Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.73 no.1 Johannesburg Fev. 2018
RESEARCH
Introductory remarks on cartilages, bones and on "Bone: formation by autoinduction"
U Ripamonti
MD, PhD Director, Bone Research Laboratory, Research Unit of the University of the Witwatersrand, Johannesburg; Full Professor, Department of Oral Medicine and Periodontology, School of Oral Health Sciences, Faculty of Health Sciences,2193 Parktown, South Africa ugo.ripamonti@wits.ac.za
PROLOGUE
Introductory remarks on cartilages, on bones and on: "Bone formation by autoinduction."
U Ripamonti
A prologue is an explanatory and introductory discourse, which in this case should then commence by asking "Why did I become interested in sharks, sharks' cartilages, sharks' teeth and evolution?"
It all started many years ago when, soon after landing in January 1983 in Africa from the cold shores of Milano University in North Italy, I met Tracey at the University of the Witwatersrand, Johannesburg, and with her hastened to the hot, feverish Natal North Coast, spending time in Umhlanga Rocks. There I visited the Natal Shark Board and was reminded during my tour that whilst sharks have an endoskeleton of cartilage there are some bony appendages on the integument, known as placoids or denticles (Fig. 1A). These render the integument into a formidable abrasive instrument which lacerates victims, causing bleeding, and provoking a full shark attack.1
Back at the University of the Witwatersrand, Johannesburg, I went to the library of the Medical School where, with difficulties, I started to manually sieve through the Index Books then printed and made available world-wide by the National Library of Medicine of the National Institutes of Health (NIH) in Bethesda, Maryland USA.
It was serendipitous that I was attracted by an interesting, perhaps alluring, title of a published paper on sharks: "How to swim with sharks: A primer"1. I read with mounting enthusiasm and scientific interest the essay by a then unknown author who signed himself as Voltaire Cousteau. As the Editor's foot note stated "the essay will make fascinating reading material and sets fundamental rules and principles that, if followed, will make it possible to swim, albeit with difficulties, amid sharks, whilst becoming experts through practice".2
Cousteau makes the wry comment "Actually, nobody wants to swim with sharks" and, reflecting on those who may have wrongly assumed that waters were not shark infested, "has by now doubtless lost any interest in learning how to swim with sharks!1
The essay "How to swim with sharks: A primer" further sparked my interest in shark biology, leading to my reading classic papers on the cartilaginous fishes and discovering that shark genera and species are fully evolved animals with superb differentiating pathways 3-5 that make the shark the most effective killer swimming in the waters of our planet. Sharks or Selachians are named Chon-drichthyes, fishes with an endoskeleton of cartilage thus including the jaws and the chondrocrania (a cartilaginous box containing the spinal nervous tissue of the shark).
Further reading across several different scientific disciplines revealed the concept that the cartilage forming the endoskeletons of Chondrichthyans such as Elasmobranchs is not a primitive condition. It had been thought that cartilage was the original structure of the endoskeleton of fishes and that "bone only appeared at a late date in the history of fishes to augment and replace the cartilage," 6,7 However, it had been reported at the beginning of last century that Elasmobranchs, including sharks, are placoderm descendants "which have degenerated in their skeletal structure from an ancestral condition in which bone was present."7
Romer concurred in 1963, stating "the purely cartilaginous condition (of Elasmobranchs) is not truly a primitive one" and the absence of bone "is not an ancestral character, but one due to the degeneration from bone-bearingancestors."6
This has obviously changed our understanding of the evolutionary skeletogenesis in animals up the vertebrate mammals. It is worth nothing then as Romer stated " that instead of beginning with a purely cartilaginous skeleton, and later gradually acquiring bone, the early vertebrates had a considerable degree of ossification which was followed in a majority of cases by a slump toward a cartilaginous condition"6Romer further stated that "Bone is an ancient, rather than a relatively new, skeletal material in the history of vertebrates"
That regression may explain how in modern vertebrates "internal skeletal structures are first formed in cartilage, and only as development proceeds does the transformation of these cartilages into bony elements take place"6
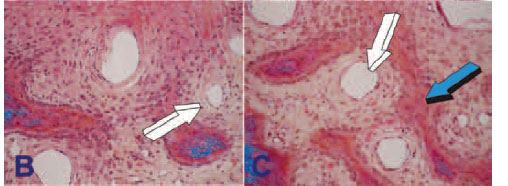
In mammals, the majority of the bones of the skeleton arise from a cartilage anlage that serves as a strut for the induction of bone formation, undergoing vascular invasion with capillary sprouting into the hypertrophic cartilage. This vascular invasion brings about chondrolysis, i.e. the death of the cartilage anlage, and the differentiation of the first waves of osteoblast-like cells. These differentiated cells lay down the early bone matrix, later to be mineralized to form the long bones of the mammalian skeleton (Fig. 1).
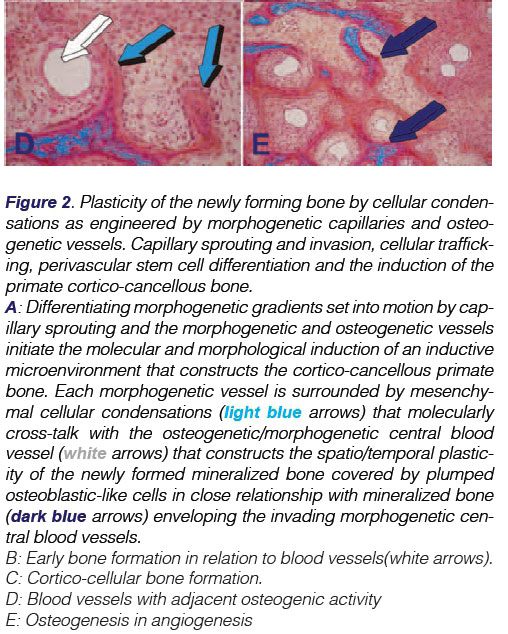
The critical role of angiogenesis in osteogenesis was described in detail in the middle of last century by the classic studies of Trueta which grandly provided the first insights into the supramolecular assembly of the extracellular matrix of bone.8 In several research experiments conducted at the Bone Research Laboratory, using either naturally-derived bone morphogenetic proteins9-11 or coral-derived macroporous constructs,12,13 the critical role of angiogenesis in osteogenesis has been characterised by defining the induction of bone formation as "osteogenesis in angiogenesis" (Fig. 2E) 10,11,14-16
Intriguingly, Aristotle (384-322BC) has been credited to have stated that forming blood vessels have a patterning function during organogenesis.16,17 This concept of "morphogenetic vessels" long predates the idea proposed by Trueta in 1963 of "osteogenetic vessels."8 The invading "organogenetic blood vessels" shape, pattern and induce the multistep cascade of the induction of bone formation, which is "osteogenesis in angiogenesis" (Fig. 2). An overview of the critical role of the vessels in bone formation was published in Science-in- Africa.18
Recently, a team of scientists has identified a specific vessel subtype in bone that links angiogenesis to osteogenesis.19 These specialized capillary sub-types, morphogenetic and osteogenet-ic vessels, provide niche signals to perivascular osteoprogenitor cells, as previously postulated by the grand insights of Aristotle and Trueta.8,11 As in mammals and as briefly discussed above, the cartilage anlage serves to initiate endochondral bone formation, that is a molecular and cellular morphogenetic event that forms the basis for the induction of endochondral bone formation, i.e. via a cartilaginous phase (Fig. 1B-D).10,11,15
Different molecular and morphogenetic events initiate the induction of bone formation via the intramembranous ossification pathway without a cartilage anlage. Angiogenesis with capillary sprouting is the three-dimensional construct for the induction of bone formation. Osteogenic precursor and other committed mes-enchymal cells aggregate and condense in selected areas of the craniofacial skeleton. The mesenchymal condensations are surfaced by multiple osteoblast-like cells and other committed precursors which embrace the invading capillaries. The blood vessels are morphogenetic since there is further differentiation of surrounding mesenchymal tissue condensations. Finally, the three-dimensional pattern of new vessels with capillary sprouting and invasion within the newly formed secreted matrix, engineers the osteonic primate cortico-cancellous bone, a process which is shown in the fascinating "morphogenetic" images presented in Figure 2. Further angiogenesis and development give rise to the majority of the cranial and craniofacial skeleton which forms via intramembranous ossification without the need for a cartilage anlage. The process is clearly both "morphogenetic" and "osteo-genetic" as per the Aristotelian and Trueta8 views and may be described as "osteogenesis in angiogenesis."11,16
We now know that the presence of a cartilaginous endoskeleton in sharks has replaced a bony skeleton in the early evolution of the Elasmobranchs. The question of course arises how indeed was degeneration of the bony skeleton possible? A recent paper in Nature5 provides important insights into the evolution of cartilaginous fishes. That molecular experimentation reports that Elasmobranchs lack a specific secretory calcium binding phos-phoprotein (SCPP).5
SCPP genes, arising from the Sparc-like1 (Sparcl1) gene family, have a crucial role in the formation of bone.5 It has been proposed, therefore, that the absence of the SCPP genes in the Elephant shark Callorhinchus milii will account for the absence of bone from the endoskeleton of the animal.5
The Feature Paper which follows describes our understanding of these evolutionary "de-differentiating" events from a bony to a cartilaginous endoskeleton invocating evolutionary speciation highly favorable to degenerate the bony endoskeleton thus blocking the induction of bone formation and skeletogenesis. These "de-differentiating" events in selachians returning to cartilaginous endoskeletons have set evolutionary specificity that resulted in more resilient animals, with higher capacities to float, more favorable to deep immersions and feeding into the oceans without breaking a bony endoskeleton.
As Moss clearly states in his essay on the "Skeletal Tissues in Sharks,"20the "cartilaginous endoskeleton of fossil Elasmobranchs is derived from phylogenetically ancestral forms with osseous endoskeletal tissues. In spite of the total lack of a bony endoskeleton, it has been emphasised that "bone does exist at the base (or pedicle) of the teeth and dermal denticles."20
Hence, and importantly, sharks may not lack osteogenic ability, or their differentiating chondroblasts may have an intrinsic or ge-nomic ability to differentiate into functional osteoblasts.20 Moss concludes that the "chondral" - or cartilaginous - state of the Elasmobranchs "was once believed to prove that cartilage preceded bone in vertebrate evolution". The acquisition however of a cartilaginous endoskeleton "does not establish a fundamental alteration of the potential and intrinsic ability of the Elasmobranchs' scleroblasts to modulate into osteoblasts."20
De-differentiation from a bony to a cartilaginous, or predominantly cartilaginous, endoskeleton in Elasmobranchs might have occurred following natural evolutionary selection resulting in the speciating of selected gene clusters encoding powerful inhibitors of angiogenesis. Cartilaginous matrices contain powerful morphogenetic signals that inhibit angiogenesis and capillary sprouting.21,22 Shark cartilages contain a substance that strongly inhibits the growth of new vessels which may well explain the rarity of malignant tumours in Elasmobranchs,23,2* and potentially could be used to inhibit tumour angiogenesis in humans.
Evolutionary pressure for superior habitats for feeding in the deeper waters of the oceans might have set in motion genetic mutations leading to the expression of several powerful inhibitors of angiogenesis, thus blocking the induction of bone formation.
The molecular and cellular cascades of osteogenesis via an en-dochondral pathway, as seen in mammals and in teleost fishes (those having an endoskeleton of bone) have been blocked in the shark by the inhibitors of angiogenesis which have been found in extant shark cartilage.
On consideration, there is an intriguing possibility that it may be possible to "reactivate" the osteogenic pathways in the shark. What biological mechanisms could be involved?
My research interest then was primarily focused on the induction of bone formation using morphogens, firstly defined in 1952 25 as "forms generating substances", and subsequently named "os-teogenin" or "bone morphogenetic proteins",9,26-28 following the colossal experimental work of several pioneers headed by Marshal Urist, at the Bone Research Laboratory at the University of California Los Angeles (UCLA). Urist published his fundamental and crucial studies in Science in a report titled "Bone: formation by autoinduction." 29 Hari A Reddi, at the Bone Cell Biology Section, NIH, Bethesda, made seminal discoveries on the chaotropic* dissociative extraction and reconstitution of the bone matrix components, published in the Proceedings of the National Academy of the Sciences, USA30,31
*A chaotropic agent is a molecule in water solution that can disrupt the hydrogen bonding network between water molecules (i.e. exerts chaotropic activity).
In this context, the question was then formulated whether the bone morphogenetic proteins would have the capacity to "force" endochondral bone formation and/or direct intramembranous bone formation by inducing bone in heterotopic intramuscular sites of cartilaginous Selachian recipients.
The idea of the experiment was conceived at the famous Oyster Box Hotel in Umhlanga. The Director of the Natal Shark Board granted permission to use their fast boats which continuously check the off-shore shark nets along the white beaches of the North Coast. (Figure 3A.) The Animal Ethics Committee of the University approved the intention to fish sharks and to harvest chon-drocrania and vertebrae to enable the extraction of whatever mor-phogenetic factors Selachians may have within their cartilaginous matrices. In three expeditions off the shores of Umhlanga several dusky sharks and a larger shark, Carcharinus taurus, were fished out the ocean and enough quantities of cartilages were then secured (Fig 3B.)
At the laboratories of the then Dental Research Institute in Johannesburg Laura Yeates and I undertook the attempt at the extraction process from cartilage taken from the large shark Carchari-nus taurus, using techniques we had learnt when studying the purification of naturally-derived bone morphogenetic proteins from bovine bones.28 Laura, already attached to the embryonic and emerging Bone Research Laboratory within the Dental Research Institute of the University, was confronted by the ultra-viscous extracted material overly rich in high molecular weight mu-co-polysaccarides that characterized the cartilaginous extracts. Several attempts to extract and purify the shark proteins resulted in partially purified morphogenetic factors.
Several months elapsed in the year 1989 without further experimentation and in 1990 I decided to invite Laura to join me at the Bone Cell Biology Section of the NIH in Bethesda. In collaboration with Dr AH Reddi, the then Chief of the Bone Cell Biology Section, Laura Yeates and I purified, to homogeneity, osteogenin, a bone morphogenetic protein, from baboon bone matrices (Fig. 1 B)9. An account of our mutual interactions during that rewarding and exhilarating scientific period was published some years later in Science in Africa?833 highlighting not only the extraction and purification of bone morphogenetic proteins from baboon bone matrices but also the grand effect of geometry on the induction of bone formation by culturing in vitro osteoprogenitor cells on specific geometric configurations of coral-derived macroporous bioreactors. The work was also published in a classic paper in Biomaterials32
An account of our endeavours was published by Science in Africa in 2012.33 Together we had cracked the purification to homogeneity of osteogenin from baboon bone matrices and defined the critical role of geometry on the induction of bone formation. These experiments were described at length in two papers in 2012.33,34
After our success with the baboon proteins, we thought to again try to extract and purify proteins from Selachian cartilages, but using different chromatographic procedures. The samples had been flown with Laura and carried in her hand luggage from Africa to Washington DC. The extracted morphogenetic factors were later implanted in the subcutaneous space of rodents and uniquely and provocatively also implanted into the muscle of adolescent dusky sharks Carcharinus obscurus in a series of in vivo experiments in the salty water ponds of the Oceanographic Research Institute, Marine Parade, Durban, under the blue skies of the subtropical African sun.
My primary dedication was then, as it is now, the induction of bone formation in non-human and human primates using bone morphogenetic proteins,33-36 as well as exploring the induction of bone formation by the mammalian transforming growth factor-p (TGF-(3) isoforms. 14,37-39 The work has continued with unique research results on the substantial induction of bone formation by the hTGF-(33 morphogen (Figs. 1 E,F), later published in the Journal of Cellular Molecular Medicine.40
Whilst working as a learning and developing scientist at the then Dental Research Institute of the University, I had become interested also in the unique dentition of shark species that showed superb evolutionary traits for survival and predation.20 Of particular intrigue was the polyphyodonty (several rows of multiple teeth) in Selachians, which is the genesis of continuously erupting teeth that migrate forward until, like rolling over the edges of the cartilaginous jaws, they are shed, leaving space for the replacement row of the newly formed and forward-moving teeth (Figs. 4 A,B). Biologically, this is rendered possible by the presence of a continuously erupting dental lamina located in the mucous membrane behind the rear rank of the phalanx (Fig. 4 C).41
It is the intention of the following Feature Paper to describe the extraction and purification of Selachian's cartilages and the implantation of the extracted morphogenetic factors both in rodents and in Carcharinus obscurus sharks at the Oceanographic Research Institute in Durban. The manuscript also details the attachment apparatus of the connective tissue matrix to dentine or dentine/ like material of the multiple shark teeth and describes the forward movement and migration of the Selachian's teeth by a mechanism of a conveyor belt of condensed mesenchymal cells packed with fine connective tissue fibres that by tractional forces between cells and secreted matrix must move the rows of teeth forward until their exfoliation.
The Feature Paper additionally highlights the fact that the loss of teeth is physiologic in sharks but pathologic in mammals, and that tooth shedding in Selachian fishes is the conditio sine qua non for the ancestral evolutionary predatory habit of the sharks amidst the richly populated waters of our oceans.
We show that the implantation of coral-derived macroporous bio-reactors induce the differentiation of chondroblastic tissue and the differentiation of cartilage within the macroporous spaces, indicating the lack of an overt osteogenetic programme within the DNA of the Selachian cartilage.
We further propose that the lack of the induction of bone formation in Selachian intramuscular sites is the result of deficient angiogen-esis and vascular invasion in the Selachian cartilages because of the overtly rich anti-angiogenic factors within the cartilaginous matrices21-24 which block "osteogenesis in angiogenesis".