Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Dental Journal
versión On-line ISSN 0375-1562
versión impresa ISSN 0011-8516
S. Afr. dent. j. vol.73 no.3 Johannesburg abr. 2018
RESEARCH
Oral squamous cell carcinoma, a growing problem
RM GarranaI; SL ShangaseII; GU MohangiIII
IRhoodie Martins Garrana (Principal Researcher): BDS, MSc (Wits) , Registrar, Department of Oral Medicine & Periodontology, School of Oral Health Sciences, Faculty of Health Science, University of the Witwatersrand, 7 York Road, Parktown, Johannesburg, 2193, South Africa, Rhoodie.Garrana@wits.ac.za
IISindisiwe Londiwe Shangase (Secondary Researcher -Writing Article): BDS, MDent(Medunsa), Professor and Head of Department, Department of Oral Medicine & Periodontology, School of Oral Health Sciences, Faculty of Health Science, University of the Witwatersrand, 7 York Road, Parktown, Johannesburg, 2193, South Africa, Sindisiwe.Shangase@wits.ac.za
IIIGovindrau Udaibhan Mohangi (Secondary Researcher -Writing article): BDS (Mys), DipOdont-Perio, DipOdont-OralMed, MSc Odont Perio, MChD-OMP (Pret) Head - Clinical Unit, Department of Oral Medicine & Periodontology, School of Oral Health Sciences, Faculty of Health Science, University of the Witwatersrand, 7 York Road, Parktown, Johannesburg, 2193, South Africa, Govindrau.Mohangi@wits.ac.za
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is a malignancy or cancer of the epithelial cell layer.1 The World Health Organisation (WHO) has defined cancer as the abnormal growth or division of cells beyond their normal anatomical boundaries, leading to invasion of other bodily compartments or organ systems, a progression known as metastasis.2 Two thirds of all OSCC cases documented in the world today present in advanced stages of the disease.3,4 Local recurrence of the disease is said to occur in up to 30% of cases; 10% of cases will experience regional recurrence and up to 20% of cases will experience distant metastasis.3,4
In 2012 as many as 690 000 new OSCC cases were documented worldwide, 375 000 of these cases proving fatal, making up approximately 46% of total cancer mortality worldwide.5,6 Oral squamous cell carcinoma is considered the fifth most common malignancy known to man.6 Tobacco smoking and alcohol consumption form the two major causative factors in the development of OSCC.7,8 This disease shows a global prevalence for older Caucasian males over the age of 45 years.5,9
OSCC of the lip, tongue, oral mucosa and gingiva presents as a major health problem. Whilst the incidence, mortality and the site may vary widely from one geographical region to the next, the tongue remains the most commonly affected site.10
Identifying risk factors such as betel quid chewing, chronic exposure to sunlight11 or chemical pollution within our population group, may provide an idea of their contribution to the occurrence of OSCC.
Current literature indicates that OSCC of the lip has a 95% five-year survival rate with the prognosis of lateral/ventral tongue lesions being half as good with a 45% five-year survival rate.10 Over the past several years there has been a significant decrease in the occurrence of OSCC in the USA and Canada and a marked increase in regions such as Portugal, France, Hungary, South America and South-east Asia.5
One may possibly attribute this effect to more stringent tobacco and alcohol laws implemented in the US and Canada as well as heightened awareness of risk factors. In contrast, many Eastern European countries have experienced steadily increasing tobacco and alcohol sales. There has been a marked rise in young smokers and in binge drinking since the 1980s, which is postulated to have played a significant role in the development of OSCC.4 Indeed, Eastern Europe has been shown to have an increased number of new OSCC cases compared with Northern and Southern Europe.5,6 French citizens, in general, have a seven-fold higher chance of developing OSCC than do Greeks.6 The literature shows that 25% of all new OSCC cases worldwide originate in India.5,6,11 This figure is closely followed by the incidence in South American populations, particularly Brazilian, Argentinean and Uruguayan males, who have the third highest prevalence for OSCC after India and France.6,12 South-east Asia and India together contribute well over 100 000 cases of OSCC per year, this being from amongst a population of over one billion citizens, all of whom are exposed to similar risk factors.5 A Libyan epidemiological study showed a mean occurrence of OSCC in males in the fifth and sixth decades of life, with the floor of the mouth being mostly affected.7 Africa has produced sparse literature on the prevalence of OSCC. A publication on the occurrence of OSCC in Sudanese civilians, however, showed a high prevalence rate.13
Two thirds of OSCC cases occur in developing countries,5 which may have conditions and problems not shared in many First World settings. As one of the leading African countries, South Africa should be basing health care protocols on local occurrences and statistics rather than those obtained from other countries which may have vastly differing dynamics. Accordingly, it would be wise to redirect efforts to achieve goals specific to the needs of the country. Understanding socio-economic, epidemiologic and pathognomonic parameters of disease patterns in our population will ensure a distinct advantage in dealing with major health care problems such as oral cancer.
This study was conducted to address the relative paucity of information in South Africa. Characterization of OSCC in the country will provide information as to who is most at risk, and that data could then inform intervention policies at all levels.
MATERIALS AND METHODS
Study design and sample
This research was designed as a retrospective study which reviewed data held within the Department of Oral Medicine and Periodontology, University of the Witwatersrand, South Africa. One hundred and seven archived files from the years 1990 -2010 were analysed.
INCLUSION CRITERIA
All cases of patients having histologically confirmed OSCC lesions between 1990 and 2010 were included.
ETHICAL CONSIDERATIONS
Ethical clearance was granted by the Wits Human Ethics Research Committee Number: M140654.
METHOD
A retrospective review of archived OSCC cases files between 1990 and 2010 was performed. Demographic, clinical and histological data such as age, gender, race, anatomical site of occurrence, alcohol consumption, smoking as a habit, snuff use, systemic diseases, lymph node involvement, size of lesion and histological differentiation were obtained. Anatomical sites of the lesion were classified as described by the International Statistical Classification of Diseases,2 which covers cancer of the tongue, gingivae, floor of the mouth, palate, and other unspecified parts of the mouth as well as related health problems.
The degree of histological differentiation was classified as well differentiated (WD), moderately differentiated (MD), or poorly differentiated (PD), according to the guidelines of the World Health Organization (WHO). 2,14
DATA COLLECTION
The data were collected utilizing prepared record sheets and were tabulated prior to entering them onto an Excel spreadsheet, severally categorized as age, gender, smoking, alcohol consumption, snuff use, dietary deficiencies, site and lymph node involvement. Two other categories were added for statistical benefit which included 'incomplete available clinical data' and 'no identifiable risk factors'. Given the relatively small sample size anticipated for the study, it was decided that the focus should be on descriptive analysis. Univariate statistics such as the mean, standard deviation, median, interquartile range for continuous variables, percentages and frequency distributions for categorical variables were to be calculated for all key variables.
For further analysis, continuous variables (e.g. age, size of lesion) were organised into clinically meaningful categories. Associations between variables were assessed by the Chi-square test. Fisher's exact test was used for two x two tables where the requirements for the Chi-square test could not be met. The strength of the associations was measured by Cramer's V (for Chi-square test) and the phi coefficient (for Fisher's exact test). Data analysis was carried out in SAS. A 5% significance level was used throughout, unless otherwise specified.
RESULTS: TRENDS:
The data from the sample of patients was compiled into meaningful categories of five-year periods to analyse trends: The mean age of the patients was 56.3 years (SD 11.9y; range 24-79y; median 57.5y, interquartile range 50-64.5y).
The number of patients presenting with OSCC appeared to have increased in the last decade of the study period.
Males dominated the study group at 73.8% and the majority of the patients (60.0%) were black (Fig. 1).

Risk Factors:
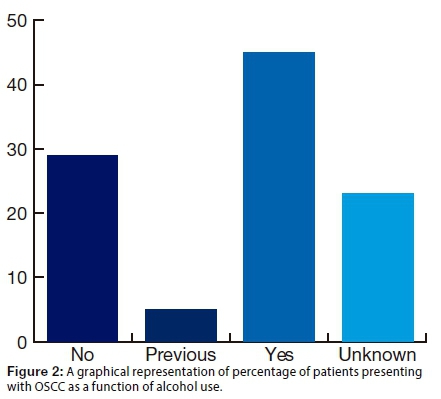
It was noted that 45.0% of the patients reported alcohol use (Fig2l).
A considerable majority of the patients (72.5%) admitted to being current smokers. This variable was categorised as light smoking (<10 per day) or heavy smoking (>= 10 per day), the latter comprising 53.5% of cases. One patient (1.3%) reported snuff use. Low calorie intake was reported in 5.0% of the study group (within the limitations of a record review). In 41.2% of the sample, systemic abnormalities or diseases were noted.
PRESENTATION / CHARACTERISTICS:
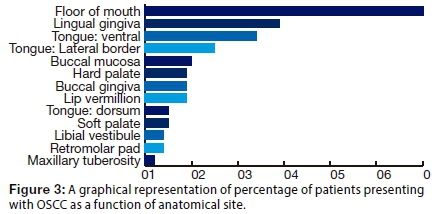
The greater proportion of patients in the sample had one (43.8%) or two (38.8%) affected sites. The floor of the mouth was most frequently involved in 60.0% of cases (Fig. 3) . (Note that percentages do not sum to 100% since some patients had more than one site affected.)
The majority of patients (70%) had moderately differentiated lesions (Fig. 4).

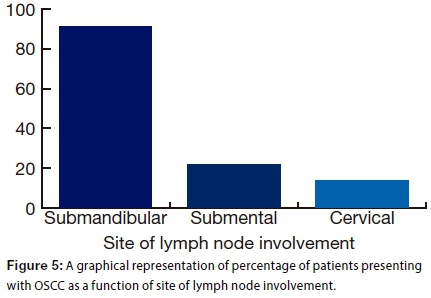
In 43.8% of the patients, lymph node involvement had occurred. The submandibular lymph node was most frequently affected (91.4% of patients) (Fig. 5). (Note that percentages do not sum to 100% as some of the patients had more than one site affected.)
ASSOCIATIONS:
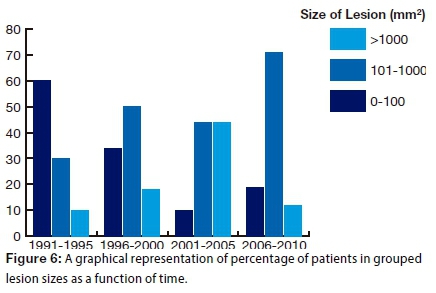
There were two significant associations made between variables in the study. A strong association was shown between categorised size of lesion and duration of presentation (p=0.029; phi coefficient=0.51): The proportion of small lesions decreased over time, while that of lesions sized between 101 and 1000 mm2 increased (Fig. 6).
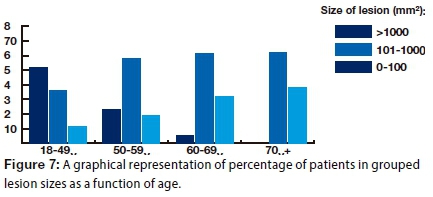
A moderate association between size of lesion and age was demonstrated (p=0.027; phi coefficient=0.48): The size of the lesion increased with age (Fig. 7).
DISCUSSION
Oral squamous cell carcinoma is considered the fifth most common malignancy known to man.6 Tobacco smoking and alcohol consumption are the two major causative factors especially in Caucasian males over 45 years old.5,7-9 The tongue remains the most commonly affected site.10
This study set out to analyse and compare the data captured from patient files over a 21 year period from 1990-2010 in an effort to characterise the patients who had presented to the Department of Oral Medicine and Periodontology with OSCC. This information is essential to the understanding of the demographics of this geographical region and will assist in improving current management. Comparison will be enabled with documentation of the disease in the rest of the world.
This study found the average age of the sample to be 56.3 years, confirmed that the most affected individuals are males (73, 8%) with the majority of lesions occurring on the floor of the mouth (60%) These data concur with global figures quoted by Ferlay et al. 2012 and by Scully et al. 2009.6,8 Contrary to the findings published by Boudewijn et al. in 2014,15 the current study concluded that the most typical race affected was Black and not Caucasian. This may have been expected, considering the population ratio in the region. The fact that alcohol and smoking were implicated in 45% and 72.5% of cases in the two groups respectively, reiterate their significance as risk factors in the aetiology in OSCC development and aligns this study with similar views held by Jaber et al. in 199816 and in 1999.17
The mean size of the lesion found on initial presentation was 915mm2 (30x30mm). Since two thirds of all lesions reported worldwide presented in advanced stages, as observed by van der Waal 2013 and by Bodner 2014, 3,4 it is not unexpected that the majority of cases in this study were rather large moderately differentiated tumours (70%). There was lymph node involvement in 43.8% of cases, with the submandibular lymph nodes being the most commonly implicated (91,4%). It may be relevant that 43% of cases presented with a single site of involvement, whereas 38.8% of cases were found to have two or more sites involved. These sites may have been independent OSCC tumours or, more likely, secondary field tumours.
The proportion of small lesions decreased over time, while that of lesions sized between 101 and 1000 mm2 increased over the 21-year period. This finding may be associated with poor access to health care facilities causing patients to allow lesions to increase in size before the condition clamoured for attention and treatment was sought. Another possibility may be the implication of HIV in OSCC progression. Oral squamous cell carcinoma is already classified as an HIV associated malignancy along with Kaposi's sarcoma and lymphoma. Further research is required to accurately assess the influence of HIV on OSCC progression in South Africa.
Of relevance may be the fact that this study revealed that 5% of the patients reported low calorie intake. Whilst this may have been caused by an underlying issue it may have been a secondary outcome of an inability to consume food effectively due to pain or tumour size. Malnourishment, as stated by Pavia et al.18 plays a major role in OSCC development.
Although the incidence of specific systemic diseases was not measured in this study, 41.2% of cases presented with one or more systemic disease or abnormality. These ranged from acquired to genetically predisposed congenital conditions which may or may not have affected the development of OSCC in this population group.
This study found that OSCC occurrence increased with age (Fig. 7). Similar findings have been reported in the literature, a study carried out in Rio de Janeiro reporting that approximately 50% of all the cases assessed were in age categories above 60-years old.19
CONCLUSIONS.
This study suggests that there is delayed recognition of the condition, possibly because the medical and dental professionals in our region are not adequately addressing the progressive disease process in OSCC and/or patients are ignoring the early signs and taking longer to present to health care facilities, which may be compounded by a lack of patient education. As clinicians we are obligated to introduce regular and thorough screenings for OSCC. More research is required into the exact contributory role of HIV/AIDS and other systemic diseases on the rate of progression and increased lesion size of OSCC. These findings may direct management protocols in health care clinics as the results of the study speak strongly to the need for early diagnosis and a rapid transition to the management/treatment phases of intervention to improve outcomes.
ACRONYMS
MD : moderately differentiated
OSSC : Oral squamous cell carcinoma
PD : poorly differentiated
WD : well differentiated
References
1. Guerra L, Guidi R, Frisan T. Do bacterial genotoxins contribute to chronic inflammation, genomic instability and tumor progression? FEBS J. 2011;278(23):4577-88. [ Links ]
2. WHO | International Classification of Diseases (ICD). http://www.who.int/classifications/icd/en/. Accessed March 24, 2016. [ Links ]
3. van der Waal, I. Are we able to reduce the mortality and morbidity of oral cancer; some considerations. Med Oral Patol Oral Cir Bucal. 2013;18(1):33-7. [ Links ]
4. Bodner L, Manor E, Friger MD V der WI. Oral squamous cell carcinoma in patients twenty years of age or younger - Review and analysis of 186 reported cases. Oral Oncol. 2014;50(2):84-9. [ Links ]
5. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309-16. [ Links ]
6. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase. No. 11 [Internet]. Lyon, Fr Int Agency Res Cancer. 2013;11:http://globocan.iarc.f. [ Links ]
7. Jaber MA, Fanas SHA. The pattern of occurrence of oral squamous cell carcinoma in Libya. Ibnosina J Med BS. 2010; 2(3):105-10. [ Links ]
8. Scully C, Bagan J. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009;15(6):388-99 [ Links ]
9. Braakhuis BJM, Visser O, René Leemans C. Oral and oropharyngeal cancer in The Netherlands between 1989 and 2006: Increasing incidence, but not in young adults. Oral Oncol. 2009;45(9):85-9. [ Links ]
10. Kumar V, Abbas AK, Fausto N MR. Robbins. Basic Pathology 8th Edition; Neoplasia. 2007;(5):155-65. [ Links ]
11. Amarasinghe HK, Usgodaarachchi US, Johnson NW, Lalloo R, Warnakulasuriya S. Betel-quid chewing with or without tobacco is a major risk factor for oral potentially malignant disorders in Sri Lanka: A case-control study. Oral Oncol. 2010;46(4):297-301. [ Links ]
12. Warnakulasuriya S. Prognostic and predictive markers for oral squamous cell carcinoma: The importance of clinical, pathological and molecular markers. Saudi J Med Med Sci. 2014;2(1):12. [ Links ]
13. Idris AM, Ahmed HM, Mukthar BI, Gander AF E-BE. Descriptive epidemiology of oral neoplasms in Sudan 1970-1985 and the role of Toombak. Int J Cancer. 1995;6(1):155-8. [ Links ]
14. Warnakulasuriya S, Johnson NW, Van Der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10): 575-80. [ Links ]
15. Braakhuis BJM, Leemans CR, Visser O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol. 2014;50(7):670-5. [ Links ]
16. Jaber MA, Porter SR, Scully C, Gilthorpe MS, Bedi R. The role of alcohol in non-smokers and tobacco in non-drinkers in the aetiology of oral epithelial dysplasia. Int J Cancer. 1998;77(3):333-6. [ Links ]
17. Jaber MA, Porter SR, Gilthorpe MS, Bedi R, Scully C. Risk factors for oral epithelial dysplasia-the role of smoking and alcohol. Oral Oncol. 1999;35(2):151-6. [ Links ]
18. Pavia M, Pileggi C, Nobile CGA, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83(5):1126-34. [ Links ]
19. Pires FR, Ramos AB, Oliveira JBC de, Tavares AS, Luz PSR da, Santos TCRB dos. Oral squamous cell carcinoma: clinicopathological features from 346 cases from a single oral pathology service during an 8-year period. J Appl Oral Sci. 2013; 21(5):460-7. [ Links ]
 Correspondence:
Correspondence:
Rhoodie Martins Garrana BDS, MSc (Wits)
Department of Oral Medicine & Periodontology, School of Oral Health Sciences, Faculty of Health Science
University of the Witwatersrand
7 York Road, Parktown, Johannesburg, 2193, South Africa. Tel:0827824838
rmgarrana@gmail.com














