Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.73 no.2 Johannesburg Mar. 2018
CLINICAL REVIEW
VELscope: shedding light on its ideal application
J Fourie
BChiD, MSc(Odontt) MChiD. Department of Periodontology and Oral Medicine, Sefako Makgatho University
SUMMARY
Oral squamous cell carcinoma (OSCC) is a deadly and disfiguring disease. Despite the fact there are readily identifiable precursor lesions and ample opportunity for detection during dental visits, patients continue to succumb to the disease due to late diagnosis. Adjunctive diagnostic aids have been designed to change the natural history of oral cancer by promoting screening practices and allowing for the early identification of OSCC and precursor lesions.' The VELscope is one such device that is available in South Africa, where it is marketed by Inter-Africa Dental. This article will look at the current evidence for the use of the VELscope in different practice scenarios.
INTRODUCTION
Oral and pharyngeal cancers, when grouped together, are the sixth most common cancer in the world.2 More than 90% of oral malignancies are squamous cell carcinomas and more than 80% of these can be attributed to tobacco or alcohol consumption.3 Despite the surgical and chemoradiotherapeutic treatment advances, the five year survival rate is still only 50%4 and this has not improved over the past three decades.3 People who do survive are often left with disfiguring and debilitating scarring from surgery or radiation therapy. Seventeen to 35% of oral squamous cell carcinomas (OSCC) develop from pre-existing leukoplakic lesions.5 Early recognition of these precursor lesions should make OSCC an essentially preventable disease.
In order to improve the outcome of OSCC, both in terms of mortality and morbidity, new cases should be prevented through behaviour modification and existing cases diagnosed at the earliest opportunity. Known risk factors, such as cigarette smoking,6 should be eliminated in order to prevent the development of precursor as well as definitive oral cancer lesions.
Despite the accessibility of the oral cavity for inspection, and the opportunities presented by routine dental appointments, many oral cancers still present at a fairly late stage. Precursor lesions such as leukoplakia may therefore have been missed by practitioners who did not examine the soft tissues thoroughly or wrongly diagnosed the lesions as innocent.
The conventional oral examination (COE) cannot reliably diagnose potentially malignant disorders (PMD) or OSCC.7 Even common mucosal pathology is wrongly diagnosed in 43% of cases and cancer in 5.6%.8 Frictional hyperkeratosis and leukoplakic lesions are admittedly very hard to tell apart.
Besides the difficulty in identifying visible lesions, it is also possible that the initial malignant transformation of keratinocytes is subclinical, and the dysplastic or molecularly altered epithelium associated with cancer progression may not be observed by COE.9-'' It has been demonstrated that dysplasia can be seen in the clinically normal appearing mucosa at sites distant from the presenting OSCC/PMD.'2'3
Diagnostic aids have been developed for the early detection of oral cancer. The VELscope (visually enhanced lesion scope) has been developed to enhance the visualisation of oral mucosal abnormalities that 'may not be apparent or visible to the naked eye, such as oral cancer or pre-malignant dysplasia' and to establish appropriate surgical margins during the removal of PMD/OSCC lesions (LED Dental, Vancouver, British Columbia, Canada).
The purpose of this article is to evaluate the current literature in the quest to identify the most appropriate application of the VELscope, considering:
1. Screening: application of a test in a population who are apparently free from disease to sort those with disease from those without disease as compared with the gold standard of the COE.14
2. Case finding: application of a test in patients with a lesion to determine the diagnosis of that lesion as compared with the gold standard of a scalpel biopsy and histopathological diagnosis.14
3. Monitoring of patients with oral dysplasia or malignancy.
SCIENTIFIC BACKGROUND OF VELSCOPE
The VELscope was first introduced by Lane et al.10 in a proof of concept study to aid in the discrimination of high risk PMD and invasive SCCs from normal oral mucosa.
The VELscope is a simple hand-held device that can be used to directly visualise the oral mucosa (Figure 1). Oral tissues are illuminated by a blue/violet light source (400-460 nm) and then visualised through long pass and notch optical filters which allow the passage of long wavelength green and red autofluoresence. Under direct fluorescent visualisation (FV), normal mucosa appears pale green and is defined as FV retained (FVR), while tissues which do not emit the natural pale green autofluoresence and therefore appear darker are classified as FV loss (FVL).10,15 The distinction should be made by comparing the suspect lesion with adjacent tissue as well as with tissue on the contra-lateral side to serve as an anatomic control.15
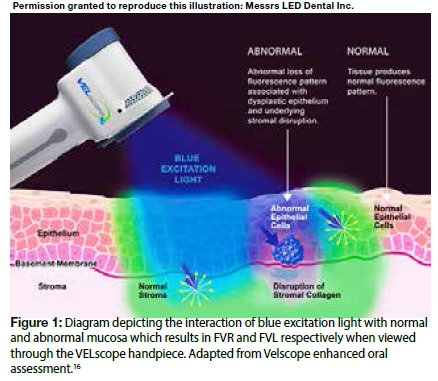
The interaction of light with the tissues highlights changes in structure and metabolic activity. FVL Fluorescent visualisation loss reflects changes in intrinsic fluorophore distribution, the breakdown of the collagen matrix, a decrease in flavin adenine dinucleotide (FAD) concentration because of tissue remodelling and increased metabolism, as well as increased haemoglobin absorption which is associated with neoplastic changes.10,17 The structural changes of the epithelium and the lamina propria associated with neoplastic development (thickening of epithelium, hyperchromatism, increased cellular pleomorphism, increased microvascularity) lead to increased absorption or scattering of light which result in altered autofluoresence.15,18 However, some of these structural and cellular changes are not confined to the malignant process, and many benign lesions also undergo changes that become accentuated during fluorescence visualisation.
Kordbacheh et al.19 correlated the fluorescence visualisation findings with different histological groups and the differentially expressed genes in these groups. The results provided molecular evidence of the cellular pathways involved in FVL, which include the immune response, cell-cell and cell-extracellular matrix adhesion, stromal remodelling and angiogenesis. The results also supported the association between fluorescent diascopy and inflammation: lesions that blanched had upregulated T-cell mediated inflammation.
The greatest differences between lesions with FVL and FVR were regarding cell cycle regulation and apoptosis-related genes. Studying a rat tongue carcinogenesis model, Ohnishi et al. 17 found that FVL of the progressive dysplastic field surrounding the tumour was primarily due to the breakdown of the collagen matrix increased haemoglobin absorption with secondary increased dispersion in the epithelium, thickening of the epithelium and a decrease in FAD concentration.
CONVENTIONAL ORAL CANCER SCREENING PRACTICES
Oral cancer screening can be defined as an oral mucosal examination that is performed together with an assessment of the individual's health history.20 The intention should be to identify asymptomatic lesions.21
Screening may be 'population based' when a sample of the general population is screened; 'opportunistic' when a patient presents to a health care provider with an unrelated problem; and 'targeted' when high risk patients are involved.22
Population: The lack of available evidence and low prevalence of oral cancer does not currently support population based screening.20,23 A population based study conducted in India24 was the only randomised controlled trial to date, and the only one to be included in the 2010 Cochrane review.22 The study demonstrated that screening of a high risk subpopulation of subjects resulted in a decreased mortality rate due to a greater proportion of cases having been diagnosed early. Similar results were obtained in Cuba with the nationally implemented Oral Cancer Case Finding Program (OCCFP) which demonstrated that focused screening is able to increase the number of oral cancer cases which were diagnosed early.25
Opportunistic: Screening during regular dental visits offer the opportunity to discuss lifestyle modifications that will lower the risk of oral cancer, such as cessation of smoking.26 Screening of high risk individuals between the age of 40 and 60 during regular dental visits has proven to be cost effective.27 However, screening should be extended to everybody as OSCC is increasingly being documented among young patients28 and patients who have never smoked or drank alcohol, especially it seems, among white females.29 Unfortunately, high risk patients may not be frequent dental attenders30,31 but people who do visit their dentist regularly are far more likely to have the advantage of early diagnosis of any OSSC which may present .32
Targeted screening can also be implemented when patients self-select to attend screening clinics due to worrying signs and symptoms, explaining why 47% of patients attending such a clinic have been found to have abnormal findings.23 Yet, only 5% were suspicious of a malignancy, and only a disappointing 50% of these patients returned for follow up.23
USE OF THE VELSCOPE AS A SCREENING ADJUNCT
The question is whether the use of adjunctive diagnostic aids, such as the VELscope, will reasonably benefit the screening process. Diagnostic aids may not even be indicated for screening which is not supposed to be a diagnostic process.33 The aim of the oral mucosal examination in general practice should simply be to detect oral mucosal abnormalities.34,35 For this purpose the VELscope may promote improved screening practices among clinicians if only by stimulating the procedure of careful mucosal examination.35,36
In any study, the intended target population as well as the skill-set of the examiner should clearly be defined as the findings of the VELscope are influenced by the risk of the population involved and the experience of the clinician. There are currently no guidelines regarding the use of the VELscope in general practice37 or settings for screening 35,38 and no studies of the diagnostic accuracy of the use of a light based detection system to screen for disease in apparently healthy individuals.39 In addition there is definitely no reliable evidence that the VELscope can identify apparently 'invisible' oral cancer lesions in the general population.40
Pilot and case studies done by specialists in cancer and dysplasia clinics are often used to demonstrate the remarkable sensitivity and specificity of the VELscope.,0,,5,,li These have been inappropriately referenced to other clinical scenarios. Case studies have never been strong enough to change general practice, because when done in specialist clinics, a spectrum bias is introduced due to the differing risk profiles of the patients attending specialist practice40 Clinical decision-making should rest on evidence-based recommendations, and so far the only criterion being satisfied is the desire of the patient to know that he/she is being screened for oral cancer.40 For the purpose of reassuring our patients a COE by a general dental practitioner is still the best method of determining the presence or absence of disease because dentists are more adept at recognising a disease-free state than necessarily classifying the presence of disease.39
In the general population, where there is a low prevalence of oral cancer, there is the very real risk of cancer over-diagnosis with the VELscope, resulting in a significant emotional and economic cost. When the VELscope is routinely used in general practice among all patients 40 years and older, it will incorrectly test positive more than 90% of the time.40
The principal weaknesses of light based detection systems are their low specificity, the fact that there is no evidence to support their cost effectiveness in comparison with COE, and the uncertainty of whether the application of the test has reduced mortality.,4,40 Therefore, multicentre controlled studies conducted by general dentists are needed to justify their application.14
When the VELscope was used in community dental clinics, the consensus was that the practitioners required more training and experience with the device as well as in mucosal pathology36,41 and that any positive findings required reassessment to limit over-diagnosis.36 Yet it was not clear swhether any clinical benefit had been derived from the use of the VELscope or whether FVL correlated with the clinical risk assessment of the lesions.35
McNamara et al.42 designed their study to accord with a general practice protocol in that ,30 consecutive patients were enrolled and that all mucosal abnormalities were included. No additional lesions were identified with the VELscope, which correctly highlighted one lesion that appeared clinically innocent, while conversely it missed one lesion that was clinically worrisome. Common inflammatory conditions and even anatomical variations will give the appearance of FVL. As such, the VELscope did not add any benefit beyond COE for routine screening of PMD/OSCC. The routine use of the VELscope in the screening of asymptomatic individuals is therefore not supported, and may even reduce the diagnostic performance of a general dentist.4,
The importance of clinical follow up and elimination of apparently benign lesions is illustrated by the decision- making protocol described by Bhatia et al.,34 which attempts to improve the low specificity of the VELscope by ruling out inconspicuous findings. In this study general practitioners found that the VELscope enhanced lesion detection by increasing the visibility and border distinctness of lesions already detected under COE, and identified additional lesions which changed the provisional diagnoses. It seems that lesions located on the lower lip were particularly amenable to FV examination as eight out of ,0 lesions which had been referred, based on FV findings, were from this site (including two that were not clinically visible) and all were finally diagnosed as actinic cheilitis with or without dysplasia. The remaining lesions from intraoral sites rendered false positives. Therefore careful interpretation of FV findings combined with the COE can improve the value of this diagnostic test.
The study undertaken by Huff et al.43 is perhaps the only investigation to have been performed in a general, independent dental practice. The authors claim that the VELscope aided the diagnosis of occult abnormal mucosal findings, but this cannot be concluded as only "clinically" abnormal lesions were investigated, and then histologically confirmed once a brush biopsy (subject to its own inherent errors) demonstrated abnormal results. There is also no evidence that the VELscope actually detected any new dysplastic lesions or even identified all lesions detected by COE.
To perform a surgical biopsy of clinically normal mucosa that displays FVL in this general population will require a strong conviction and burden of proof as it is arguably unethical to biopsy apparently healthy mucosa in an evidently healthy individual.33
USE OF THE VELSCOPE AS A DIAGNOSTICADJUNCT DURING CASE FINDING
When the general clinician identifies an area of mucosal abnormality a differential diagnosis is established which determines the need for histological confirmation of the lesion. At this point, the clinician can choose to either do nothing based on the perceived innocence of the lesion, in which case a PMD/OSCC may go undiagnosed, or biopsy a truly innocent lesion incurring unnecessary financial cost and causing emotional distress. The ideal diagnostic device would determine the appropriate action at this critical juncture.
The reality is that about ,5% of patients seen in general dental practice have some mucosal pathology,35,44 with 4.2% of those lesions regarded as malignant or potentially malignant.30 The clinical features of PMD and early OSCC are varied and may be misdiagnosed as other conditions, such as mucosal inflammation, hyperkeratosis or traumatic ulceration."45 Chronic ulceration, induration and rolled margins, the classical signs of oral cancer, occur late in the progress of the condition.2,
Leukoplakia and erythroplakia are PMD which offer an ideal opportunity for the diagnosis and monitoring of high risk patients. The diagnosis of these conditions is made by excluding other white and red lesions that carry no increased risk for oral cancer.46
White lesions to consider include hyperplastic and pseudomembranous candidosis, frictional hyperkeratosis, leukoedema and plaque type oral lichen planus (OLP) while red lesions include erythematous candidosis, erosive OLP, discoid lupus erythematosus and inflammation secondary to trauma. The question is whether the VELscope can help in making this distinction.
A number of studies have tested the application of the VELscope in a case-finding scenario, and according to a recent Cochrane review, light based detection systems had a sensitivity and specificity of 91% and 58% respectively in the accuracy of the diagnosis of PMD/ OSCC relative to the gold standard of a scalpel biopsy. This means that among a population of 1000 patients, 45 patients with OSCC would wrongly be told that they are healthy, while 210 healthy patients would wrongly be told that they have OSCC. The studies were criticised for having a high risk of bias due to subject selection being restricted to high risk patients.47
The merit of some of studies using the VELscope as a case-finding adjunct should be considered more closely (Table 1).
Scheer et al.49 evaluated the use of the VELscope in patients referred to a specialist clinic to rule out PMD/OSCC which included patients with known histories of PMD/OSCC. Despite the 100% success rate in identifying dysplasia, the authors felt that the high rate of false positives limited the positive predictive value (PPV) to such an extent that the decision to biopsy or not should always rest on the clinical judgement of the clinician.
While Rana et al.52 also conducted their study among a high risk population with PMD, surprisingly, they also included patients with pemphigus vulgaris without clarifying how these provisional diagnoses were reached. Nor were all of these provisional diagnoses confirmed as only a select number of cases received biopsies. The VElscope was not used to supplement all examinations, instead the group was arbitrarily split into subjects that would or would not receive the examination and a side-by-side comparison can therefore not be made. In addition, greater care was exercised to eliminate false positives within the VELscope group by delaying biopsies for two weeks if there were any suspicions of an acute inflammatory reaction. Despite the impressive sensitivity rate achieved the authors concede that 64.23% of all lesions examined showed FVL while only 4.88% of these lesions were dysplastic and at these levels the VELscope is considered not acceptable for clinical use.
Both Awan et al.51 and Farah et al.53 evaluated the use of the VELscope among a population of patients with red and/or white oral mucosal lesions suspected of being PMD. The VELscope enhanced lesion visualisation51,53 and had a high sensitivity for detecting any mucosal disorder.51 However, the instrument could not discriminate between high risk and low risk lesions,51,53 and wrongly indicated 69.2% of frictional hyperkeratosis as high risk.51 This poor performance could only be enhanced through experienced clinical interpretation. Indeed, an oral medicine specialist relying on COE alone proved more accurate in identifying dysplastic lesions compared with the VELscope alone.53 The VELscope could therefore neither allay patient fears as to the absence of PMD/OSCC53 nor safeguard against unnecessary biopsies.51 It instead burdened the patients with even more unnecessary biopsies since 80% of clinically occult lesions were false positives.53
These findings resonated with the study by Mehrotra et al.48 which was conducted among a population with clinically innocuous lesions. In such a low risk population the poor specificity of the VELscope really came to the fore with more than half of patients incorrectly diagnosed with dysplasia, while half of dysplastic lesions were also missed. The VELscope can therefore not be relied upon to determine the benign/malignant nature or lesions. Since no new lesions were identified with the VELscope the authors concluded that it is of no additional benefit beyond the COE.
Koch et al.50 set out to establish whether the VELscope can distinguish between suspicious high risk and benign oral lesions and compared that performance with the findings of an expert examiner and histology. By distinguishing two patterns of FVL according to intensity and uniformity of the image obtained Koch et al. were able to increase the specificity, but at the cost of decreasing the sensitivity the test. Yet, the VELscope could not improve upon the accuracy of a specialist examiner.
The above studies demonstrate that the VELscope and COE are subject to the same shortfalls, with a generally good sensitivity (ability to visualise) but poor specificity (inability to distinguish)7 and although the VELscope may be useful in the hands of an experienced specialist,49,53 its routine use is not justified due to the high risk of false positives, high cost and the lack of scientific evidence.54 Clinical interpretation remains the most limiting factor in general dental practice if the practitioner is not experienced and trained in oral mucosal pathology,53 so that the practitioner who cannot make a clinical diagnosis without the VELscope, also will not be able to make it with VELscope. None of these studies concluded that the VELscope is a worthwhile adjunct to help in distinguishing between mucosal lesions. The interpretation of VELscope findings are variable and subjective49 and may result in inter-observer disagreement53 although others feel that it is easy to use with limited operator variability.51
Fluorescent diascopy (the blanching of tissues due to the application of pressure when viewed through the VELscope) was introduced in an attempt to distinguish between FVL due to inflammation or dysplastic change. While some still support this practice49 others feel that it may hide true angiogenesis associated with dysplasia, while failing to reduce submucosal blood associated with minor trauma.48 Fluorescent diascopy therefore cannot be used to eliminate suspicious-looking lesions as dysplastic. Notably, OSCC lesions were among those which blanched.53 It is a cumbersome technique to use and generates findings that are difficult to interpret.
The clinical features of lesions determine the interpretation of findings. The keratin of verrucous lesions reveals intensifying FV, while red, ulcerated and darkly pigmented lesions are favoured by FVL.35,49,50 The preferential detection of red lesions implies that erythroplakia is unlikely to be missed but many inflammatory lesions will be falsely identified, whilst alternatively, hyperkeratotic lesions may elude autofluorescence detection and therefore there is a failure to distinguish between frictional hyperkeratosis and leukoplakia.50,51 The VELscope also favours non-homogenous leukoplakia diagnosis over homogenous leukoplakias, specifically due to a lack of or only partial blanching which will be problematic in cases of lichenoid or dysplastic lesions with an inflammatory component.53 Other benign keratotic lesions that are accompanied by hyperplasia and inflammation are also more likely to result in false positive identification by the VELscope.55,56 The bright red autofluoresence signal that is sometimes observed is attributed to porphyrin which is a product of bacterial metabolism.18,50 Interestingly, coffee and liquorice consumption can alter the autofluorescence results and a quick water rinse before examination can reduce false positives.56
MANAGEMENT OF PATIENT WITH PMD/ OSCC
All patients diagnosed with a PMD should be intensively monitored regardless of any cessation of high risk behaviour or clinical resolution of the lesion,57,58 even though there is no consensus regarding the recommended frequency or length of follow up.5,57,59 During the monitoring of patients with dysplasia, , the lesions may either disappear, remain stable or progress to malignancy.Subjects exposed to known risk factors such as tobacco use are more likely to have a recurrence after surgical excision.60
Leukoplakia can be managed through surgery, chemotherapeutics, or observation, although the evidence supporting an individual approach is not robust and none of the treatments are able to prevent oral cancer from developing.61 The outcome of the surgical management of leukoplakia is uncertain and not necessarily beneficial, with recurrences and malignant transformation possible even if a clinically healthy mucosa remains after surgery58,60,61 due to the high risk molecular field which is left behind.62 Therefore, in the absence of proven surgical or medical measures that reduce the risk of recurrence or malignant transformation of PMD,63,64 careful monitoring is most strongly advised.
There are also no reliable laboratory means of predicting which lesions will progress to cancer, although the histological confirmation of dysplasia,5 degree of dysplasia,59,65 and molecular features such as loss of heterozygosity (LOH), can be used to stratify individual risk to a degree.64 These techniques are not absolutely predictive, even more so because biopsies are not always completely representative.59 Conventional clinical factors such as the site of the lesion or tobacco habit cannot be relied upon, although larger (> 200mm2), non-homogenous leukoplakias are more likely to transform into malignancy.66
In patients who were successfully managed for a primary OSCC, the very real risk of a recurrence or second primary tumour (SPT) exists, so that monitoring is integrally important. Over the course of ten years, up to 7% of patients will experience a recurrence and 15% an SPT.67 In patients with PMD/OSCC high risk, molecular alterations may spread across a large as well as distant mucosal fields,68 so that dysplasia and even micro-invasive OSCC may be found at the clinically healthy oral site contra-lateral to that of the initially presenting dysplastic or OSCC lesion.12,55
It is within this context that an adjunctive diagnostic aid finds value by: diagnosing disease early, perhaps even within clinically normal tissue; identifying high risk change in mucosa with PMD so that the need and ideal location of the biopsy site is determined; and allowing for the complete removal of an OSCC by identifying the surrounding area of field change. Patients with PMD and OSCC are frequently subjected to biopsies during follow- up, resulting in significant anxiety and discomfort while not guaranteeing that the mucosa is healthy or safe. According to Balevi 40 the ideal application of the VELscope is in patients monitored in cancer and dysplasia clinics where the probability of OSCC is greater than 10%.
Lane et al.10 conducted the first proof of concept pilot study for the use of the VELscope among patients with a history of dysplasia or OSCC. All lesions deemed suspicious on clinical grounds and toluidine blue findings were biopsied, and the histopathological diagnosis then retrospectively matched to FV. In this setting, the VELscope achieved a sensitivity of 98% and specificity of 100%, demonstrating the significant benefit within this particular patient population. These findings were subsequently confirmed by Ohnishi et al.17 who found that the VELscope had a 91% sensitivity and 100% specificity for detecting dysplasia and OSCC.
Subsequent case reports demonstrate some remarkable features of the VELscope. Poh et al.15 demonstrated that the VELscope can delineate the clinically occult high risk field surrounding the primary tumour before surgical resection. The field reliably corresponded to molecular risk (as determined by LOH at 3p and 9p) even before it was histologically visible, hinting at the fact that histology may not always be considered as the gold standard. The VELscope also proved to be of value in the monitoring of cancer and dysplasia patients by identifying new lesions in clinically healthy tissue, even at sites distant from the original lesion, emphasising the importance of whole mouth monitoring.18 Tsui et al.69 compared the VELscope with toluidine blue in visualising the field surrounding a tumour. While toluidine blue stained only isolated areas corresponding to early OSCC, the VELscope also identified a wider area corresponding to dysplasia.
These case reports15,18,69 show that the VELscope is useful in the monitoring of patients with a history of dysplasia as well as delineating the high risk field that surrounds the primary lesion. These reports are also used to substantiate the claim that the VELscope can detect clinically occult disease. However, inspection of the photographic documentation of the cases reveal clinically visible mucosal changes in the areas deemed to be 'clinically occult'.
Delineating the surgical margin of OSCC and dysplasia significantly impacts on the lives of patients 55,56,58,70 by reducing the rate of tumour recurrences in the first year to 0% as opposed to 25% among patients in whom the margin was clinically determined.70
Using a prototype of the VELscope, Moro et al.56 also confirmed the benefit of using FVL to delineate the field surrounding the primary lesion as well as in identifying more lesions than is clinically possible in the monitoring of patients with PMD/OSCC. Although it is arguably not feasible to define false negatives as the entire mucosa cannot be biopsied,55 patients in whom there was no FVL did not develop new tumours during the study. If true negatives are considered in this context, then a sensitivity of 100%, specificity of 93%, PPPV of 92% and NPPV of 100% can be derived. The monitoring of patients with a history of PMD/OSCC is often complicated by the mucosal changes associated with previous surgery and radiation. These mucosal changes make clinical as well as fluorescent interpretation difficult so that in this setting the VELscope actually had a very limited sensitivity of 27.3% and specificity of 77.8%.71 The poor sensitivity that this study obtained is very worrisome as the sample was of the high risk population in which recurrences should be conclusively diagnosed. The subjective interpretation of the VELscope findings were again considered as an important limitation so that the VELscope was not deemed to be of any benefit in the monitoring of high risk patients.71
CONCLUSION
Based upon these studies, Fedele's37 standpoint still stands true, that the use of the VELscope in general practice is anecdotal, and that its primary use is to help experienced clinicians improve their ability to detect PMD/OSCC in high risk individuals attending specialist centres. The primary difficulty in general practice is to reliably distinguish between lesions. To this end, there is no substitute for proper training and experience in oral mucosal pathology. Because a dentist may detect only one OSCC in 7-10 years,40 the danger of complacency and cursory oral mucosal examinations is very real. However, the impact on the life of that particular patient is devastating, and worth the extra two minutes of the time of the attending clinician.
DEFINITIONS
Sensitivity: Probability that a patient with PMD/OSCC will generate a positive result when measured against the gold standard of scalpel biopsy.
Specificity: The probability that a patient who does not have a PMD/OSCC will generate a negative finding.
Positive predictive value: Probability that a patient with a positive test result actually has a PMD/OSCC.
Negative predictive value: Probability that a patient with a negative test result does not have a PMD/OSCC.
ACRONYMS
COE : Conventional oral examination
FAD : Flavin adenine dinucleotide
FVI : Fluorescence visualisation intensified
FVL: Fluorescence visualisation loss
FVR fluorescence visualisation retained
LOH : loss of heterozygosity
OCCFP : Oral Cancer Case Finding Program
OLP : oral lichen planus - plaque type or erosive type
OSCC Oral squamous cell carcinoma
PMD : Potentially malignant disorder
PPV : positive predictive value
SPT : second primary tumour
VELscope : Visually Enhanced Lesion scope
Reference
1. Patton LL, Epstein JB, Kerr AR. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc 2008;139:896-905; quiz 93-4. [ Links ]
2. Warnakulasuriya S. Living with oral cancer: epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol 2010;46:407-10. [ Links ]
3. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009;45:309-16. [ Links ]
4. Brinkman BM, Wong DT. Disease mechanism and biomarkers of oral squamous cell carcinoma. Curr Opin Oncol 2006;18:228-33. [ Links ]
5. van der Waal I, Schepman KP, van der Meij EH, Smeele LE. Oral leukoplakia: a clinicopathological review. Oral Oncol 1997;33:291-301. [ Links ]
6. Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med 2008;37:1-10. [ Links ]
7. Epstein JB, Guneri P, Boyacioglu H, Abt E. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. J Am Dent Assoc 2012;143:1332-42. [ Links ]
8. Kondori I, Mottin RW, Laskin DM. Accuracy of dentists in the clinical diagnosis of oral lesions. Quintessence Int 2011;42:575-7. [ Links ]
9. Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, Kummer JA, Leemans CR, Braakhuis BJ. Genetically altered fields as origin of locally recurrent head and neck cancer: a retrospective study. Clin Cancer Res 2004;10:3607-13. [ Links ]
10. Lane PM, Gilhuly T, Whitehead P, Zeng H, Poh CF, Ng S, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt 2006;11:024006. [ Links ]
11. Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol 2008;44:10-22. [ Links ]
12. Thomson PJ. Field ch ange and oral cancer: new evidence for widespread carcinogenesis? Int J Oral Maxillofac Surg 2002;31:262-6. [ Links ]
13. Jayaraj R, Thomas M, Kavanagh D, d'Abbs P, Mayo L, Thomson V, et al. Study protocol: Screening and Treatment of Alcohol-Related Trauma (START) - a randomised controlled trial. BMC Health Serv Res 2012;12:371. [ Links ]
14. Dios PD, Leston JS. Oral cancer pain. Oral Oncol 2010;46:448-51. [ Links ]
15. Poh CF, Zhang L, Anderson DW, Durham JS, Williams PM, Priddy RW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res 2006;12:6716-22. [ Links ]
16. Velscope enhanced oral assessment. Atlanta, Georgia: Fifth Gear; 2016 [cited 2016]; Available from: http://www.velscope.com/velscope-technology/tissue-fluorescence/. [ Links ]
17. Ohnishi Y, Fujii T, Ugaki Y, Yasui H, Watanabe M, Dateoka S, et al. Usefulness of a fluorescence visualization system for the detection of oral precancerous and early cancerous lesions. Oncol Rep 2016;36:514-20. [ Links ]
18. Poh CF, Ng SP, Williams PM, Zhang L, Laronde DM, Lane P, et al. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head Neck 2007;29:71-6. [ Links ]
19. Kordbacheh F, Bhatia N, Farah CS. Patterns of differentially expressed genes in oral mucosal lesions visualised under autofluorescence (VELscope() ). Oral Dis 2016;22:285-96. [ Links ]
20. Ford PJ, Farah CS. Early detection and diagnosis of oral cancer: Strategies for improvement. Journal of Cancer Policy 2013:e2-e7. [ Links ]
21. Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol 2010;46:414-7. [ Links ]
22. Brocklehurst P, Kujan O, Glenny AM, Oliver R, Sloan P, Ogden G, et al. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev 2010:CD004150. [ Links ]
23. Shuman AG, Entezami P, Chernin AS, Wallace NE, Taylor JM, Hogikyan ND. Demographics and efficacy of head and neck cancer screening. Otolaryngol Head Neck Surg 2010;143:353-60. [ Links ]
24. Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet 2005;365:1927-33. [ Links ]
25. Santana JC, Delgado L, Miranda J, Sanchez M. Oral Cancer Case Finding Program (OCCFP). Oral Oncol 1997;33:10-2. [ Links ]
26. Scully C, Petti S. Overview of cancer for the healthcare team: aetiopathogenesis and early diagnosis. Oral Oncol 2010;46:402-6. [ Links ]
27. Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al. The cost-effectiveness of screening for oral cancer in primary care. Health Technol Assess 2006;10:1-144, iii-iv. [ Links ]
28. Bodner L, Manor E, Friger MD, van der Waal I. Oral squamous cell carcinoma in patients twenty years of age or younger--review and analysis of 186 reported cases. Oral Oncol 2014;50:84-9. [ Links ]
29. Harris SL, Kimple RJ, Hayes DN, Couch ME, Rosenman JG. Never-smokers, never-drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck 2010;32:499-503. [ Links ]
30. Lim K, Moles DR, Downer MC, Speight PM. Opportunistic screening for oral cancer and precancer in general dental practice: results of a demonstration study. Br Dent J 2003;194:497-502; discussion 493. [ Links ]
31. Netuveli G, Sheiham A, Watt RG. Does the 'inverse screening law' apply to oral cancer screening and regular dental check-ups? J Med Screen 2006;13:47-50. [ Links ]
32. Elwood JM, Gallagher RP. Factors influencing early diagnosis of cancer of the oral cavity. CMAJ 1985;133:651-6. [ Links ]
33. Downer MC, Moles DR, Palmer S, Speight PM. A systematic review of test performance in screening for oral cancer and precancer. Oral Oncol 2004;40:264-73. [ Links ]
34. Bhatia N, Matias MA, Farah CS. Assessment of a decision making protocol to improve the efficacy of VELscope in general dental practice: a prospective evaluation. Oral Oncol 2014;50:1012-9. [ Links ]
35. Laronde DM, Williams PM, Hislop TG, Poh C, Ng S, Bajdik C, et al. Influence of fluorescence on screening decisions for oral mucosal lesions in community dental practices. J Oral Pathol Med 2014;43:7-13. [ Links ]
36. Laronde DM, Poh CF, Williams PM, Hislop TG, Zhang L, MacAulay C, et al. A magic wand for the community dental office? Observations from the British Columbia Oral Cancer Prevention Program. J Can Dent Assoc 2007;73:607-9. [ Links ]
37. Fedele S. Diagnostic aids in the screening of oral cancer. Head Neck Oncol 2009;1:5. [ Links ]
38. Guneri P, Epstein JB. Late stage diagnosis of oral cancer: components and possible solutions. Oral Oncol 2014;50:1131-6. [ Links ]
39. Walsh T, Liu JL, Brocklehurst P, Glenny AM, Lingen M, Kerr AR, et al. Clinical assessment to screen for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database Syst Rev 2013:CD010173. [ Links ]
40. Balevi B. Assessing the usefulness of three adjunctive diagnostic devices for oral cancer screening: a probabilistic approach. Community Dent Oral Epidemiol 2011;39:171-6. [ Links ]
41. Jane-Salas E, Blanco-Carrion A, Jover-Armengol L, Lopez-Lopez J. Autofluorescence and diagnostic accuracy of lesions of oral mucosa: A pilot study. Braz Dent J 2015;26:580-6. [ Links ]
42. McNamara KK, Martin BD, Evans EW, Kalmar JR. The role of direct visual fluorescent examination (VELscope) in routine screening for potentially malignant oral mucosal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:636-43. [ Links ]
43. Huff K, Stark PC, Solomon LW. Sensitivity of direct tissue fluorescence visualization in screening for oral premalignant lesions in general practice. Gen Dent 2009;57:34-8. [ Links ]
44. Scully C, Bagan JV, Hopper C, Epstein JB. Oral cancer: current and future diagnostic techniques. Am J Dent 2008;21:199-209. [ Links ]
45. Rosin MP, Poh CF, Elwood JM, Williams PM, Gallagher R, MacAulay C, et al. New hope for an oral cancer solution: together we can make a difference. J Can Dent Assoc 2008;74:261-6. [ Links ]
46. Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007;36:575-80. [ Links ]
47. Macey R, Walsh T, Brocklehurst P, Kerr AR, Liu JL, Lingen MW, et al. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev 2015:CD010276. [ Links ]
48. Mehrotra R, Singh M, Thomas S, Nair P, Pandya S, Nigam NS, et al. A cross-sectional study evaluating chemiluminescence and autofluorescence in the detection of clinically innocuous precancerous and cancerous oral lesions. J Am Dent Assoc 2010;141:151-6. [ Links ]
49. Scheer M, Neugebauer J, Derman A, Fuss J, Drebber U, Zoeller JE. Autofluorescence imaging of potentially malignant mucosa lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;111:568-77. [ Links ]
50. Koch FP, Kaemmerer PW, Biesterfeld S, Kunkel M, Wagner W. Effectiveness of autofluorescence to identify suspicious oral lesions-- a prospective, blinded clinical trial. Clin Oral Investig 2011;15:975-82. [ Links ]
51. Awan KH, Morgan PR, Warnakulasuriya S. Evaluation of an autofluorescence based imaging system (VELscope) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncol 2011;47:274-7. [ Links ]
52. Rana M, Zapf A, Kuehle M, Gellrich NC, Eckardt AM. Clinical evaluation of an autofluorescence diagnostic device for oral cancer detection: a prospective randomized diagnostic study. Eur J Cancer Prev 2012;21:460-6. [ Links ]
53. Farah CS, McIntosh L, Georgiou A, McCullough MJ. Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck 2012;34:856-62. [ Links ]
54. Trullenque-Eriksson A, Munoz-Corcuera M, Campo-Trapero J, Cano-Sanchez J, Bascones-Martinez A. Analysis of new diagnostic methods in suspicious lesions of the oral mucosa. Med Oral Patol Oral Cir Bucal 2009;14:E210-6. [ Links ]
55. Jayaprakash V, Sullivan M, Merzianu M, Rigual NR, Loree TR, Popat SR, et al. Autofluorescence-guided surveillance for oral cancer. Cancer Prev Res (Phila) 2009;2:966-74. [ Links ]
56. Moro A, Di Nardo F, Boniello R, Marianetti TM, Cervelli D, Gasparini G, et al. Autofluorescence and early detection of mucosal lesions in patients at risk for oral cancer. J Craniofac Surg 2010;21:1899-903. [ Links ]
57. Poh CF, Ng S, Berean KW, Williams PM, Rosin MP, Zhang L. Biopsy and histopathologic diagnosis of oral premalignant and malignant lesions. J Can Dent Assoc 2008;74:283-8. [ Links ]
58. Holmstrup P, Dabelsteen E. Oral leukoplakia-to treat or not to treat. Oral Dis 2016;22:494-7. [ Links ]
59. Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Oral premalignant lesions: is a biopsy reliable? J Oral Pathol Med 2007;36:262-6. [ Links ]
60. Arduino PG, Surace A, Carbone M, Elia A, Massolini G, Gandolfo S, et al. Outcome of oral dysplasia: a retrospective hospital-based study of 207 patients with a long follow-up. J Oral Pathol Med 2009;38:540-4. [ Links ]
61. Lodi G, Franchini R, Warnakulasuriya S, Varoni EM, Sardella A, Kerr AR, et al. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst Rev 2016;7:CD001829. [ Links ]
62. Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953;6:963-8. [ Links ]
63. Lodi G, Porter S. Management of potentially malignant disorders: evidence and critique. J Oral Pathol Med 2008;37:63-9. [ Links ]
64. Brennan M, Migliorati CA, Lockhart PB, Wray D, Al-Hashimi I, Axell T, et al. Management of oral epithelial dysplasia: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103 Suppl:S19 e1-2. [ Links ]
65. Bouquot JE, Speight PM, Farthing PM. Epithelial dysplasia of the oral mucosa-diagnostic problems and prognostic features. Current Diagnostic Pathology 2006;12:11-21. [ Links ]
66. Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Long-term treatment outcome of oral premalignant lesions. Oral Oncol 2006;42:461-74. [ Links ]
67. Montebugnoli L, Gissi DB, Flamminio F, Gentile L, Dallera V, Leonardi E, et al. Clinicopathologic parameters related to recurrence and locoregional metastasis in 180 oral squamous cell carcinomas. Int J Surg Pathol 2014;22:55-62. [ Links ]
68. Ha PK, Califano JA. The molecular biology of mucosal field cancerization of the head and neck. Crit Rev Oral Biol Med 2003;14:363-9. [ Links ]
69. Tsui IF, Garnis C, Poh CF. A dynamic oral cancer field: unravelling the underlying biology and its clinical implication. Am J Surg Pathol 2009;33:1732-8. [ Links ]
70. Poh CF, MacAulay CE, Zhang L, Rosin MP. Tracing the "at-risk" oral mucosa field with autofluorescence: steps toward clinical impact. Cancer Prev Res (Phila) 2009;2:401-4. [ Links ]
71. Scheer M, Fuss J, Derman MA, Kreppel M, Neugebauer J, Rothamel D, et al. Autofluorescence imaging in recurrent oral squamous cell carcinoma. Oral Maxillofac Surg 2016;20:27-33. [ Links ]
 Correspondence:
Correspondence:
Dr J Fourie
PO Box 230, Irene 0062.
Tel 082 460 8368














