine, an immunosuppressant, is often considered in the treatment of EBA.1,5 Studies have revealed that some EBA patients responded to cyclosporine use. However, patients require high doses (>6mg/kg) of the drug. Cyclosporine has long-term toxicity, but is a valuable treatment modality in patients who are non-responsive to EBA therapy.5
Systemic glucocorticoids in high doses have proven to be successful in the management of some EBA patients.1,5 Other Immunosuppressants such as methotrexate, azathioprine, and cyclophosphamide may also be used.11 Prednisone and dapsone may also assist some EBA patients.5
Photophoresis was efficient in a cohort of EBA patients, including one in a critical condition. Photophoresis is used to treat mycosis fungoides and Sezary syndrome (which is a malignancy of skin-homing CD4++ memory Τ cells that is clinically described as erythroderma, lymphadenopathy, and blood involvement12) and numerous autoimmune bullous disorders.5
Blister formation following mild mechanical trauma characterizes most of major types of EB and most EB patients may reveal systemic complications, such as genital, ocular and oropharyngeal infections with difficulty in swallowing.13
Implications for the oral health worker
EB patients need particular precautions during dental treatment due to the probability of soft tissue injury during their examination of the oral mucosa and skin.7 These patients are predisposed to dental caries as a result of their cariogenic diet; poor oral hygiene worsened by pain and limited mouth opening.11 Regular visits/ contact with the oral health team can help to avoid complex procedures and treatments.7
Several alternative treatments are often used as first aid therapies for blisters. Aloe vera gel application decreases the sub-dermal temperature, affords a refreshed sensation, promotes antimicrobial activity and diminishes the healing period.7 Biotene® mouthwash minimizes blister formation by providing oral moisturizing and saliva stimulation, providing buffering capacity and antimicrobial activity.14
Nowadays, researchers are investigating treatments such as gene and cell therapy, intradermal injections of allogeneic fibroblasts, recombinant protein infusions and stem cell transplantation.7 Other developing treatments for EB patients are focusing on the improvement of wound healing and good quality of life.7,15
It is essential that all EBA patients receive supportive care to decrease the possibility of skin trauma and to improve quality of life. This supportive care involves appropriate wound management and approaches for preventing trauma. Patients should be educated that harsh soaps, vigorous rubbing of skin during washing specifically with hot water, may aggravate the lesions and result in trauma. The application of sunscreen may reduce the exacerbation or stimulation of new lesions due to prolonged sun exposure. Finally, the patient should be well informed to recognize superimposed infections of the skin and to seek urgent medical care should they develop.
ACRONYMS
BMZ: basement membrane zone
DEJ : dermal-epithelial junction
DIF: Direct immunofluorescence
EBA: Epidermolysis bullosa aquisita
EB : Epidermolysis bullosa
ELISA: enzyme linked immunosorbent assay.
IBD inflammatory bowel disease
IF: Indirect immunofluorescence
SLE : systemic lupus erythematosus
References
1. Woodley DT, Gammon WR, Briggaman RA. Epidermolysis bullosa acquisita. Immunologic Diseases of the Skin. Norwalk (CT): Appleton & Lange. 1991;321-33. Available from https://www.uptodate.com/contents/epidermolysis-bullosa- acquisita> [ Links ]
2. Woodley DT, Chang C, Saadat P, et al. Evidence that anti-type VII collagen antibodies are pathogenic and responsible for the clinical, histological, and immunological features of epidermolysis bullosa acquisita. J Invest Dermatol. 2005; 124(5):958-64. Available from < https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3411315/> [ Links ]
3. Callot-Mellot C, Bodemer C, Caux F, Bourgault-Villada I, Fraitag S, Goudié G, Heller M, de Prost Y, Prost C. Epidermolysis bullosa acquisita in childhood. Archives of Dermatology. 1997;133(9):1122-6. [ Links ]
4. Sakai LY, Keene DR, Morris NP, Burgeson RE. Type VII collagen is a major structural component of anchoring fibrils. The Journal of Cell Biology. 1986;103(4):1577-86. [ Links ]
5. Chen M, Kim GH, Prakash L, Woodley DT. Epidermolysis bullosa acquisita: autoimmunity to anchoring fibril collagen. Autoimmunity 2012;45(1):91-101. [ Links ]
6. Luke MC, Darling TN, Hsu R, Summers RM, Smith JA, Solomon Bl, Thomas GR, Yancey KB. Mucosal morbidity in patients with epidermolysis bullosa acquisita. Archives of Dermatology 1999;135(8):954-9. [ Links ]
7. Goyal N, Rao R, Shenoi SD, Pai S, Kumar P, Bhogal BS, Schmidt E, Zillikens D. Epidermolysis bullosa acquisita and anti-p200 pemphigoid as major subepidermal autoimmune bullous diseases diagnosed by floor binding on indirect immunofluorescence microscopy using human salt-split skin. Indian Journal of Dermatology, Venereology, and Leprology. 2017;1;83(5):550-5. [ Links ]
8. Scheidt L, Sanabe ME, Diniz MB. Oral manifestations and dental management of epidermolysis bullosa simplex. International Journal of Clinical Pediatric Dentistry 2015;8(3):239-41. [ Links ]
9. Mekori YA, Chowers Y, Ducker I, Klajman A. Inhibition of delayed hypersensitivity reactions by colchicine. II. Colchicine inhibits interferon-gamma induced expression of HLA-DR on gut epithelial cell line. Clinical and Experimental Immunology 1989;78(2):230-2. [ Links ]
10. Megahed M, Scharffetter-Kochanek K. Epidermolysis bullosa acquisita-successful treatment with colchicine. Archives of Dermatological Research 1994;286(1):35-40. [ Links ]
11. Kirtschig G, Murreil D, Wojnarowska F, Khumal N. Interventions for mucous membrane pemphigoid/cicatricial pemphigoid and epidermolysis bullosa acquisita: a systematic literature review. Archives of Dermatology. 2002;138(3):380-4. [ Links ]
12. van Doorn R, Slieker RC, Boonk SE, Zoutman WH, Goeman JJ, Bagot Μ, Michel L, Tensen CP, Willemze R, Heijmans BT, Vermeer ΜΗ. Epigenomic analysis of Sezary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol.. 2016;136:1876-84. [ Links ]
13. Babaee N, Zabihi E, Mohseni S, Moghadamnia AA. Evaluation of the therapeutic effects of Aloe vera gel on minor recurrent aphthous stomatitis. Dent Res J. 2012;9(4):381-5 [ Links ]
14. dos Santos KK, Difabio LFG, Santos MTBR, Junior LAVS. Efetividade no uso de substÄ¢ ncias lubrificantes oráis em pacientes com epidermÄ3lise bolhosa/Effectiveness of oral lubricants in patients with epidermolysis bullosa. 2011;59;2. [ Links ]
15. Kummer TR, Nagano HC, Tavares SS, Santos BZ, Miranda C. Oral manifestations and challenges in dental treatment of epidermolysis bullosa dystrophica. J Dent Child. 2013;80(2):97-100. [ Links ]
16. A El Nassry, A EIGedaily. Epidermolysis bullosa acquisita. Int J Dent Health Sei 2014;1(2): 265-271. [ Links ]
 Correspondence:
Correspondence:
Dr. Haly Holmes
Division of Oral Medicine and Periodontology
University of the Western Cape Dental Faculty
Francie Van Zyl Drive, Tygerberg Campus
Tel: 27 21 9373102
Email hholmes@uwc.ac.za














